Projection Micro-Stereolithography to Manufacture a Biocompatible Micro-Optofluidic Device for Cell Concentration Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
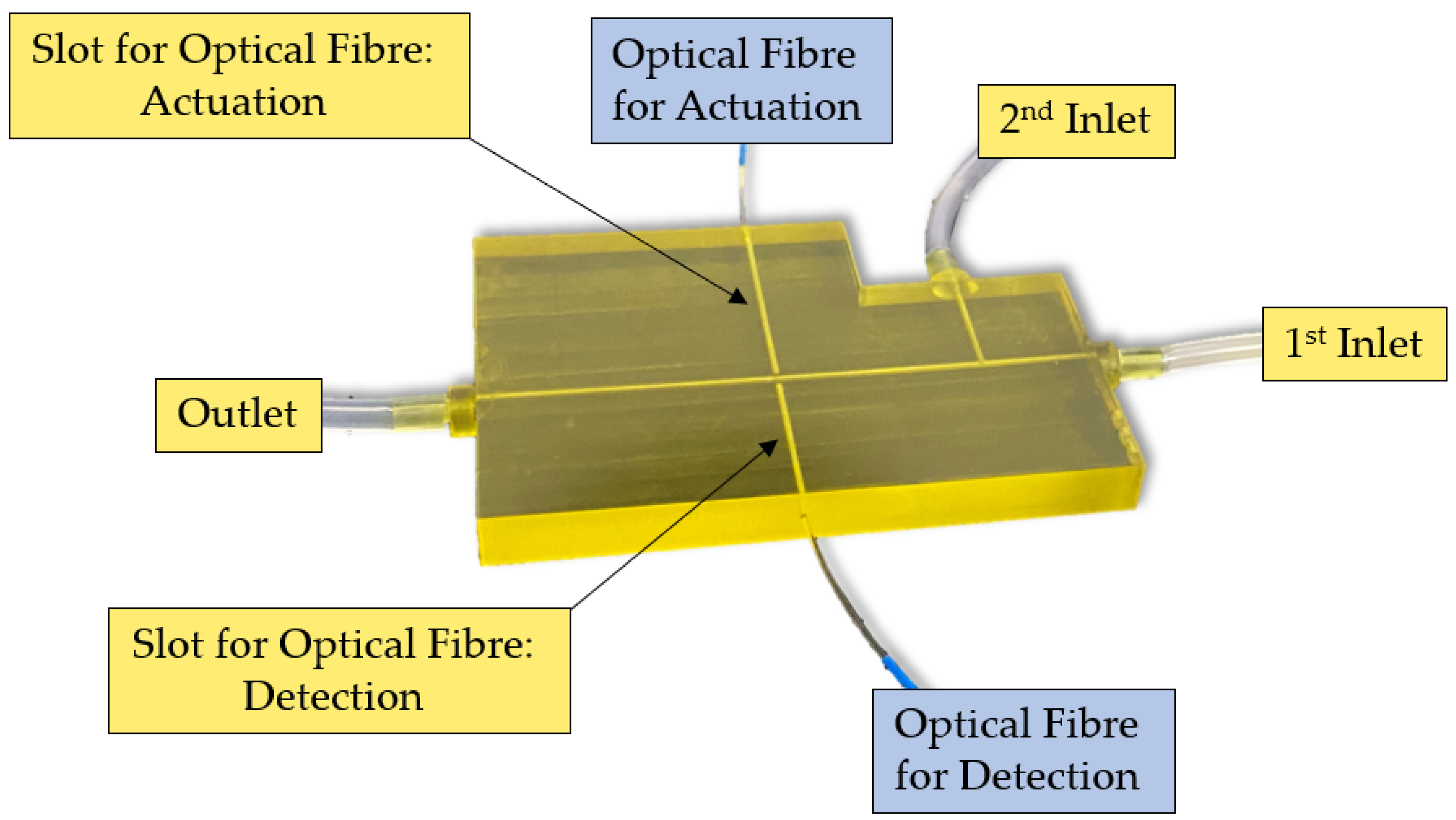
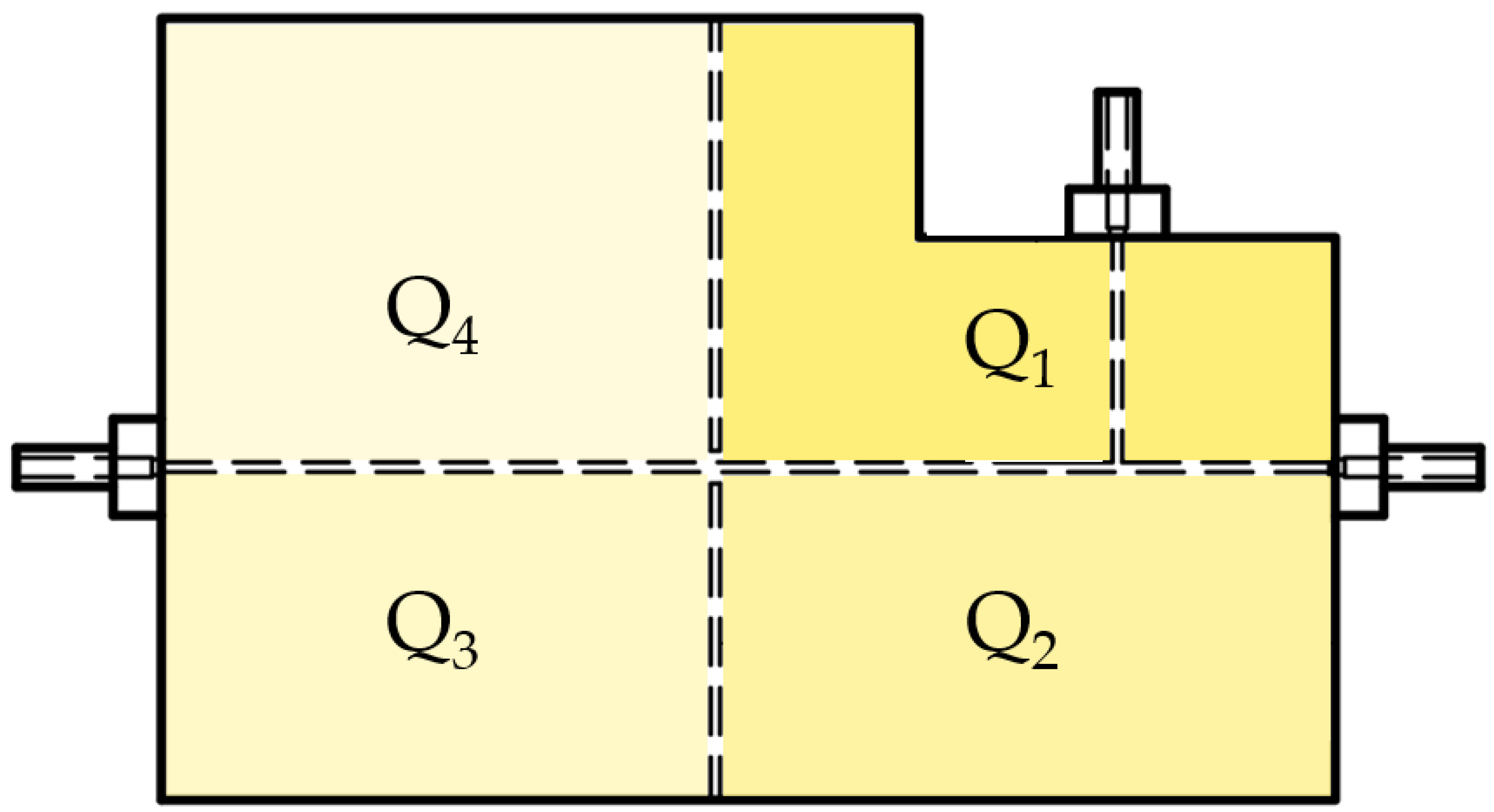
2.2. MoF Device: Design, Manufacturing, and Working Principle
- (i)
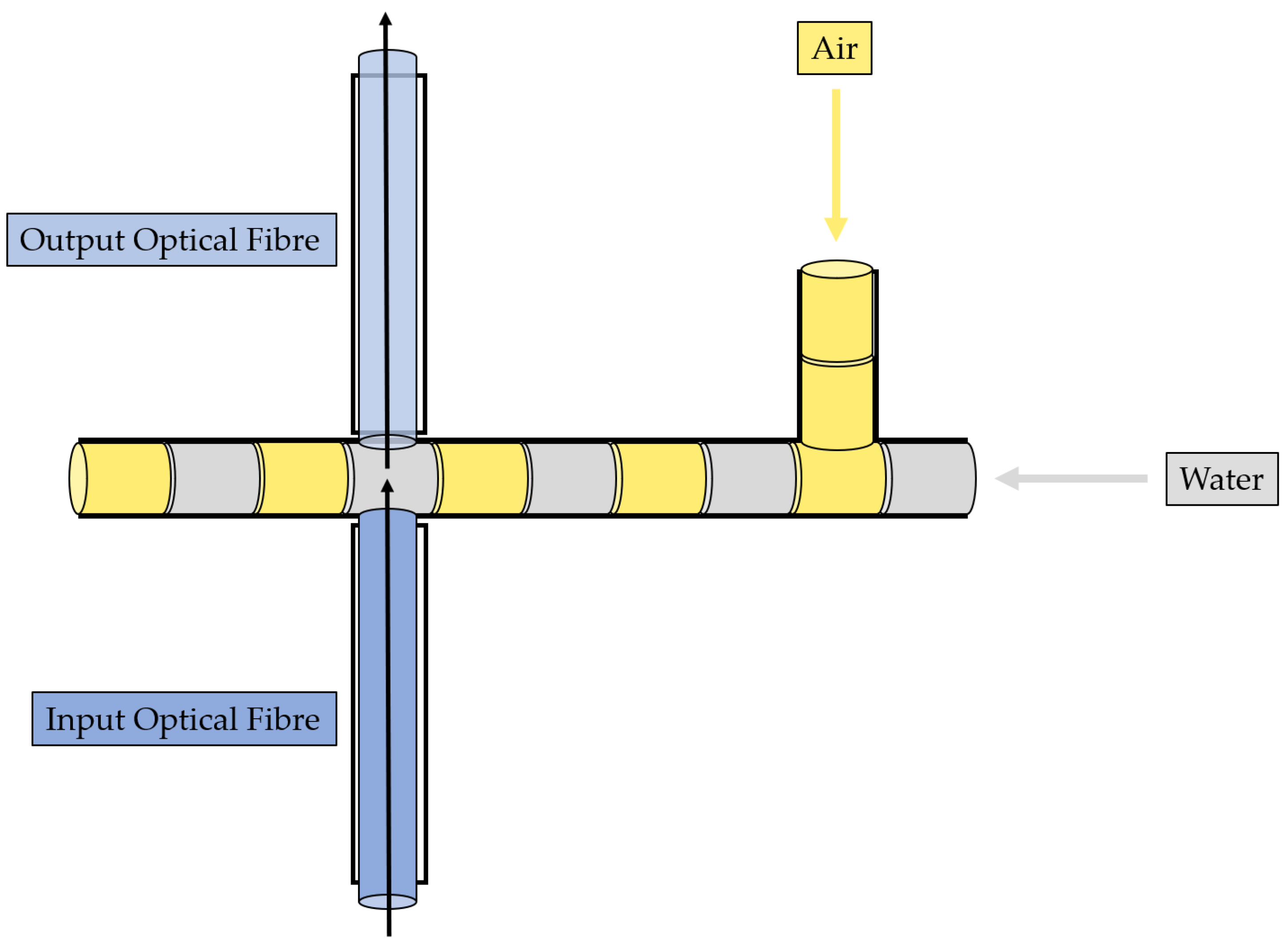
- Immiscible gas–liquid two-phase flow detection—its working principle is reported in Figure 2 and relies on the absorption phenomenon. In fact, depending on the fluid’s refractive index value, its interaction with the incident laser beam determines a different nature of light transmission. Thus, in turn, the acquired optical signal has a different amplitude depending on the fluid with which it is interacting at a precise moment. More deeply, the acquired optical signal has a square wave shape, characterized by two levels corresponding to each fluid making up the two-phase flow;
- (ii)
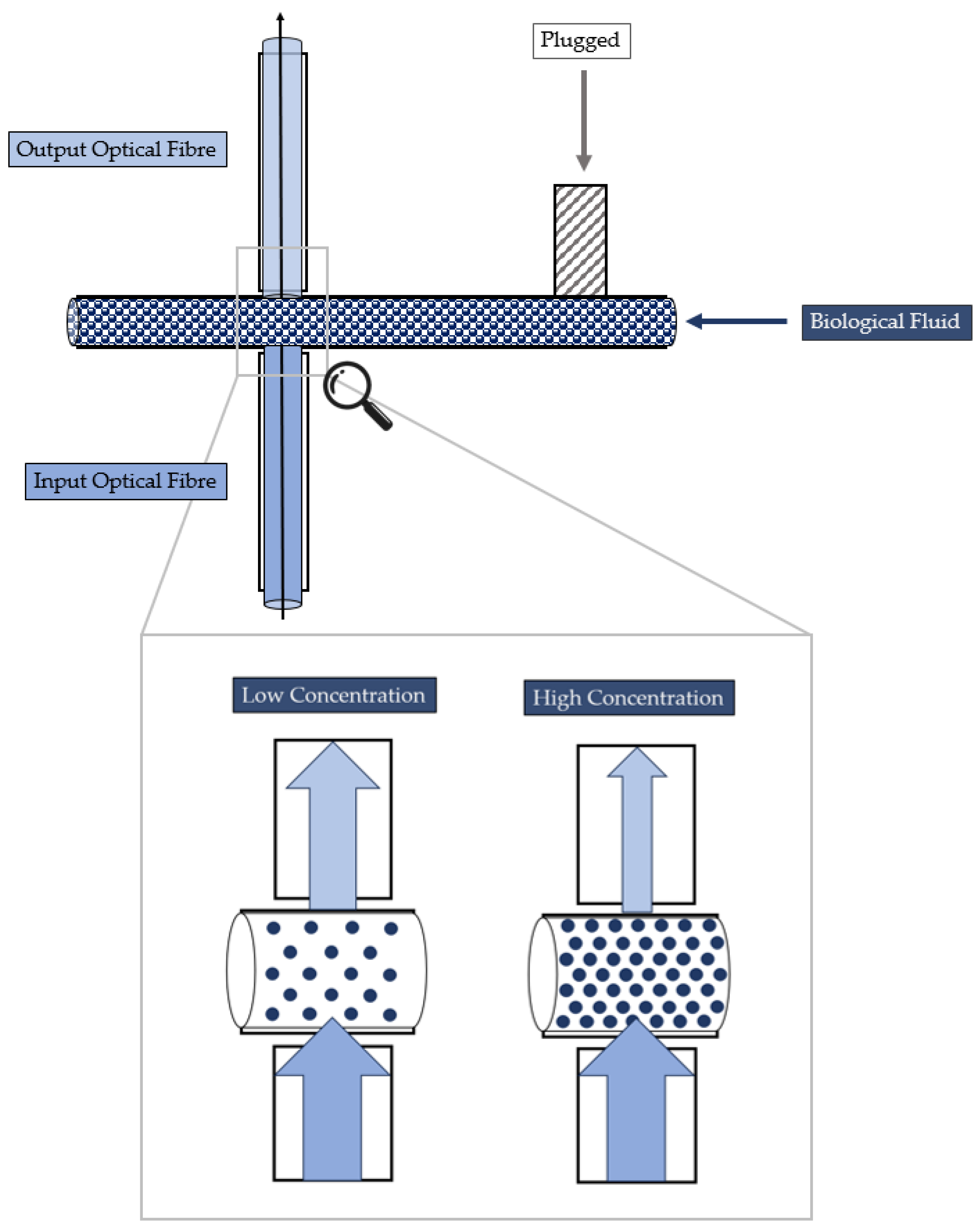
- Cell concentration monitoring—its working principle is reported in Figure 3 and exploits the cell–light interaction linked to the different cell concentration. The higher the concentration, the greater the number of cells that interact with the light, which, in turn, causes the light’s back-scattering. Thus, as consequence, the beam does not reach the outgoing optical fibre. Consequently, with increasing concentrations, there is a corresponding decrease in the measured levels of light intensity.
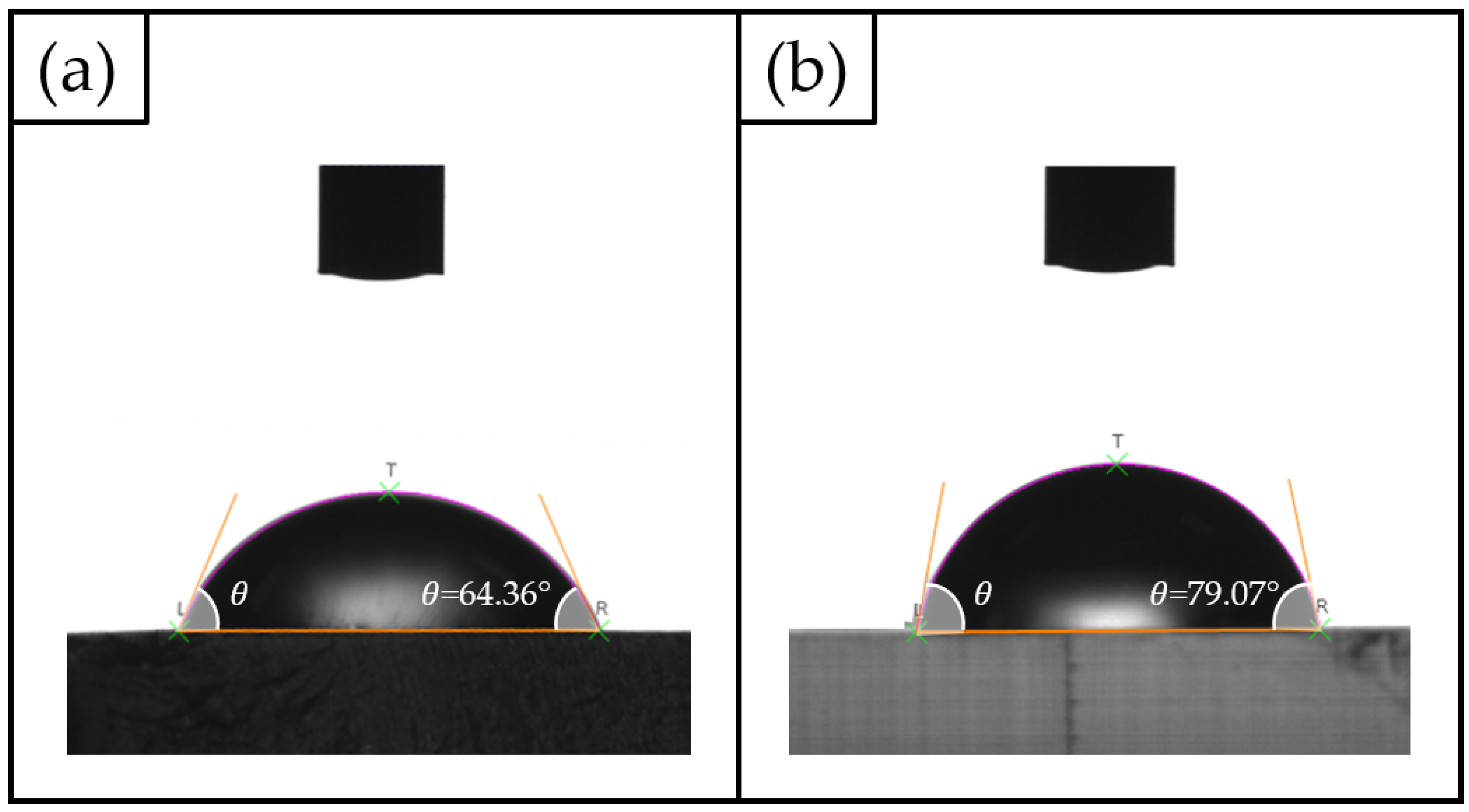
2.3. Surface Characterization: Static Water Contact Angle and Roughness Measurements
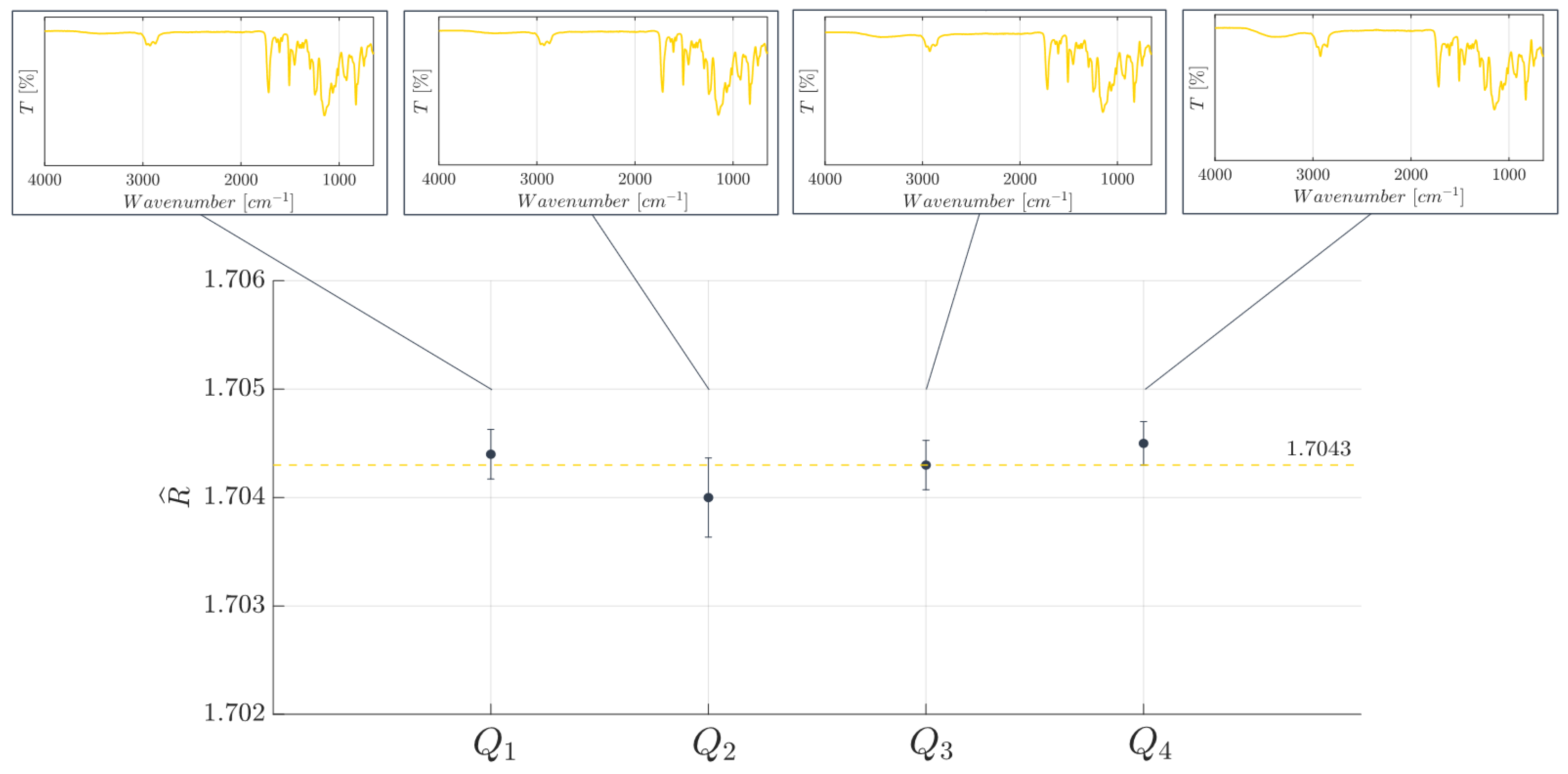
2.4. Cross-Linking State of BIO Resin: FT-IR ATR Analysis and Refractive Index Estimation
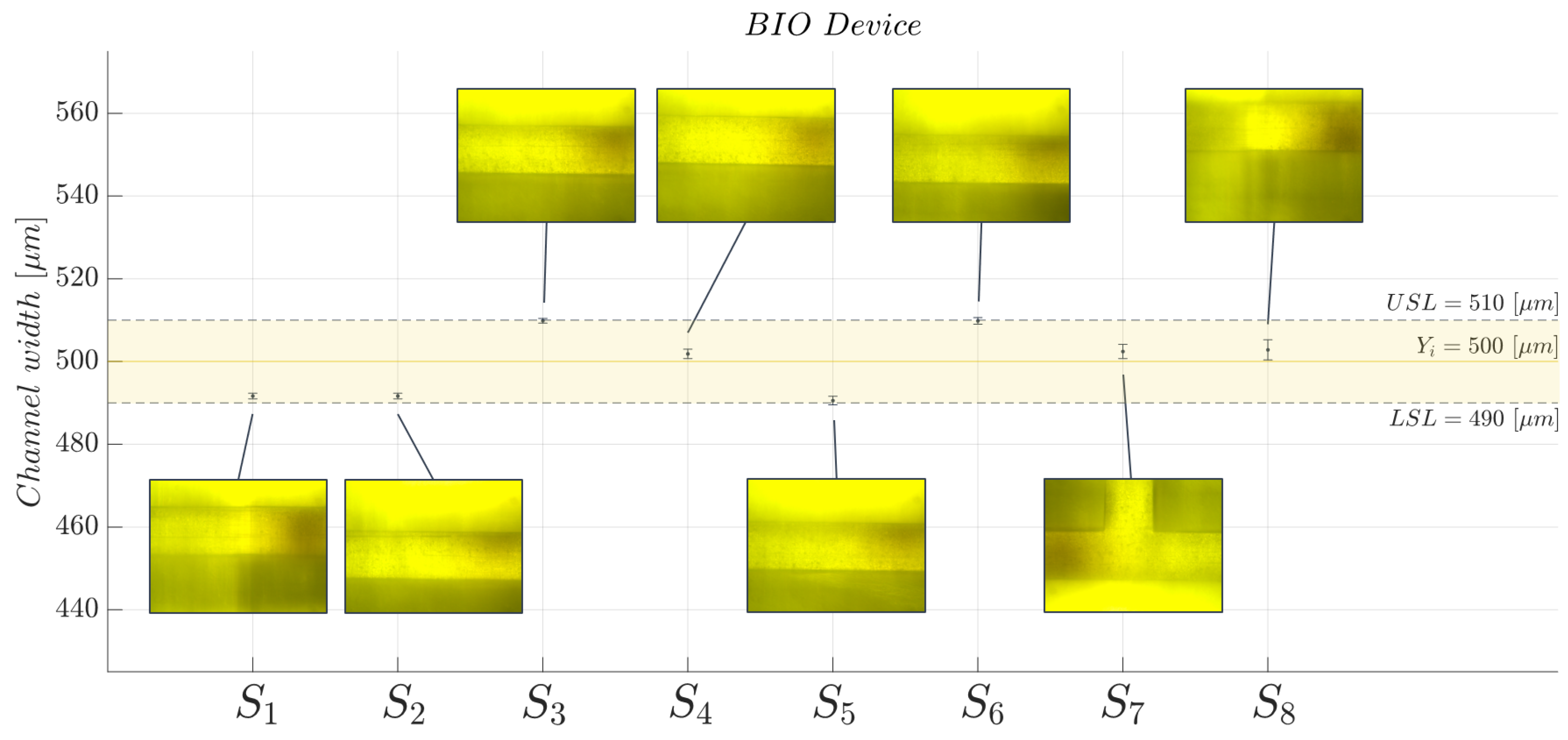
2.5. Three-Dimensional Printed Microchannel: Quality Monitoring
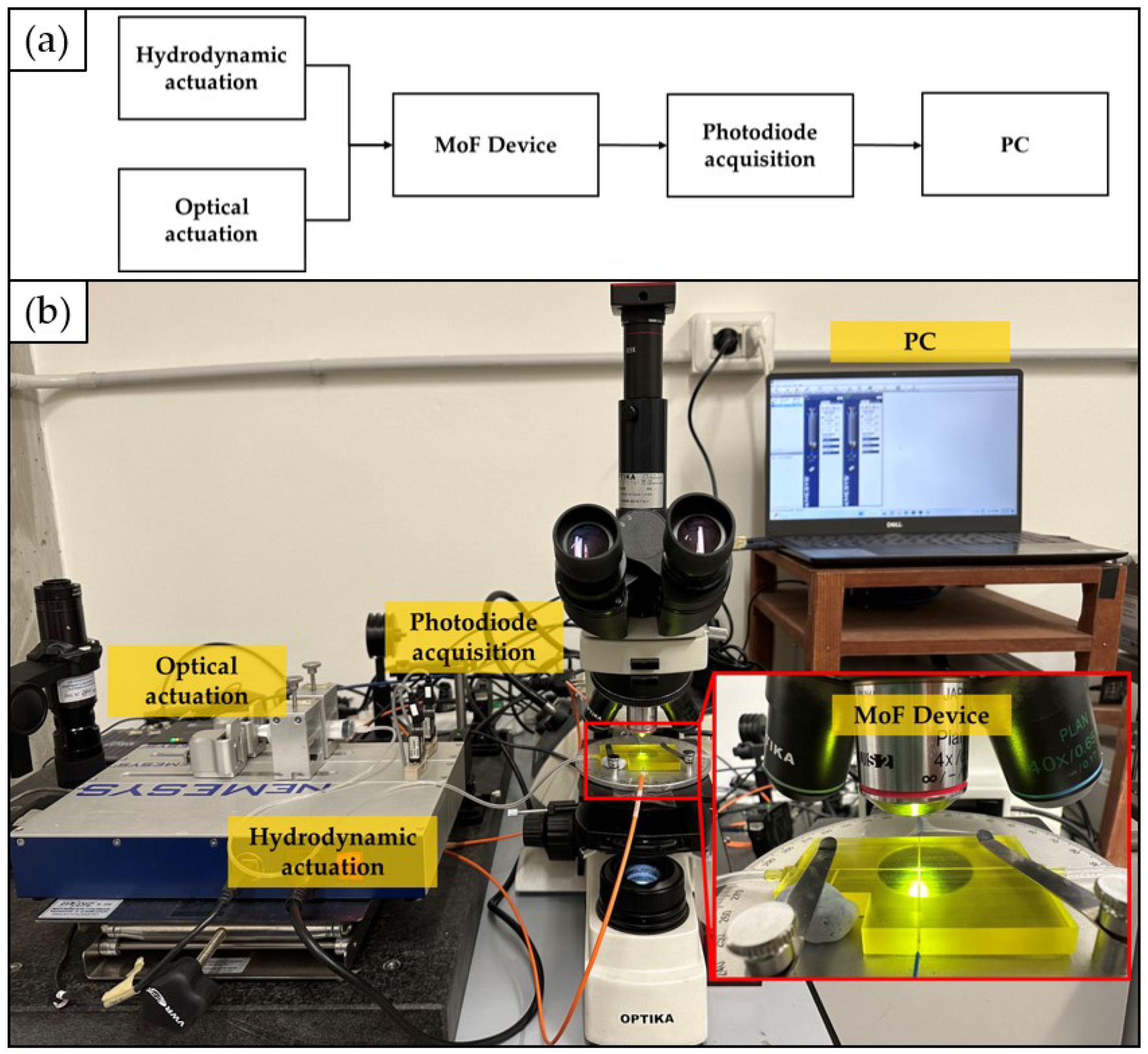
2.6. Experimental Setup: Two-Phase Flow Process and Cell Concentration Monitoring
2.7. Acquired Optical Signals’ Post-processing and Investigated Responses’ Calculation
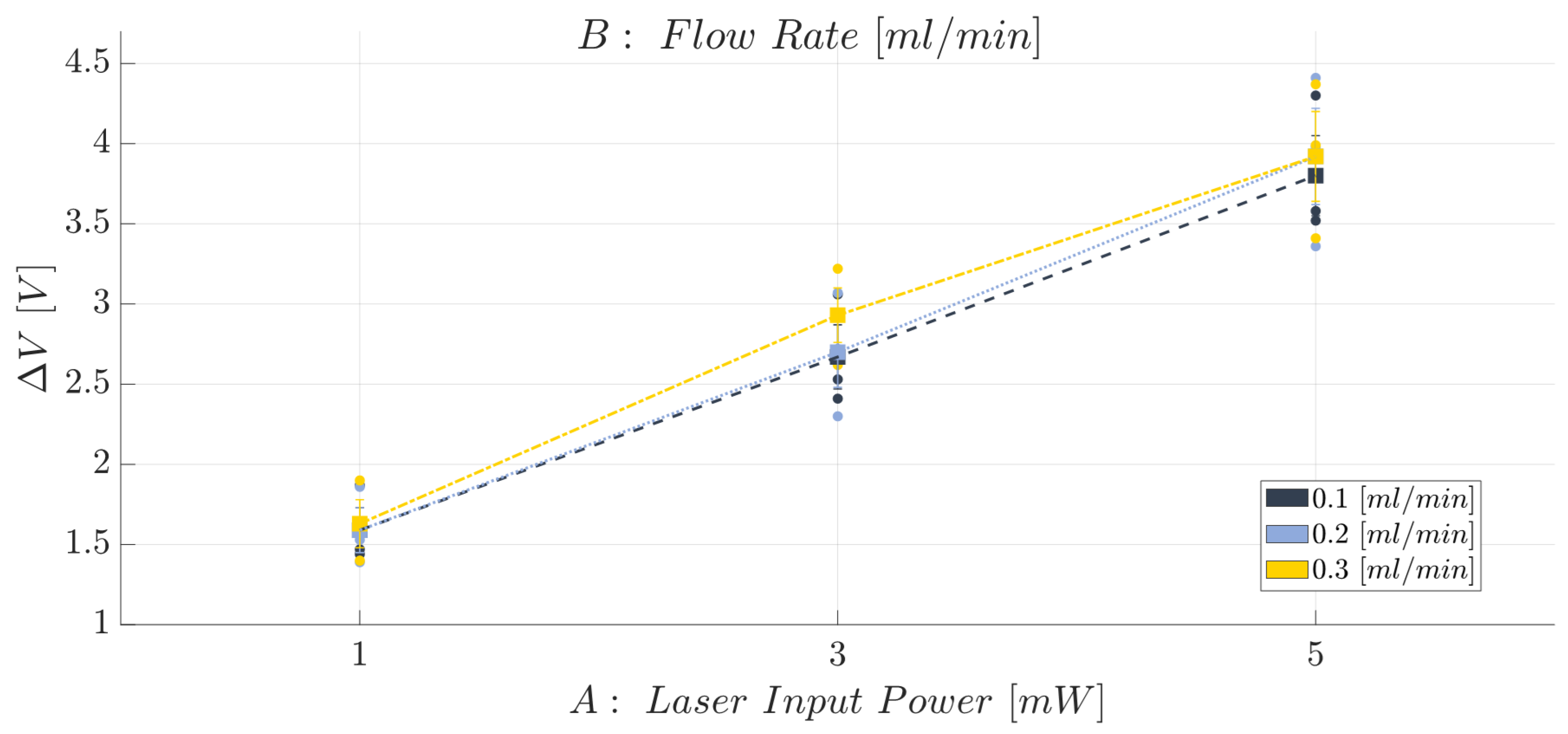
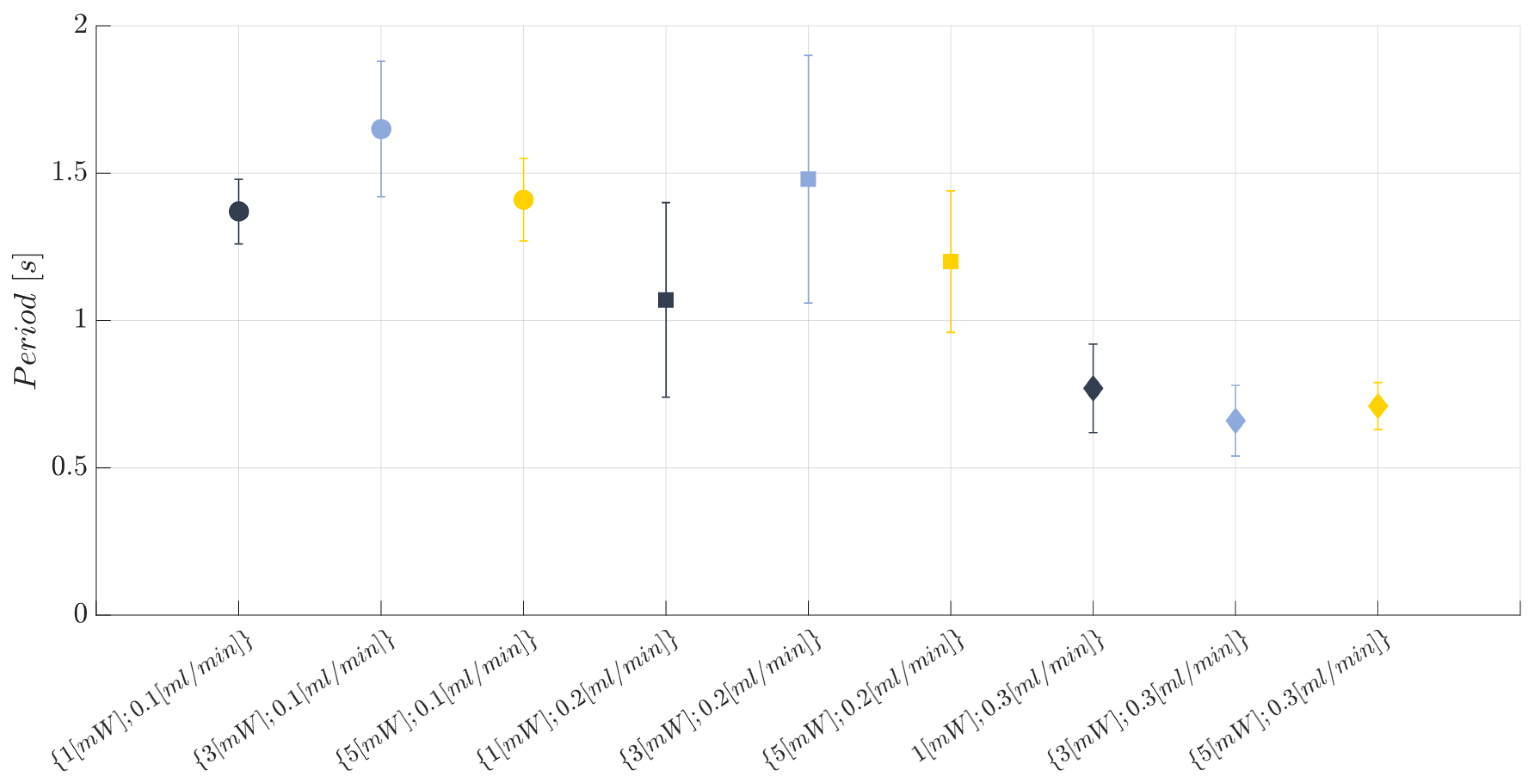
2.8. Two-Phase Flow Process: Experimental Campaign BIO Device
- Laser input power (factor A)—Quantitative factor varied at three levels (a = 3) corresponding to {1, 3, 5} mW;
- Fluid flow rate (factor B)—Quantitative factor varied at three levels (b = 3) corresponding to {0.1, 0.2, 0.3} mL/min.
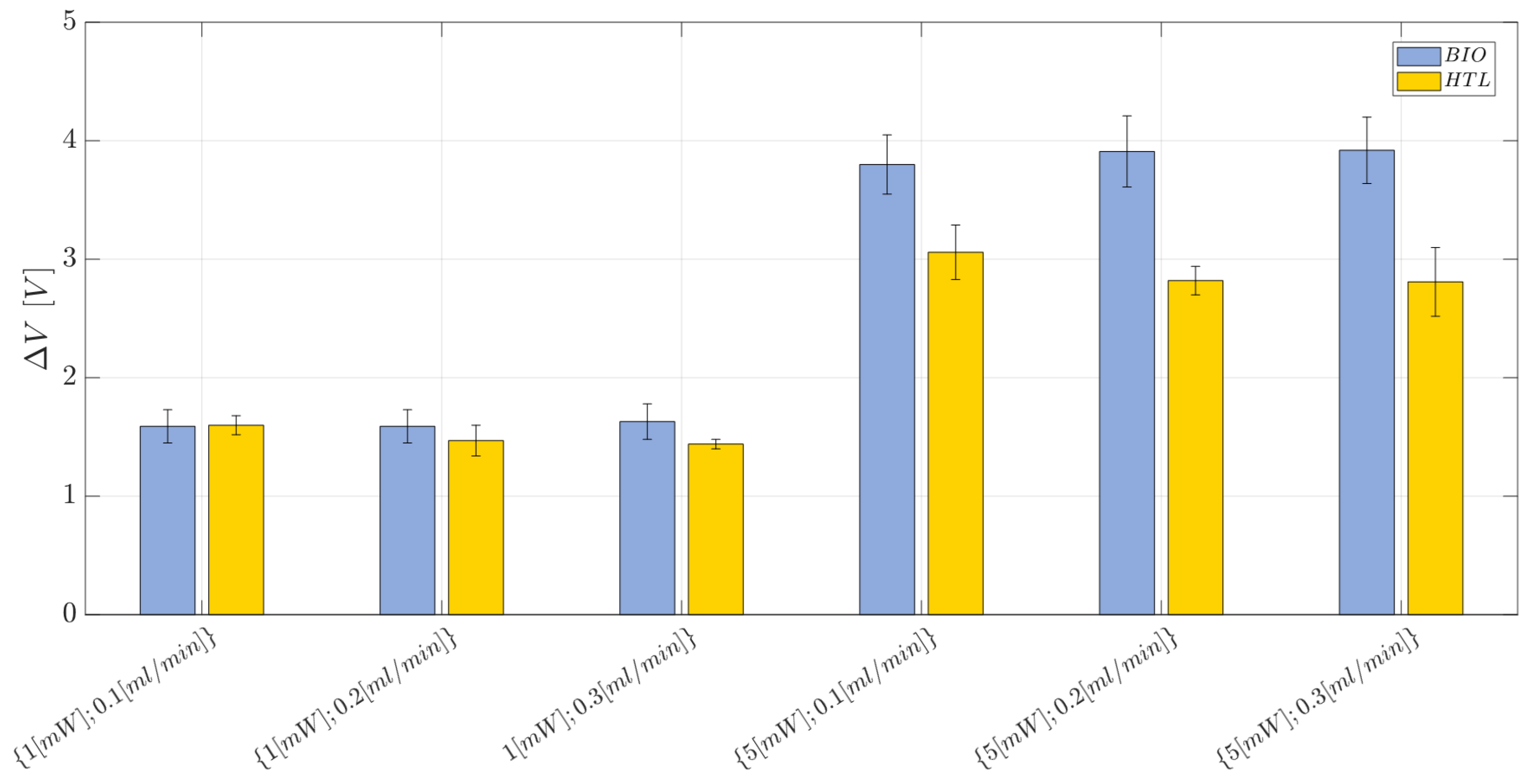
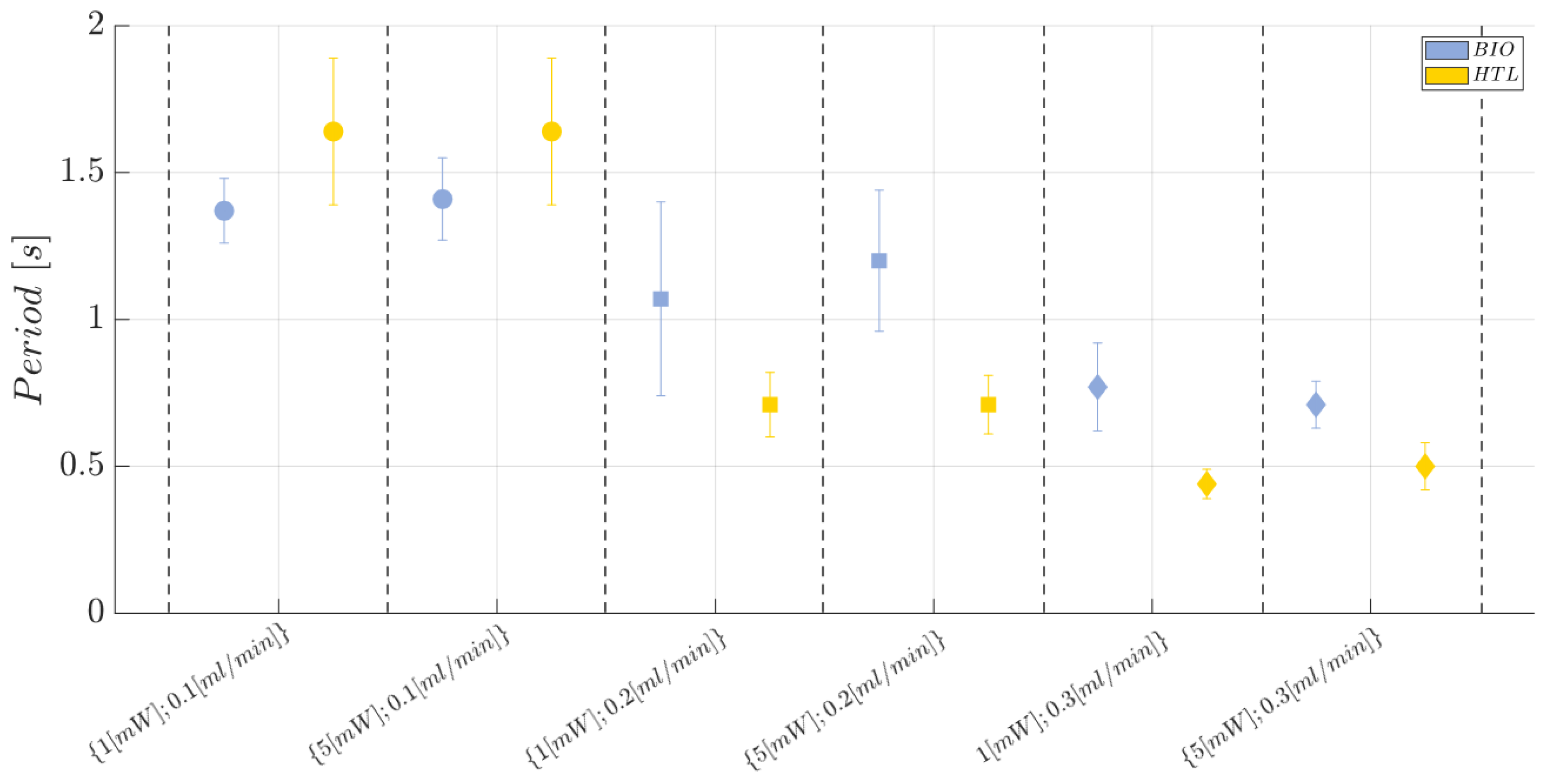
2.9. Two-Phase Flow Process: Experimental Campaign and Comparative Analysis between BIO Device and HTL Device
- Laser input power (factor A)—Quantitative factor varied at two levels (a = 2) corresponding to {1, 5} mW;
- Fluid flow rate (factor B)—Quantitative factor varied at three levels (b = 3) corresponding to {0.1,0.2,0.3} mL/min;
- Material (factor C)—Categorical factor varied at two levels (c = 2) corresponding to {HTL, BIO}.
2.10. Cell Concentration Monitoring: Experimental Campaign (BIO Resin)
- Laser input power (factor A)—Quantitative factor varied at three levels (a = 3) corresponding to {1, 3, 5} mW;
- Concentration of yeast cells (factor B)—Quantitative factor varied at two levels (b = 4) corresponding to {0, 106, 107, and 108} in 10 mL PBS;
- Fluid flow rate (factor C)—Quantitative factor varied at two levels (c = 2) corresponding to {0.05, 0.1}mL/min.
3. Results and Discussion
3.1. Immiscible Gas–Liquid Two-Phase Flow Process
3.2. BIO Device: Characterization Results
3.2.1. BIO Device
3.2.2. HTL and BIO Resins
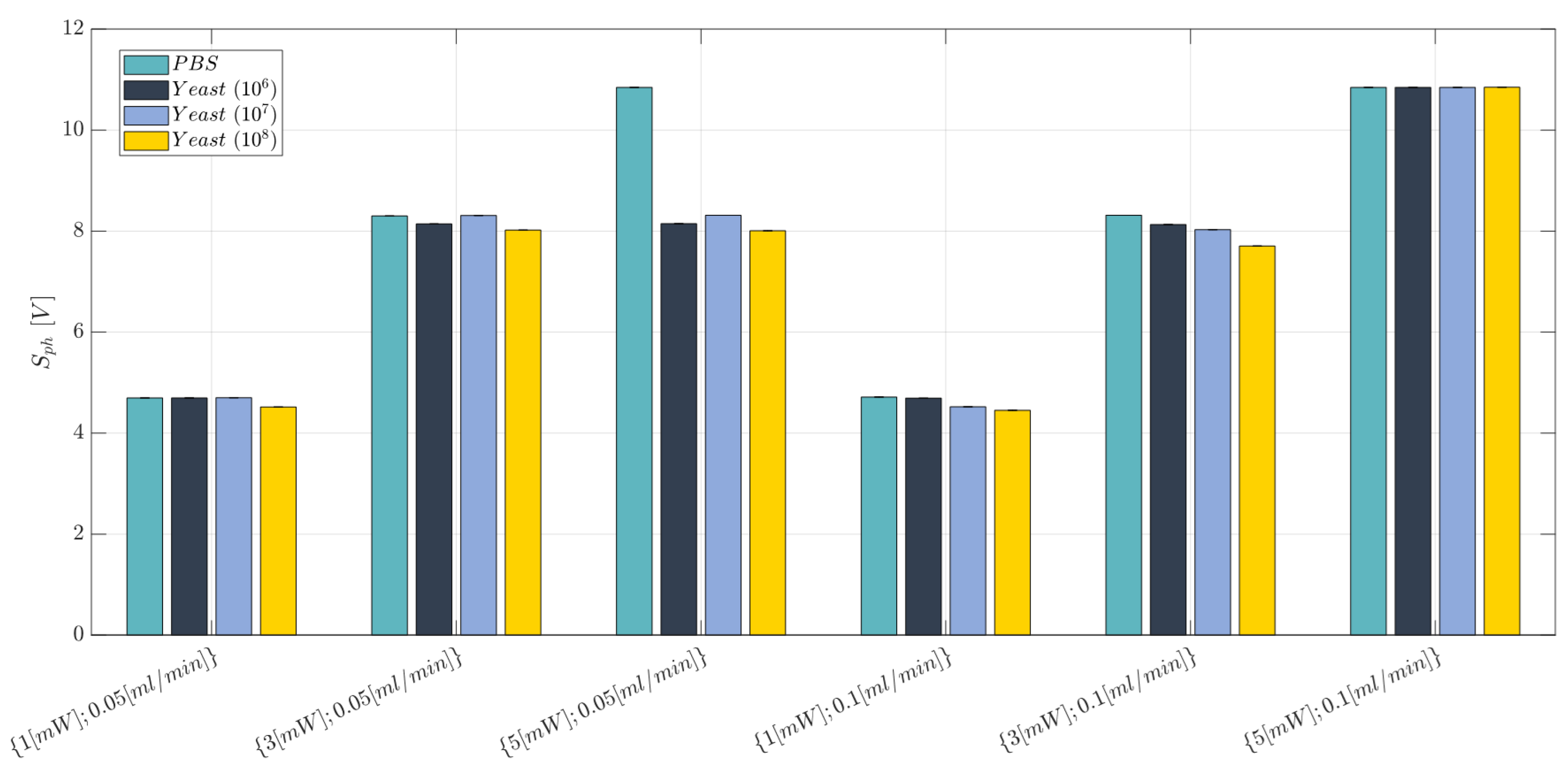
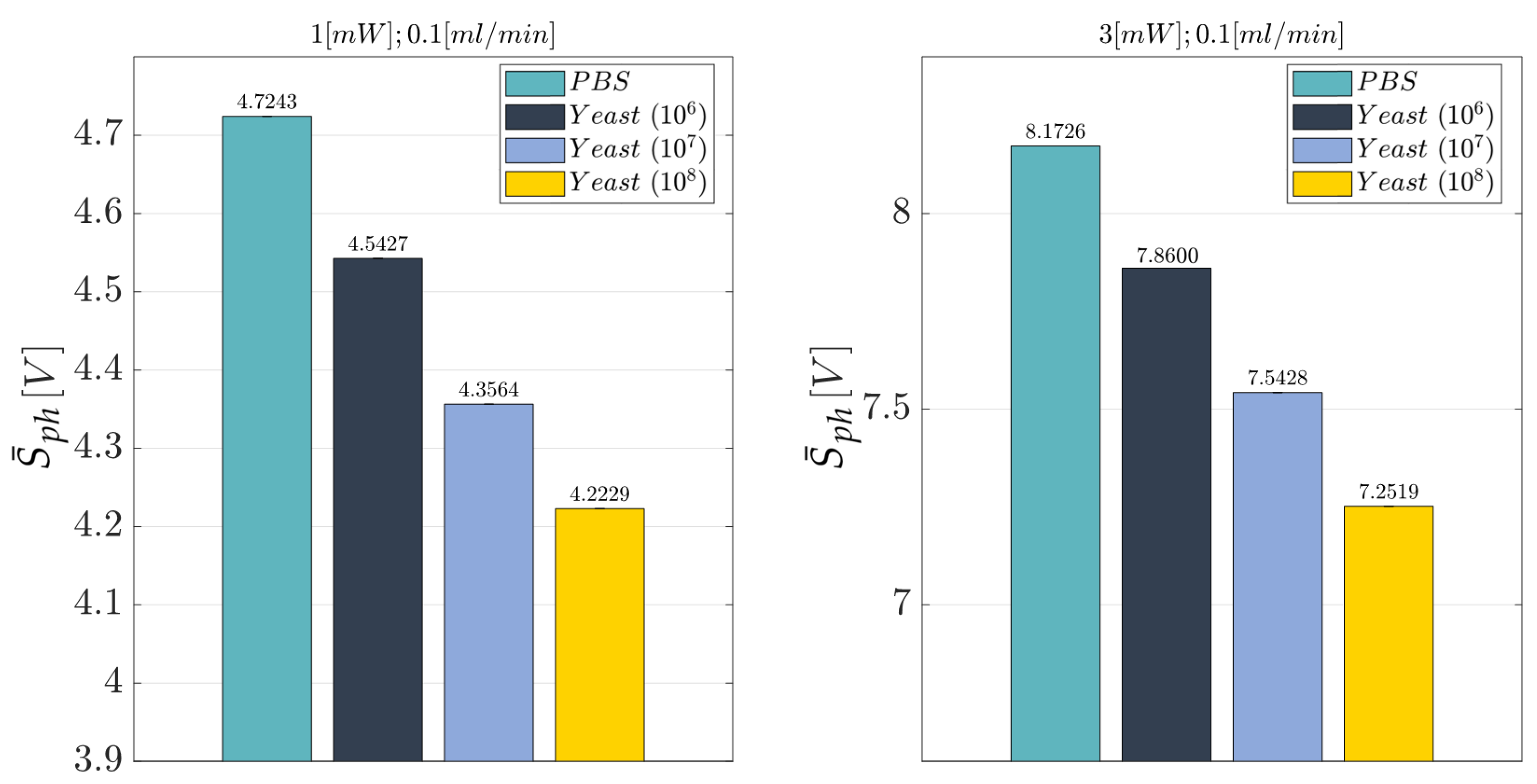
3.3. Cell Concentration Monitoring
- Laser Input Power (factor A)—Quantitative factor varied at two levels (a = 2) corresponding to {1, 3} mW;
