A Fluorine-Containing Main-Chain Benzoxazine/Epoxy Co-Curing System with Low Dielectric Constants and High Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
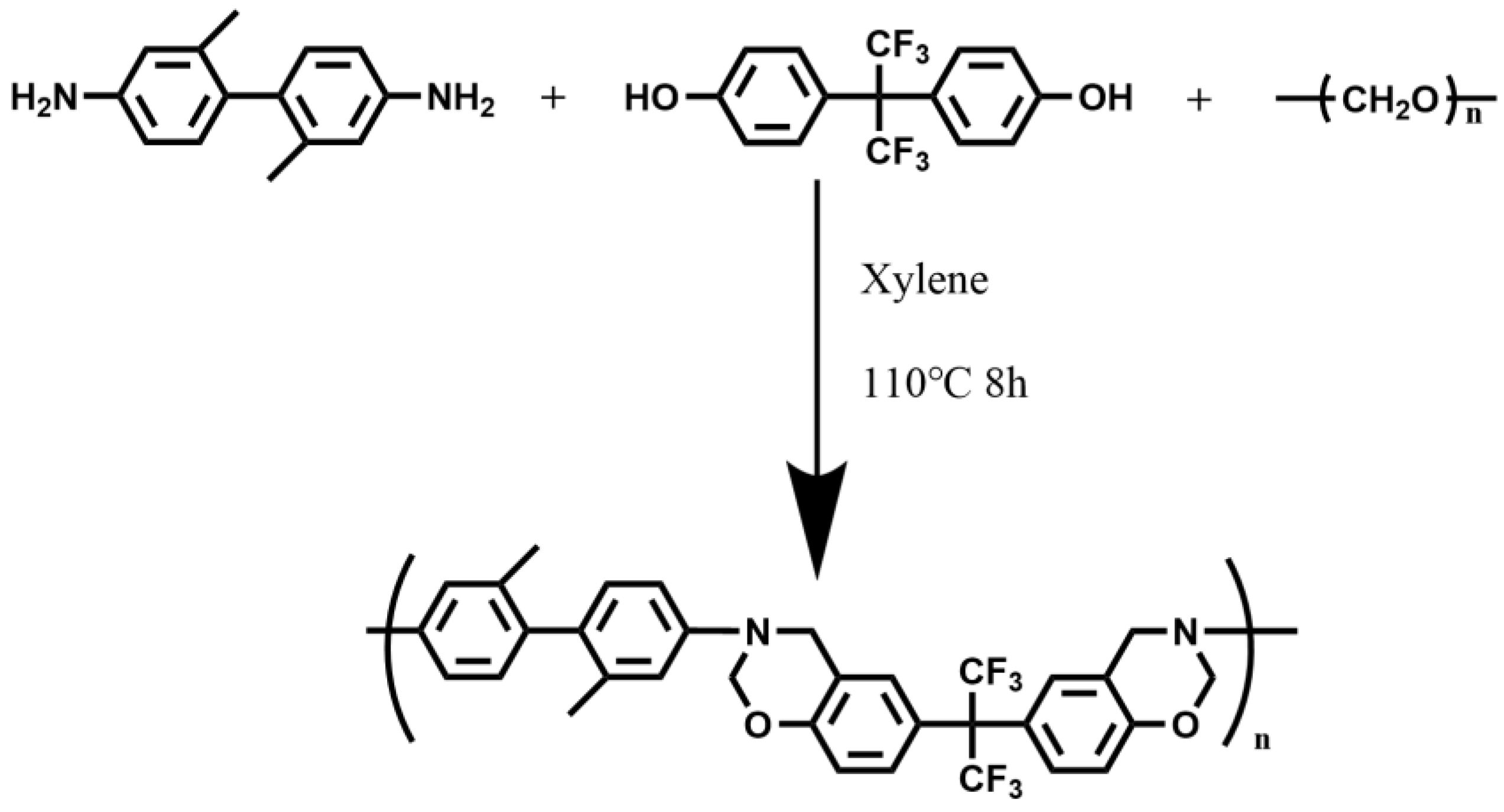
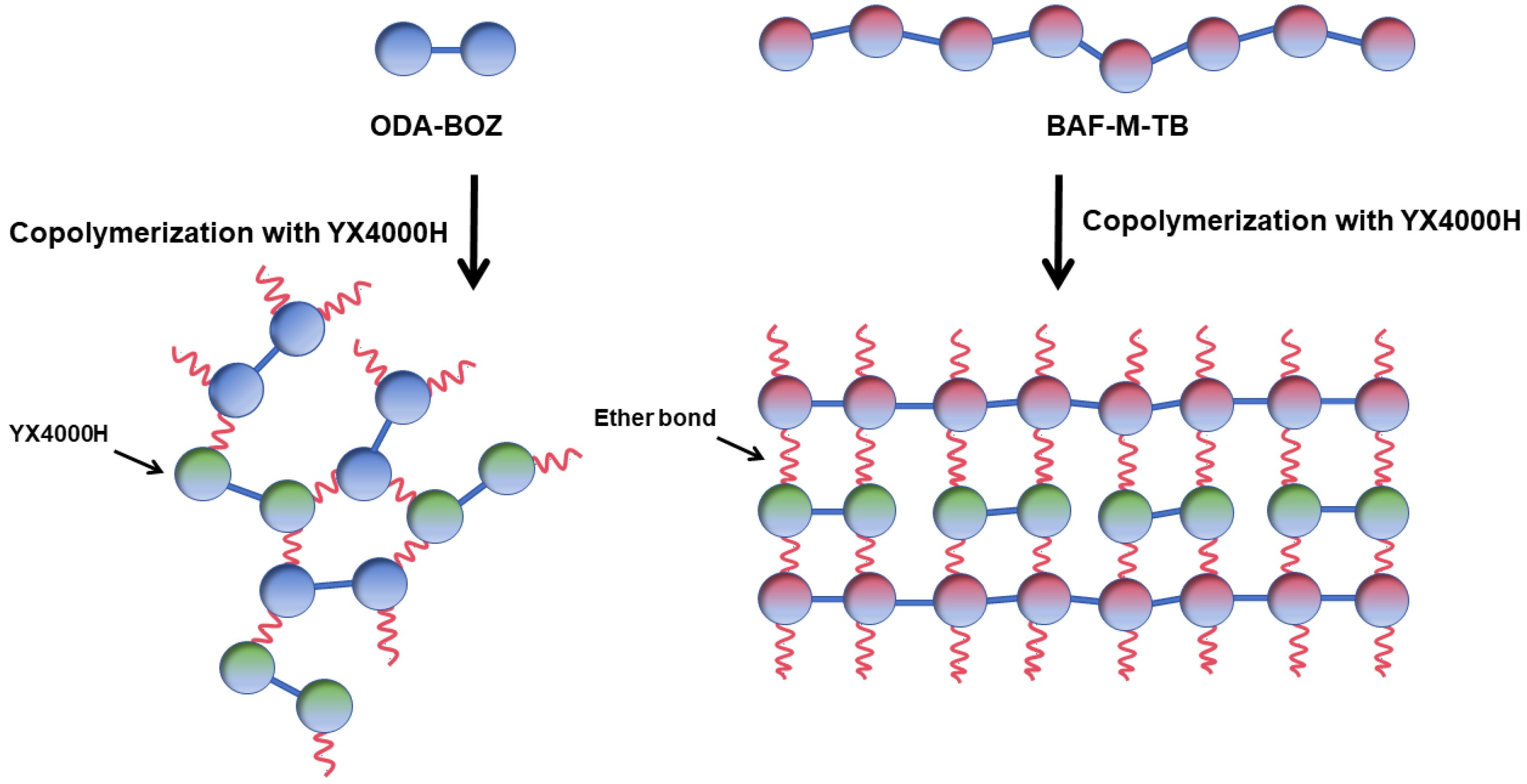
2.2. Synthesis of BAF-M-TB
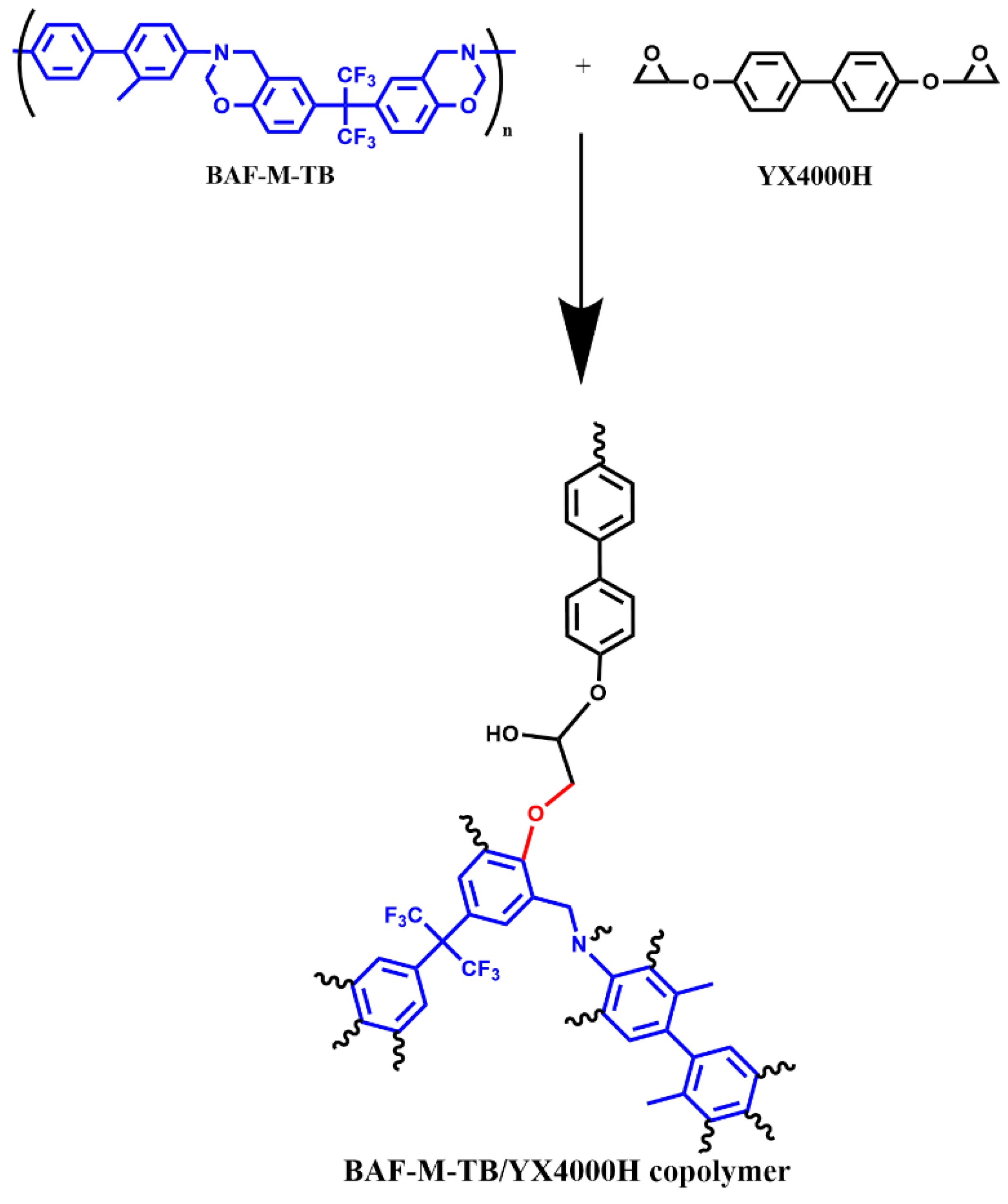
2.3. Preparation of the EP/BAF-M-TB and EP/ODA-BOZ Blend
2.4. Preparation of EP/BAF-M-TB QFRP Composites
2.5. Preparation of EP/ODA-BOZ QFRP Composites
2.6. Representation
2.6.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6.2. Nuclear Magnetic Resonance (NMR)
2.6.3. Size Exclusion Chromatography (SEC)
2.6.4. Differential Scanning Calorimetry (DSC)
2.6.5. Dynamic Rheological Analysis
2.6.6. Dynamic Thermal Mechanical Analysis (DMA)
2.6.7. Thermogravimetric Analysis (TGA)
2.6.8. Flexural Test
2.6.9. Dielectric Property Measurement
3. Results and Discussion
3.1. Chemical Structural Characterization of BAF-M-TB
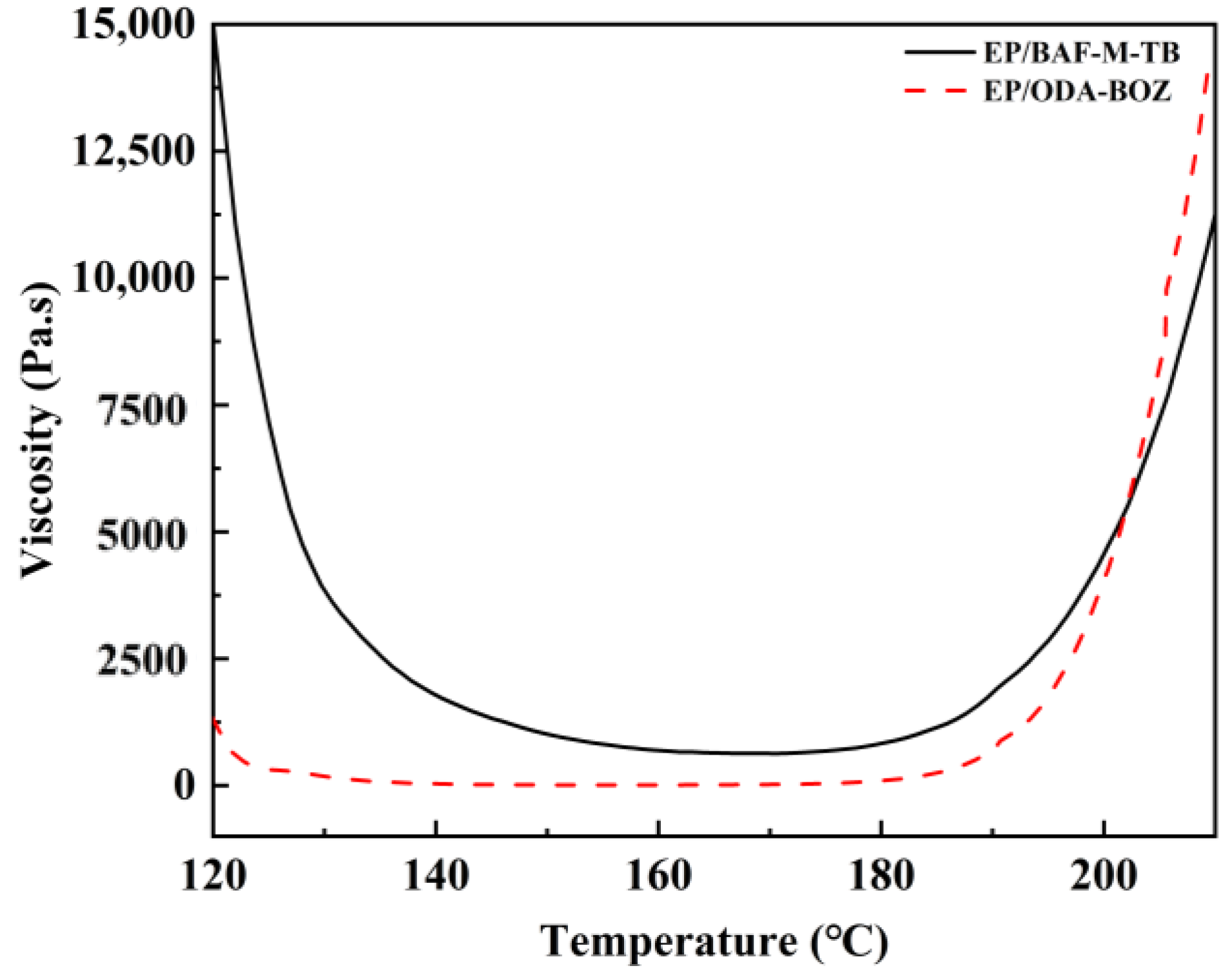
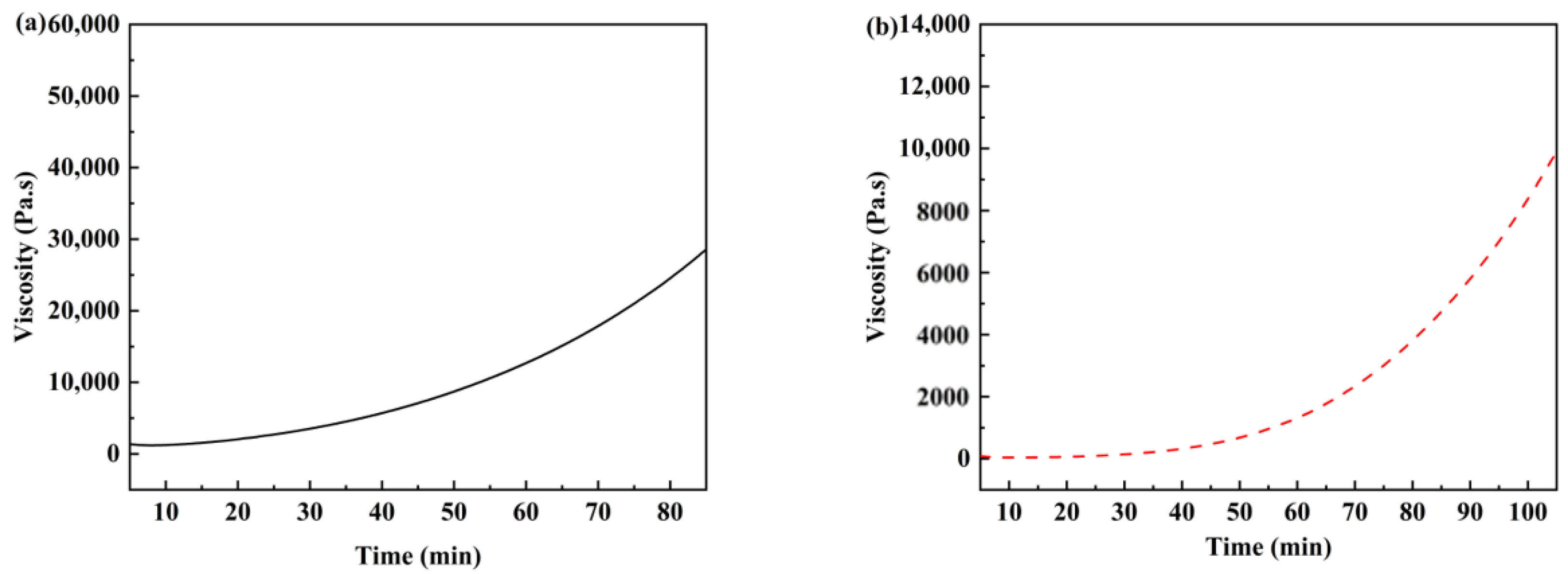
3.2. Curing, Rheological Behavior, and Processing of the Copolymer
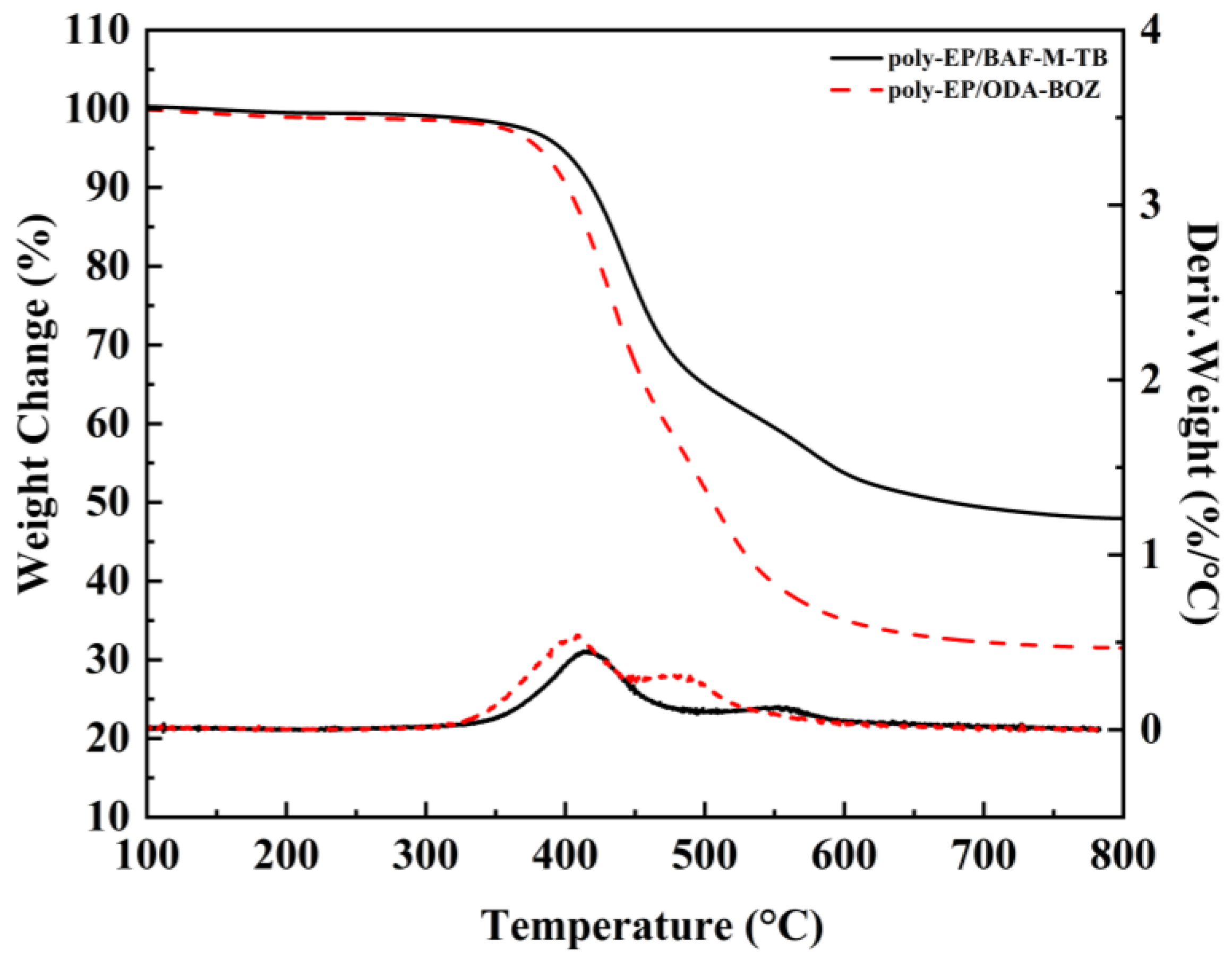
3.3. Thermal Stability of Poly-EP/BAF-M-TB and Poly-EP/ODA-BOZ
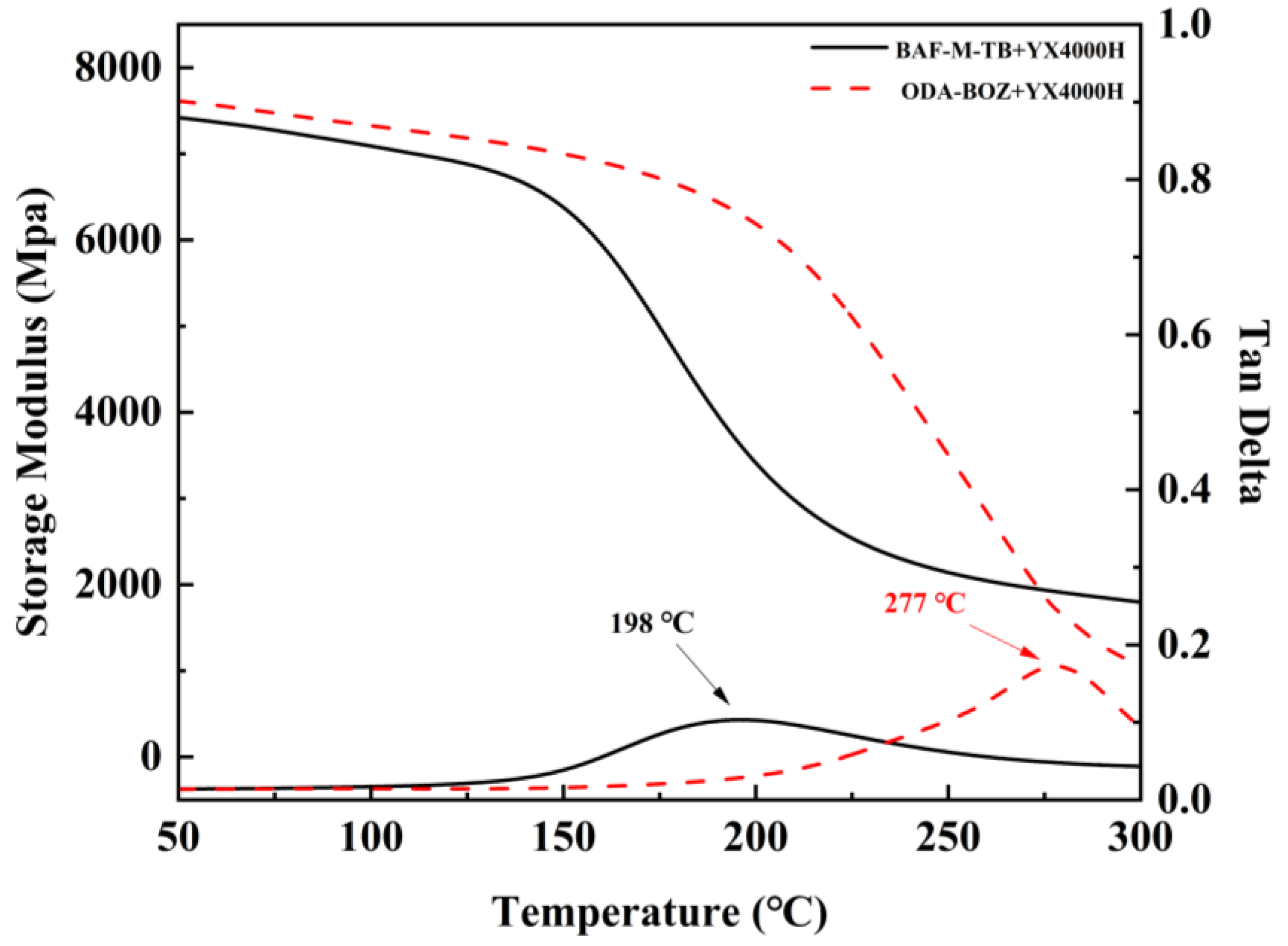
3.4. Thermal and Mechanical Properties of Poly-EP/BAF-M-TB QFRP and Poly-EP/ODA-BOZ QFRP
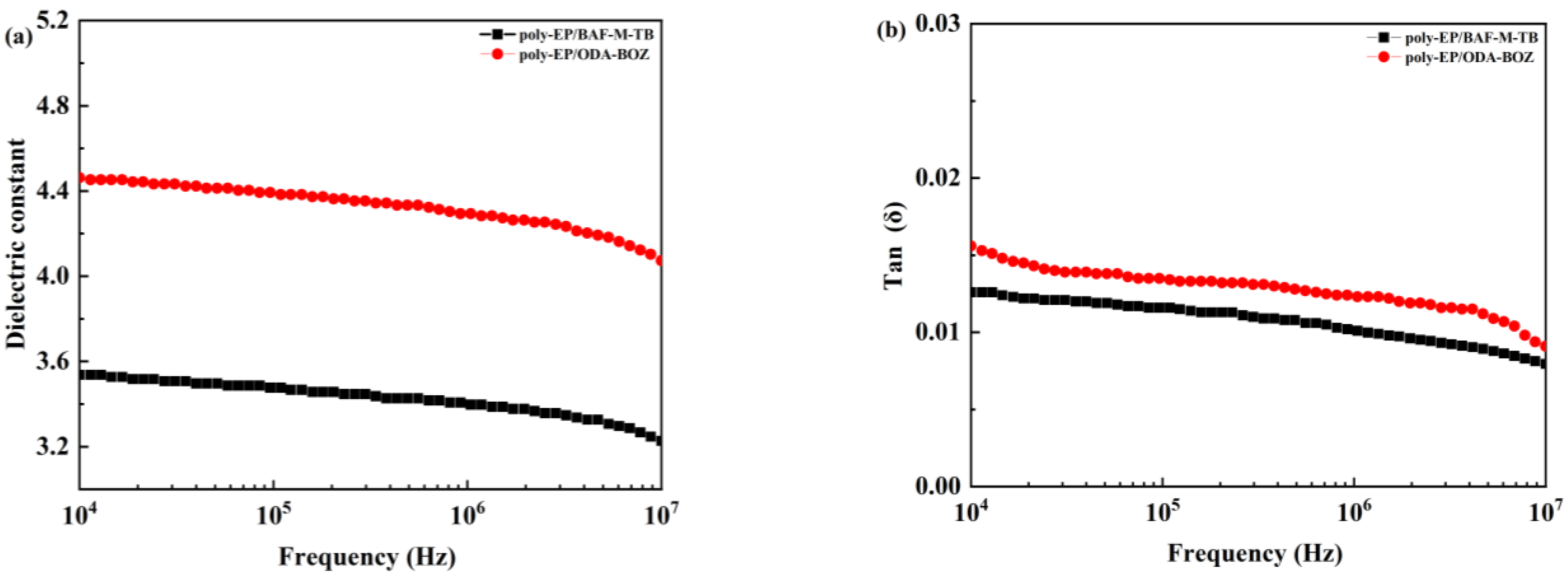
3.5. Dielectric Properties of Poly-EP/BAF-M-TB QFRP and Poly-EP/ODA-BOZ QFRP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amiri Delouei, A.; Emamian, A.; Sajjadi, H.; Atashafrooz, M.; Li, Y.; Wang, L.-P.; Jing, D.; Xie, G. A comprehensive review on multi-dimensional heat conduction of multi-layer and composite structures: Analytical Solutions. J. Therm. Sci. 2021, 30, 1875–1907. [Google Scholar] [CrossRef]
- Groby, J.P.; De Ryck, L.; Leclaire, P.; Wirgin, A.; Lauriks, W.; Gilbert, R.P.; Xu, Y.S. Use of specific Green’s functions for solving direct problems involving a heterogeneous rigid frame porous medium slab solicited by Acoustic Waves. Math. Methods Appl. Sci. 2006, 30, 91–122. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Yang, S. Quartz fiber cloth-reinforced semi-aromatic thermosetting polyimide composite with high-frequency low dielectric constant. High Perform. Polym. 2020, 32, 91–102. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Feng, Z.; Yin, D.; Huang, Y.; Chen, Y.; Yan, C.; Li, R.; Gu, Y. Understanding the effects of carboxylated groups of functionalized graphene oxide on the curing behavior and intermolecular interactions of benzoxazine nanocomposites. RSC Adv. 2016, 6, 31484–31496. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, Y.S.; Lin, H.T.; Wang, C.F. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Zeng, M.; Tan, D.; Feng, Z.; Chen, J.; Lu, X.; Huang, Y.; Xu, Q. Multistructural Network Design Enables Polybenzoxazine to Achieve Low-Loss-Grade Super-High-Frequency Dielectric Properties and High Glass Transition Temperatures. Ind. Eng. Chem. Res. 2021, 61, 115–129. [Google Scholar] [CrossRef]