- Concentration of Yeast cells (factor B)—Quantitative factor varied at four levels (b = 4) corresponding to {0, 106, 107, 108} in 10 mL PBS.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based Biosensors: Recent Trends, Challenges and Future Perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Lin, R.; Lee, L.P. Continuous Perfusion Microfluidic Cell Culture Array for High-Throughput Cell-Based Assays. Biotechnol. Bioeng. 2005, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rantataro, S.; Parkkinen, I.; Airavaara, M.; Laurila, T. Real-time Selective Detection of Dopamine and Serotonin at Nanomolar Concentration from Complex in vitro Systems. Biosens. Bioelectron. 2023, 241, 115579. [Google Scholar] [CrossRef] [PubMed]
- Uhl, C.G.; Yaling, L. Microfluidic Device for Expedited Tumor Growth towards Drug Evaluation. Lab A Chip 2019, 19, 1458–1470. [Google Scholar] [CrossRef]
- Peng, W.W.; Liu, Y.; Sha, H.H.; Wen, S.D.; Fang, Y.; Zhou, G.R. Relationship between Plasma Circulating Cell-free DNA Concentration and Treatment Outcomes including Prognosis in Patients with Advanced Non-small Cell Lung Cancer. BMC Pulm. Med. 2023, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, M.; Wang, Y.; Brunel, A.; Beffara, F.; Akil, H.; Verdier, M.; Bessette, B.; Crunteanu, A.; Ho, H.; Humbert, G.; et al. Ultra-sensitive Real-time Detection of Cancer-derived Exosomes Directly from Cell Supernatants by a Large Goos–Hänchen Signal Generation on Plasmonic Sensing Interface. Biosens. Bioelectron. 2023, 15, 100391. [Google Scholar] [CrossRef]
- Li, J.; Reimers, A.; Dang, K.; Brunk, M.; Drewes, J.; Hirsch, U.; Willems, C.; Schmelzer, C.; Groth, T.; Nia, A.; et al. 3D Printed Neural Tissues with In Situ Optical Dopamine Sensors. Biosens. Bioelectron. 2023, 222, 114942. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, T.; Meng, H.; Wang, X.; Peng, H.; Liu, G.; Wei, S.; Lu, Q.; Wang, Y.; Wang, A.; et al. 3D Gel-printed Porous Magnesium Scaffold Coated with Dibasic Calcium Phosphate Dihydrate for Bone Repair In Vivo. J. Orthop. Transl. 2022, 33, 13–23. [Google Scholar] [CrossRef]
- Krafft, B.; Tycova, A.; Urban, R.; Dusny, C.; Belder, D. Microfluidic Device for Concentration and SERS-based Detection of Bacteria in Drinking Water. Electrophoresis 2021, 42, 86–94. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, K.; Ekinci, K. All-electrical Monitoring of Bacterial Antibiotic Susceptibility in a Microfluidic Device. Proc. Natl. Acad. Sci. USA 2020, 117, 10639–10644. [Google Scholar] [CrossRef]
- Coluccio, M.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.; Cuda, G.; Candeloro, P.; et al. Microfluidic Platforms for Cell Cultures and Investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Achille, C.; Parra-Cabrera, C.; Dochy, R.; Ordutowski, H.; Piovesan, A.; Piron, P.; Van Looy, L.; Kushwaha, S.; Reynaerts, D.; Verboven, P.; et al. 3D Printing of Monolithic Capillarity-Driven Microfluidic Devices for Diagnostics. Adv. Mater. 2021, 33, 2008712. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Heidari, A.; Nadi, M.; Fallahy, M.; Nezamabadi, S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic Devices for Detection of RNA Viruses. Rev. Med. Virol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.; Garcia-Granada, A.; Reilly, G.; Claeyssens, F. Design and Evaluation of an Osteogenesis-on-a-Chip Microfluidic Device Incorporating 3D Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 557111. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.; Rzhetsky, A.; White, K.; Tay, S. Automated Microfluidic Platform for Dynamic and Combinatorial Drug Screening of Tumor Organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Jiang, R.; Fu, Y.; Jin, N. Automated Blood Cell Detection and Counting via Deep Learning for Microfluidic Point-of-Care Medical Devices. IOP Conf. Ser. Mater. Sci. Eng. 2019, 646, 012048. [Google Scholar] [CrossRef]