- Lin, C.H.; Huang, S.J.; Wang, P.J.; Lin, H.T.; Dai, S.A. Miscibility, Microstructure, and Thermal and Dielectric Properties of Reactive Blends of Dicyanate Ester and Diamine-Based Benzoxazine. Macromolecules 2012, 45, 7461–7466. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Thermosets: Structure, Properties, and Applications, 2nd ed.; Elsevier: Amsterdam, Howland, 2018. [Google Scholar]
- Dunkers, J.P. Vibrational, Crystallographic, and Mechanistic Studies of Benzoxazine Monomers and Their Resulting Polybenzoxazines as Novel Phenolic Resins. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 1994. [Google Scholar]
- Rao, B.; Rajavardhana Reddy, K.; Pathak, S.K.; Pasala, A. Benzoxazine–epoxy copolymers: Effect of molecular weight and crosslinking on thermal and viscoelastic properties. Polym. Int. 2005, 54, 1371–1376. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Lee, H.; Kristoffer, N. Handbook of Epoxy Resins; Mcgraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Xu, M.; Yang, X.; Zhao, R.; Liu, X. Copolymerizing behavior and processability of benzoxazine/epoxy systems and their applications for glass fiber composite laminates. J. Appl. Polym. Sci. 2013, 128, 1176–1184. [Google Scholar] [CrossRef]
- Peng, C.; Gao, C.; Yuan, Y.; Wu, Z.; Zhou, D. Synthesis and application of a benzoxazine-type phosphorus-containing monomer on epoxy/benzoxazine copolymer: Thermal stability and compatibility with Liquid Oxygen. Polym. Degrad. Stabil. 2018, 157, 131–142. [Google Scholar] [CrossRef]
- Rimdusit, S.; Kunopast, P.; Dueramae, I. Thermomechanical Properties of Arylamine-Based Benzoxazine Resins Alloyed with Epoxy Resin. Polym. Eng. Sci. 2011, 51, 1797–1807. [Google Scholar] [CrossRef]
- Alhassan, S.M.; Qutubuddin, S.; Schiraldi, D.A.; Agag, T.; Ishida, H. Preparation and thermal properties of graphene oxide/main chain benzoxazine polymer. Eur. Polym. J. 2013, 49, 3825–3833. [Google Scholar] [CrossRef]
- Chernykh, A.; Agag, T.; Ishida, H. Effect of Polymerizing Diacetylene Groups on the Lowering of Polymerization Temperature of Benzoxazine Groups in the Highly Thermally Stable, Main-Chain-Type Polybenzoxazines. Macromolecules 2009, 42, 5121–5127. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Chen, J.; Zeng, S.; Huang, Y.; Feng, Z.; Xu, Q.; Yan, C.; Gu, Y. Synthesis, polymerization kinetics, and high-frequency dielectric properties of novel main-chain benzoxazine copolymers. React. Funct. Polym. 2018, 122, 158–166. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Li, S.; Shang, Z.; Wang, J. Remarkable improvement of thermal stability of main-chain benzoxazine oligomer by incorporating o-norbornene as terminal functionality. J. Appl. Polym. Sci. 2017, 134, 45408. [Google Scholar] [CrossRef]
- Wang, M.W.; Jeng, R.J.; Lin, C.H. The robustness of a thermoset of a main-chain type polybenzoxazine precursor prepared through a strategy of A–A and B–B polycondensation. RSC Adv. 2016, 6, 18678–18684. [Google Scholar] [CrossRef]