- Vembadi, A.; Menachery, A.; Qasaimeh, M. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Palma-Florez, S.; Lopez-Canosa, A.; Moralez-Zavala, F.; Castano, O.; Kogan, M.; Samitier, J.; Lagunas, A.; Mir, M. BBB-on-a-Chip with Integrated Micro-TEER for Permeability Evaluation of Multi-functionalized Gold Nanorods Against Alzheimer’s Disease. J. Nanobiotechnology 2023, 21, 115. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.C.; Paul, S.; Hong, E.; Suk, J.M. High-Content Screening of Drug-Induced Cardiotoxicity Using Quantitative Single Cell Imaging Cytometry on Microfluidic Device. Lab A Chip 2011, 11, 104–114. [Google Scholar]
- Mansor, M.; Takeuchi, M.; Nakajima, M.; Hasegawa, Y.; Ahmad, M. Electrical Impedance Spectroscopy for Detection of Cells in Suspensions Using Microfluidic Device with Integrated Microneedles. Appl. Sci. 2017, 7, 170. [Google Scholar] [CrossRef]
- Martinez Teruel, J.; García Sánchez, T.; Sosa, A.; Bragós Bardia, R. Electrical Impedance Spectroscopy Cell Monitoring in a Miniaturized Bioreactor. In Proceedings of the 19th IMEKO TC 4 Symposium Measurements of Electrical Quantities, 17th TC 4 Workshop IWADC on ADC and DAC Modeling and Testing, Barcelona, Spain, 18–19 July 2013; pp. 446–450. [Google Scholar]
- Brunauer, G.; Meindl, A.; Rotter, B.; Gruber, A.; Slouka, C.; Schnabel, T.; Petutschnigg, A. Electrochemical Impedance Spectroscopy for Microbiological Processes: On the Way to a Monitoring Tool for the Determination of Biomass. Biomed. J. Sci. Technol. Res. 2021, 35, 27548–27557. [Google Scholar]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab A Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Sun, L. Digital microfluidics: A promising technique for biochemical applications. Front. Mech. Eng. 2017, 12, 510–525. [Google Scholar] [CrossRef]
- Pang, L.; Ding, J.; Liu, X.X.; Fan, S.K. Digital microfluidics for cell manipulation. TrAC Trends Anal. Chem. 2019, 117, 291–299. [Google Scholar] [CrossRef]
- Dixon, C.; Lamanna, J.; Wheeler, A. Direct Loading of Blood for Plasma Separation and Diagnostic Assays on a Digital Microfluidic Device. Lab A Chip 2020, 20, 1845–1855. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Q.; Zhang, X.; Liu, X.; Lu, L.; Wei, J.; Li, Y.; Wang, Y.; Zheng, G. A Digital Microfluidic Diluter-Based Microalgal Motion Biosensor for Marine Pollution Monitoring. Biosens. Bioelectron. 2019, 143, 111597. [Google Scholar] [CrossRef]
- Barbaresco, F.; Racca, L.; Spigarelli, L.; Cocuzza, M.; Marasso, S.; Pirri, C.; Canavese, G. Focalization Performance Study of a Novel Bulk Acoustic Wave Device. Nanomaterials 2021, 11, 2630. [Google Scholar] [CrossRef]
- Devendran, C.; Choi, K.; Han, J.; Ai, Y.; Neild, A.; Collins, D. Diffraction-Based Acoustic Manipulation in Microchannels Enables Continuous Particle and Bacteria Focusing. Lab A Chip 2020, 20, 2674–2688. [Google Scholar] [CrossRef]
- Torrisi, F.; Stella, G.; Guarino, F.; Bucolo, M. Cell Counting and Velocity Algorithms for Hydrodynamic Study of Unsteady Biological Flows in Micro-Channels. Biomicrofluidics 2023, 17, 014105. [Google Scholar] [CrossRef]
- Bills, M.; Nguyen, B.; Yoon, J. Simplified White Blood Cell Differential: An Inexpensive, Smartphone-and Paper-Based Blood Cell Count. IEEE Sensors J. 2019, 19, 7822–7828. [Google Scholar] [CrossRef] [PubMed]
- Hengoju, S.; Shvydkiv, O.; Tovar, M.; Roth, M.; Rosenbaum, M. Advantages of Optical Fibers for Facile and Enhanced Detection in Droplet Microfluidics. Biosens. Bioelectron. 2022, 200, 113910. [Google Scholar] [CrossRef]
- Pires, N.; Dong, T.; Hanke, U.; Hoivik, N. Recent Developments in Optical Detection Technologies in Lab-on-a-Chip Devices for Biosensing Applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, L.; Martínez-Martínez, E.; Pazos-Solís, D.; Aguado-Preciado, J.; Dutt, A.; Chávez-Ramírez, A.; Korgel, B.; Sharma, A.; Oza, G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors 2023, 23, 439. [Google Scholar] [CrossRef]
- Cairone, F.; Davi, S.; Stella, G.; Guarino, F.; Recca, G.; Cicala, G.; Bucolo, M. 3D-Printed Micro-Optofluidic Device for Chemical Fluids and Cells Detection. Biomed. Microdevices 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Stella, G.; Saitta, L.; Ongaro, A.; Cicala, G.; Kersaudy-Kerhoas, M.; Bucolo, M. Advanced Technologies in the Fabrication of a Micro-Optical Light Splitter. Micro 2023, 3, 338–352. [Google Scholar] [CrossRef]
- Cairone, F.; Gallo Afflitto, F.; Stella, G.; Cicala, G.; Ashour, M.; Kersaudy-Kerhoas, M.; Bucolo, M. Micro-Optical Waveguides Realization by Low-Cost Technologies. Micro 2022, 2, 123–136. [Google Scholar] [CrossRef]
- Cairone, F.; Gagliano, S.; Carbone, D.; Recca, G.; Bucolo, M. Micro-optofluidic switch realized by 3D printing technology. Microfluid Nanofluidics 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Kristinn, B.; Minzioni, P.; Osellame, R. Roadmap for Opto-Fluidics. J. Opt. 2017, 19, 093003. [Google Scholar]
- Schembri, F.; Bucolo, M. Periodic Input Flows Tuning Nonlinear Two-Phase Dynamics in a Snake Microchannel. Microfluid Nanofluidics 2011, 11, 189–197. [Google Scholar] [CrossRef]
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Moore, A.W.; Ramsey, J.M. Fused quartz substrates for microchip electrophoresis. Anal. Chem. 1995, 67, 2059–2063. [Google Scholar] [CrossRef]
- Matzke, C.M.; Kottenstette, R.J.; Casalnuovo, S.A.; Frye-Mason, G.C.; Hudson, M.L.; Sasaki, D.Y.; Manginell, R.P.; Wong, C.C. Microfabricated silicon gas chromatographic microchannels: Fabrication and performance. In Proceedings of the Micromachining and Microfabrication Process Technology IV, Santa Clara, CA, USA, 20–24 September 1998; SPIE: Cergy Pontoise, France, 1998; Volume 3511, pp. 262–268. [Google Scholar]
- Moore, A.W.; Jacobson, S.C.; Ramsey, J.M. Microchip separations of neutral species via micellar electrokinetic capillary chromatography. Anal. Chem. 1995, 67, 4184–4189. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Rath, S.N. 3D printed microfluidic devices: A review focused on four fundamental manufacturing approaches and implications on the field of healthcare. Bio-Des. Manuf. 2021, 4, 311–343. [Google Scholar] [CrossRef]
- Su, C.K. Review of 3D-Printed functionalized devices for chemical and biochemical analysis. Anal. Chim. Acta 2021, 1158, 338348. [Google Scholar] [CrossRef]
- Zhu, F.; Wigh, A.; Friedrich, T.; Devaux, A.; Bony, S.; Nugegoda, D.; Kaslin, J.; Wlodkowic, D. Automated lab-on-a-chip technology for fish embryo toxicity tests performed under continuous microperfusion (μFET). Environ. Sci. Technol. 2015, 49, 14570–14578. [Google Scholar] [CrossRef]
- Valentin, T.M.; DuBois, E.M.; Machnicki, C.E.; Bhaskar, D.; Cui, F.R.; Wong, I.Y. 3D printed self-adhesive PEGDA–PAA hydrogels as modular components for soft actuators and microfluidics. Polym. Chem. 2019, 10, 2015–2028. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab A Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef]
- Alessandri, K.; Feyeux, M.; Gurchenkov, B.; Delgado, C.; Trushko, A.; Krause, K.H.; Vignjević, D.; Nassoy, P.; Roux, A. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab A Chip 2016, 16, 1593–1604. [Google Scholar] [CrossRef]
- Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. Moving from millifluidic to truly microfluidic sub-100-μm cross-section 3D printed devices. Anal. Bioanal. Chem. 2017, 409, 4311–4319. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 μm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Castiaux, A.D.; Pinger, C.W.; Hayter, E.A.; Bunn, M.E.; Martin, R.S.; Spence, D.M. PolyJet 3D-printed enclosed microfluidic channels without photocurable supports. Anal. Chem. 2019, 91, 6910–6917. [Google Scholar] [CrossRef]