- Agag, T.; Geiger, S.; Alhassan, S.M.; Qutubuddin, S.; Ishida, H. Low-Viscosity Polyether-Based Main-Chain Benzoxazine Polymers: Precursors for Flexible Thermosetting Polymers. Macromolecules 2010, 43, 7122–7127. [Google Scholar] [CrossRef]
- Yi, G.; Qi Chao, R. Polybenzoxazine—Principle, Performance and Application; Science Press: Beijing, China, 2018. [Google Scholar]
- Li, J.; Chen, M.; Wang, Y. Preparation and properties of a fluorinated epoxy resin with low dielectric constant. J. Appl. Polym. Sci. 2022, 139, 52132. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Han, E.; Niu, H.; Wu, D. Structure-property relationship of low dielectric constant polyimide fibers containing fluorine groups. Polymer 2020, 206, 122884. [Google Scholar] [CrossRef]
- Pattharasiriwong, P.; Jubsilp, C.; Mora, P.; Rimdusit, S. Dielectric and thermal behaviors of fluorine-containing dianhydride-modified polybenzoxazine: A molecular design flexibility. J. Appl. Polym. Sci. 2017, 134, 45204. [Google Scholar] [CrossRef]
- Parveen, A.S.; Thirukumaran, P.; Sarojadevi, M. Low dielectric materials from fluorinated polybenzoxazines. Polym. Adv. Technol. 2014, 25, 1538–1545. [Google Scholar] [CrossRef]
- Lu, A.; Lin, H.; Yuan, M.; Shao, L.; Lu, X.; Xin, Z. Preparation of biphenyl-containing polybenzoxazines with low dielectric constants and high thermal stability. Polymer 2023, 280, 126018. [Google Scholar] [CrossRef]