- Best Industrial-Grade 3D Printer—Pro2|$3499|Raise3D—raise3d.com. Available online: https://www.raise3d.com/products/pro2-3d-printer/ (accessed on 9 November 2023).
- UltiMaker S3—ultimaker.com. Available online: https://ultimaker.com/3d-printers/s-series/ultimaker-s3/ (accessed on 9 November 2023).
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Advanced Additive Manufacturing Solutions—bmf3d.com. Available online: https://bmf3d.com/ (accessed on 9 November 2023).
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection micro stereolithography based 3D printing and its applications. Int. J. Extrem. Manuf. 2020, 2, 022004. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing microfluidic performance of three-dimensional (3D) printing platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Sochol, R.; Sweet, E.; Glick, C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K.; et al. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab A Chip 2016, 16, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Childs, E.H.; Latchman, A.V.; Lamont, A.C.; Hubbard, J.D.; Sochol, R.D. Additive assembly for PolyJet-based multi-material 3D printed microfluidics. J. Microelectromech. Syst. 2020, 29, 1094–1096. [Google Scholar] [CrossRef]
- Lin, X.; Wu, H.; Zeng, S.; Peng, T.; Zhang, P.; Wan, X.; Lang, Y.; Zhang, B.; Jia, Y.; Shen, R.; et al. A self-designed device integrated with a Fermat spiral microfluidic chip for ratiometric and automated point-of-care testing of anthrax biomarker in real samples. Biosens. Bioelectron. 2023, 230, 115283. [Google Scholar] [CrossRef] [PubMed]
- Saitta, L.; Celano, G.; Cicala, G.; Fragalà, M.; Stella, G.; Barcellona, M.; Tosto, C.; Bucolo, M. Projection Micro-Stereolithography Versus Master–Slave Approach to Manufacture a Micro-Optofluidic Device for Slug Flow Detection. Int. J. Adv. Manuf. Technol. 2022, 120, 4443–4460. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The Recent Development of Vat Photopolymerization: A Review. Addit. Manuf. 2021, 48, 102426. [Google Scholar] [CrossRef]
- Detamornrat, U.; McAlister, E.; Hutton, A.; Larraneta, E.; Donnelly, R. The Role of 3D Printing Technology in Microengineering of Microneedles. Small 2022, 18, e2106392. [Google Scholar] [CrossRef]
- Martin, N.; Pirie, A.; Ford, L.; Callaghan, C.; McTurk, K.; Lucy, D.; Scrimger, D. The use of phosphate buffered saline for the recovery of cells and spermatozoa from swabs. Sci. Justice J. Forensic Sci. Soc. 2006, 46, 179–184. [Google Scholar] [CrossRef]
- Rines, D.R.; Thomann, D.; Dorn, J.F.; Goodwin, P.; Sorger, P.K. Live cell imaging of yeast. Cold Spring Harb. Protoc. 2011, 2011, pdb.top065482. [Google Scholar] [CrossRef]
- Hoang, V.T.; Stępniewski, G.; Czarnecka, K.H.; Kasztelanic, R.; Long, V.C.; Xuan, K.D.; Shao, L.; Śmietana, M.; Buczyński, R. Optical properties of buffers and cell culture media for optofluidic and sensing applications. Appl. Sci. 2019, 9, 1145. [Google Scholar] [CrossRef]
- Saitta, L.; Cutuli, E.; Celano, G.; Tosto, C.; Stella, G.; Cicala, G.; Bucolo, M. A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications. Polymers 2023, 15, 2690. [Google Scholar] [CrossRef]
- Choi, C.; Westin, K.; Breuer, K. To Slip or Not to Slip: Water Flows in Hydrophilic and Hydrophobic Microchannels. In Proceedings of the ASME 2002 International Mechanical Engineering Congress and Exposition, New Orleans, LO, USA, 17–22 November 2002; pp. 557–564. [Google Scholar]
- Sendner, C.; Horinek, D.; Bocquet, L.; Netz, R. Interfacial Water at Hydrophobic and Hydrophilic Surfaces: Slip, Viscosity, and Diffusion. Langmuir 2009, 25, 10768–10781. [Google Scholar] [CrossRef]
- Zhou, G.; Yao, S. Effect of Surface Roughness on Laminar Liquid Flow in Micro-channels. Appl. Therm. Eng. 2011, 2–3, 228–234. [Google Scholar] [CrossRef]