- Shang, C.-Y.; Zhao, X.-J.; Yang, X.; Zhang, Y.; Huang, W. Epoxy resin containing trifluoromethyl and pendant polyfluorinated phenyl groups: Synthesis and properties. High Perform. Polym. 2012, 24, 683–691. [Google Scholar] [CrossRef]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and Characterization of Highly Fluorinated Polymer with the Benzoxazine Moiety in the Main Chain. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- GB/T 9341-2008/ISO 178; Plastics-Determination of Flexural Properties. Chinese Standard Press: Beijing, China, 2001.
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. Critical investigation of methods for kinetic analysis of thermoanalytical data. J. Therm. Anal. 1975, 7, 601–617. [Google Scholar] [CrossRef]
- Jubsilp, C.; Punson, K.; Takeichi, T.; Rimdusit, S. Curing kinetics of Benzoxazine–epoxy copolymer investigated by non-isothermal differential scanning calorimetry. Polym. Degrad. Stabil. 2010, 95, 918–924. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Chen, Y.F.; Liu, H.B. Pyrolysis of Phenolic Resin by TG-MS and FTIR Analysis. Adv. Mat. Res. 2013, 631–632, 104–109. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Vautard, F.; Meyer, H.M.; Messman, J.M.; Tolnai, B.; Naskar, A.K. Methanol Fractionation of Softwood Kraft Lignin: Impact on the Lignin Properties. ChemSusChem 2014, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, M.; Ran, Q. The study on curing and weight-loss mechanisms of benzoxazine during thermal curing process. Polym. Degrad. Stabil. 2020, 179, 109279. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Q.; Gu, Y. Preparation and properties of benzoxazine blends with intumescent flame retardancy. Polym. Degrad. Stabil. 2019, 163, 15–24. [Google Scholar] [CrossRef]
- Hamerton, I.; Brendan, J.H.; Alyssa, D.M.; Lisa, T.M.; Shinji, T. Systematic examination of thermal, mechanical and dielectrical properties of aromatic polybenzoxazines. React. Funct. Polym. 2012, 72, 736–744. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Cheng, W.; Zou, J.; Zhao, D. Progress on Polymer Composites with Low Dielectric Constant and Low Dielectric Loss for High-Frequency Signal Transmission. Front. Mater. 2021, 8, 774843. [Google Scholar] [CrossRef]




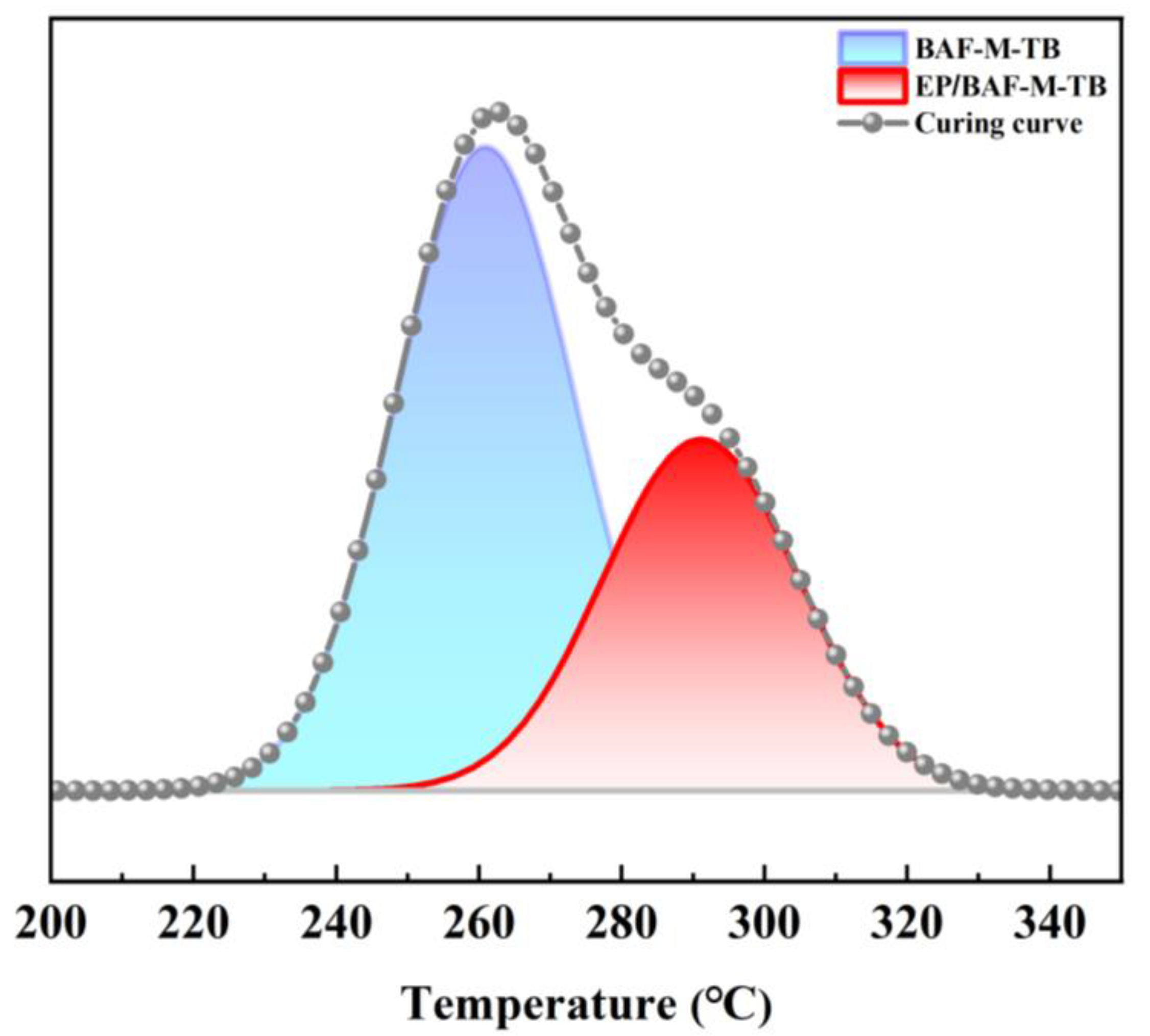





| Curing Behavior | Heating Rate (°C/min) | Ti (°C) | Tp (°C) | Td (°C) | Ea (kJ/mol) (Kissinger Method) | Ea (kJ/mol) (Ozawa Method) | Ea (kJ/mol) (Average) |
|---|---|---|---|---|---|---|---|
| BAF-M-TB self-polymerization | 5 | 207.1 | 243.8 | 281.7 | 103 | 106.3 | 104.65 |
| 10 | 220.3 | 260.9 | 294.3 | ||||
| 15 | 236 | 269.1 | 300 | ||||
| 20 | 229 | 270.8 | 307.4 | ||||
| EP/BAF-M-TB copolymerization | 5 | 251.3 | 283.3 | 313.3 | 189.6 | 189.1 | 189.35 |
| 10 | 259 | 291 | 322.3 | ||||
| 15 | 271 | 296.3 | 327 | ||||
| 20 | 283.3 | 302.1 | 318 | ||||
| EP/ODA-BOZ copolymerization | 5 | 175 | 229 | 271.3 | 78.3 | 82.6 | 80.45 |
| 10 | 184.5 | 244.7 | 287 | ||||