- Vitale, A.; Cabral, J. Frontal Conversion and Uniformity in 3D Printing by Photopolymerisation. Materials 2016, 9, 760. [Google Scholar] [CrossRef]
- Tran, T.; Di Mauro, C.; Malbured, S.; Graillot, A.; Mija, A. Dual Cross-linking of Epoxidized Linseed Oil with Combined Aliphatic/Aromatic Diacids Containing Dynamic S-S Bonds Generating Recyclable Thermosets. ACS Appl. Bio. Mater. 2020, 3, 7550–7561. [Google Scholar] [CrossRef] [PubMed]
- Weilin, Q.; Mala, G.; Dongqing, L. Pressure-driven Water Flows in Trapezoidal Silicon Microchannels. Int. J. Heat Mass Transf. 2000, 43, 353–364. [Google Scholar] [CrossRef]
- Pfund, D.; Rector, D.; Shekarriz, A.; Popescu, A.; Welty, J. Pressure Drop Measurements in a Microchannel. AIChE J. 2000, 46, 1496–1507. [Google Scholar] [CrossRef]
- Cennamo, N.; Saitta, L.; Tosto, C.; Arcadio, F.; Zeni, L.; Fragalà, M.; Cicala, G. Microstructured Surface Plasmon Resonance Sensor Based on Inkjet 3D Printing Using Photocurable Resins with Tailored Refractive Index. Polymers 2021, 13, 2518. [Google Scholar] [CrossRef]
- Saitta, L.; Arcadio, F.; Celano, G.; Cennamo, N.; Zeni, L.; Tosto, C.; Cicala, G. Design and Manufacturing of a Surface Plasmon Resonance Sensor Based on Inkjet 3D Printing for Simultaneous Measurements of Refractive Index and Temperature. Int. J. Adv. Manuf. Technol. 2023, 124, 2261–2278. [Google Scholar] [CrossRef]






| Cell Concentration Monitoring Technique | Working Principle | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Flow Cytometry | It works by suspending cells in a flowing fluid, passing them through a focused laser beam, and detecting the emitted fluorescence. By measuring the intensity and properties of this fluorescence, it quantifies cell concentration and can differentiate between different cell types based on labeled markers. | (i). High Throughput; (ii). Single-Cell Analysis; (iii). Real-Time Monitoring; (iv). Automation and Precision; (v). Labeling Flexibility | (i). Complex Instrumentation; (ii). Time-consuming sample preparation; (iii). Limited Detection Range; (iv). Complexity of Data Analysis; (v). Invasive technique (label-based using dyes). | [19,20] |
| Impedance Spectroscopy | It relies on measuring the electrical impedance of a microchannel or electrode when cells flow through it. As cells pass through the channel, they alter the impedance due to their size, shape, and dielectric properties. Cell concentration can be monitored by analyzing the impedance changes at different frequencies. | (i). Real-Time monitoring; (ii). Label-free; (iii). High sensitivity and accuracy; (iv). Miniaturization and integration; (v). Multiparametric analysis. | (i). High instrumentation costs; (ii). Invasive; (iii). Complex data interpretation; (iv). Sensitivity to Environmental Factors; (v). Limited cell types compatibility. | [21,23] |
| Digital Microfluidics | It uses a grid of electronically actuated electrodes to manipulate discrete microdroplets containing cells. By precisely moving, splitting, or merging these droplets, it enables dynamic control of cell concentrations within the droplets. | (i). Real-Time Monitoring; (ii). High Throughput; (iii). Precise Control; (iv). Integration with Sensors; (v). Reduced Sample Volume. | (i). Complex Instrumentation; (ii). Limited Droplet Size Range; (iii). Limited Sample Volume; (iv). Electrode Wear; (v). Sensitivity to Environmental Factors. | [28,29] |
| Acoustic-based Microfluidics | It operates by generating acoustic waves within a microchannel, so that as cells flow through it, they experience acoustic forces that push them towards specific positions or nodes within the channel. By monitoring the distribution of cells at these nodes, the method can determine cell concentration. | (i). Label-free; (ii). Noninvasive; (iii) High Precision; (iv). Cell types compatibility; (v). Real-Time monitoring. | (i). Limited Information; (ii). Complex and Expensive Equipment; (iii) Sensitivity to Environmental Factors; (iv). Limited Sample Throughput; (v). Acoustic Noise. | [30,31] |
| Microscopy and Image Analysis | It involves capturing images of cells within microchannels. Image analysis software then processes these images to count and analyze the cells, determining their concentration by measuring cell density or counting individual cells. | (i). Real-Time Monitoring; (ii). Noninvasive; (iii). High Precision; (iv). Multiparametric Analysis; (v). Cell types compatibility. | (i). Limited Throughput; (ii). Data Processing; (iii). High Instrumentation costs; (iv). Complexity; (v). Setup Compatibility. | [32,33] |
| Optical Detection | It involves illuminating cells within a microchannel with light and measuring the resulting optical signals. As cells pass through the detection zone, changes in light absorption, scattering, or fluorescence are detected and analyzed. The magnitude of these optical signals is proportional to the cell concentration, enabling quantitative monitoring and analysis. | (i). High Sensitivity; (ii). Real-Time Monitoring; (iii). Label-free; (iv). Noninvasive; (v). Integration. | (i). Sensitivity to Sample Properties; (ii). Phototoxicity; (iii). Background Noise; (iv). Temperature Sensitivity; (v). Calibration Challenges. | [34,35,36,37] |
| 3D Printing Process | ||
|---|---|---|
| Parameter | Value | Unit |
| Layer Thickness | 15 | (m) |
| Exposure Time | 1 | (s) |
| Print Time | 48 | (h) |
| Washing | ||
| Description | ||
| Washing in isopropyl alcohol (IPA) solution for about min, by changing the solutions several times. | ||
| Post-processing | ||
| Curing Type | Description | |
| Thermal Curing | at C for 2 h | |
| UV Curing | gradient radiation with UV light power (80 mW/cm2) for 150 s | |
| Factor | Symbol | Type | Unit | Levels | Low Level (−1) | Central Level (0) | High Level (+1) |
|---|---|---|---|---|---|---|---|
| Laser Input Power | A | Quantitative | (mW) | a = 3 | 1 | 3 | 5 |
| Flow Rate | B | Quantitative | (mL/min) | b = 3 | 0.1 | 0.2 | 0.3 |
| Factor | Symbol | Type | Unit | Levels | Low Level (−1) | Central Level (0) | High Level (+1) |
|---|---|---|---|---|---|---|---|
| Laser Input Power | A | Quantitative | (mW) | a = 2 | 1 | - | 5 |
| Flow Rate | B | Quantitative | (mL/min) | b = 3 | 0.1 | 0.2 | 0.3 |
| Material | C | Categorical | (-) | c = 2 | HTL | - | BIO |
| Factor | Symbol | Type | Unit | Levels | Level (1) | Level (2) | Level (3) | Level (4) |
|---|---|---|---|---|---|---|---|---|
| Laser Input Power | A | Quantitative | (mW) | a = 3 | 1 | 3 | 5 | - |
| Concentration of Yeast Cells | B | Quantitative | (-) | b = 4 | 0 in 10 mL PBS | in 10 mL PBS | in 10 mL PBS | in 10 mL PBS |
| Flow Rate | C | Quantitative | (mL/min) | c = 2 | 0.05 | 0.1 | - | - |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Block | 2.07 | 2 | 1.03 | |||
| Model | 23.43 | 8 | 2.93 | 113.76 | <0.0001 | significant |
| (A) Laser Input Power | 23.28 | 2 | 11.64 | 452.05 | <0.0001 | |
| (B) Flow Rate | 0.091 | 2 | 0.045 | 1.76 | 0.2030 | |
| AB | 0.062 | 4 | 0.016 | 0.60 | 0.6662 | |
| Residual | 0.41 | 16 | 0.026 | |||
| Cor Total | 25.91 | 26 | ||||
| Std. Dev. | 0.16 | R-Squared | 0.9827 | |||
| Mean | 2.75 | Adj R-Squared | 0.9741 | |||
| C.V. % | 5.84 | Adeq Precision | 29.257 | |||
| PRESS | 1.17 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Block | 0.36 | 2 | 0.18 | |||
| Model | 2.58 | 8 | 0.32 | 4.59 | 0.0113 | significant |
| (A) Laser Input Power | 0.029 | 2 | 0.015 | 0.21 | 0.8149 | |
| (B) Flow Rate | 2.27 | 2 | 1.14 | 16.17 | 0.00005 | |
| AB | 0.27 | 4 | 0.067 | 0.95 | 0.4728 | |
| Residual | 0.77 | 11 | 0.070 | |||
| Cor Total | 3.72 | 21 | ||||
| Std. Dev. | 0.27 | R-Squared | 0.7694 | |||
| Mean | 1.17 | Adj R-Squared | 0.6017 | |||