| 15 | 188.3 | 253.3 | 296.8 | ||||
| 20 | 193.3 | 265.7 | 301 |
| Sample | Td5 (°C) | Td10 (°C) | CY800 (%) |
|---|---|---|---|
| Poly-EP/BAF-M-TB | 398 | 420 | 47.95 |
| Poly-EP/ODA-BOZ | 380 | 401 | 31.50 |
| Sample | Dielectric Constant at 5 GHz | Dielectric Loss at 5 GHz |
|---|---|---|
| Poly-EP/BAF-M-TB | 3.16 | 0.016 |
| Poly-EP/ODA-BOZ | 4.01 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, W.; Ding, Z.; Jiao, J.; Wang, J.; Zhang, W. A Fluorine-Containing Main-Chain Benzoxazine/Epoxy Co-Curing System with Low Dielectric Constants and High Thermal Stability. Polymers 2023, 15, 4487. https://doi.org/10.3390/polym15234487
Zhang T, Zhang W, Ding Z, Jiao J, Wang J, Zhang W. A Fluorine-Containing Main-Chain Benzoxazine/Epoxy Co-Curing System with Low Dielectric Constants and High Thermal Stability. Polymers. 2023; 15(23):4487. https://doi.org/10.3390/polym15234487
Chicago/Turabian StyleZhang, Tinghao, Wenzheng Zhang, Zichun Ding, Jianjian Jiao, Jinyan Wang, and Wei Zhang. 2023. "A Fluorine-Containing Main-Chain Benzoxazine/Epoxy Co-Curing System with Low Dielectric Constants and High Thermal Stability" Polymers 15, no. 23: 4487. https://doi.org/10.3390/polym15234487
APA StyleZhang, T., Zhang, W., Ding, Z., Jiao, J., Wang, J., & Zhang, W. (2023). A Fluorine-Containing Main-Chain Benzoxazine/Epoxy Co-Curing System with Low Dielectric Constants and High Thermal Stability. Polymers, 15(23), 4487. https://doi.org/10.3390/polym15234487