| C.V. % | 22.69 | Adeq Precision | 6.856 | |||
| PRESS | 3.58 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Block | 0.35 | 2 | 0.17 | |||
| Model | 34.81 | 11 | 3.16 | 28.35 | <0.0001 | significant |
| (A) Laser Input Power | 30.20 | 1 | 30.20 | 270.58 | <0.0001 | |
| (B) Flow Rate | 0.032 | 2 | 0.016 | 0.14 | 0.8684 | |
| (C) Material | 2.65 | 1 | 2.65 | 23.76 | <0.0001 | |
| AB | 5.00 × | 2 | 2.50 × | 2.24 × | 0.9998 | |
| AC | 1.76 | 1 | 1.76 | 15.81 | 0.0006 | |
| BC | 0.14 | 2 | 0.071 | 0.64 | 0.5393 | |
| ABC | 0.021 | 2 | 0.010 | 0.092 | 0.9122 | |
| Residual | 2.46 | 22 | 0.11 | |||
| Cor Total | 37.61 | 35 | ||||
| Std. Dev. | 0.33 | R-Squared | 0.9341 | |||
| Mean | 2.47 | Adj R-Squared | 0.9012 | |||
| C.V. % | 13.52 | Adeq Precision | 12.957 | |||
| PRESS | 6.57 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Block | 0.65 | 2 | 0.33 | |||
| Model | 6.27 | 11 | 0.57 | 9.79 | <0.0001 | significant |
| (A) Laser Input Power | 2.77 × | 1 | 2.77 × | 0.047 | 0.8298 | |
| (B) Flow Rate | 4.55 | 2 | 2.27 | 39.03 | <0.0001 | |
| (C) Material | 0.27 | 1 | 0.27 | 4.71 | 0.0429 | |
| AB | 0.052 | 2 | 0.026 | 0.45 | 0.6446 | |
| AC | 0.03 | 1 | 0.03 | 0.52 | 0.4806 | |
| BC | 1.18 | 2 | 0.59 | 10.10 | 0.0010 | |
| ABC | 5.04 × | 2 | 2.52 × | 0.043 | 0.9578 | |
| Residual | 1.11 | 19 | 0.058 | |||
| Cor Total | 8.03 | 32 | ||||
| Std. Dev. | 0.24 | R-Squared | 0.8500 | |||
| Mean | 1.04 | Adj R-Squared | 0.7632 | |||
| C.V. % | 23.21 | Adeq Precision | 10.289 | |||
| PRESS | 3.19 |
| Factor | Symbol | Type | Unit | Levels | Level (1) | Level (2) | Level (3) | Level (4) |
|---|---|---|---|---|---|---|---|---|
| Laser Input Power | A | Quantitative | (mW) | a = 2 | 1 | 3 | - | - |
| Concentration of Yeast Cells | B | Quantitative | (-) | b = 4 | 0 in 10 mL PBS | in 10 mL PBS | in 10 mL PBS | in 10 mL PBS |
| Concentration of Yeast Cells | |||||
|---|---|---|---|---|---|
| 0 in 10 mL PBS | 106 in 10 mL PBS | 107 in 10 mL PBS | 108 in 10 mL PBS | ||
| Laser Input Power (mW) | 1 | ||||
| 3 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Block | 0.47 | 2 | 0.24 | |||
| Model | 65.04 | 7 | 9.29 | 156.58 | <0.0001 | significant |
| (A) Laser Input Power | 63.19 | 1 | 63.19 | 1064.80 | <0.0001 | |
| (B) Concentration of Yeast Cells | 1.71 | 3 | 0.57 | 9.60 | 0.0011 | |
| AB | 0.14 | 3 | 0.048 | 0.81 | 0.5070 | |
| Residual | 0.83 | 16 | 0.059 | |||
| Cor Total | 66.35 | 23 | ||||
| Std. Dev. | 0.24 | R-Squared | 0.9874 | |||
| Mean | 6.08 | Adj R-Squared | 0.9811 | |||
| C.V. % | 4.00 | Adeq Precision | 27.119 | |||
| PRESS | 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitta, L.; Cutuli, E.; Celano, G.; Tosto, C.; Sanalitro, D.; Guarino, F.; Cicala, G.; Bucolo, M. Projection Micro-Stereolithography to Manufacture a Biocompatible Micro-Optofluidic Device for Cell Concentration Monitoring. Polymers 2023, 15, 4461. https://doi.org/10.3390/polym15224461
Saitta L, Cutuli E, Celano G, Tosto C, Sanalitro D, Guarino F, Cicala G, Bucolo M. Projection Micro-Stereolithography to Manufacture a Biocompatible Micro-Optofluidic Device for Cell Concentration Monitoring. Polymers. 2023; 15(22):4461. https://doi.org/10.3390/polym15224461
Chicago/Turabian StyleSaitta, Lorena, Emanuela Cutuli, Giovanni Celano, Claudio Tosto, Dario Sanalitro, Francesca Guarino, Gianluca Cicala, and Maide Bucolo. 2023. "Projection Micro-Stereolithography to Manufacture a Biocompatible Micro-Optofluidic Device for Cell Concentration Monitoring" Polymers 15, no. 22: 4461. https://doi.org/10.3390/polym15224461
APA StyleSaitta, L., Cutuli, E., Celano, G., Tosto, C., Sanalitro, D., Guarino, F., Cicala, G., & Bucolo, M. (2023). Projection Micro-Stereolithography to Manufacture a Biocompatible Micro-Optofluidic Device for Cell Concentration Monitoring. Polymers, 15(22), 4461. https://doi.org/10.3390/polym15224461









