Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. Formation of Scaffolds via Rotary Jet Spinning System
2.4. Physical/Chemical Characterization
2.5. In Vitro Assay
2.5.1. Cultivation of 3T3 Fibroblasts
2.5.2. Cytotoxicity of RJS Scaffolds in 3T3 Fibroblasts by the Thiazolyl Blue (MTT) Method
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition of Pracaxi Seed Oil (Pentaclethra macroloba)
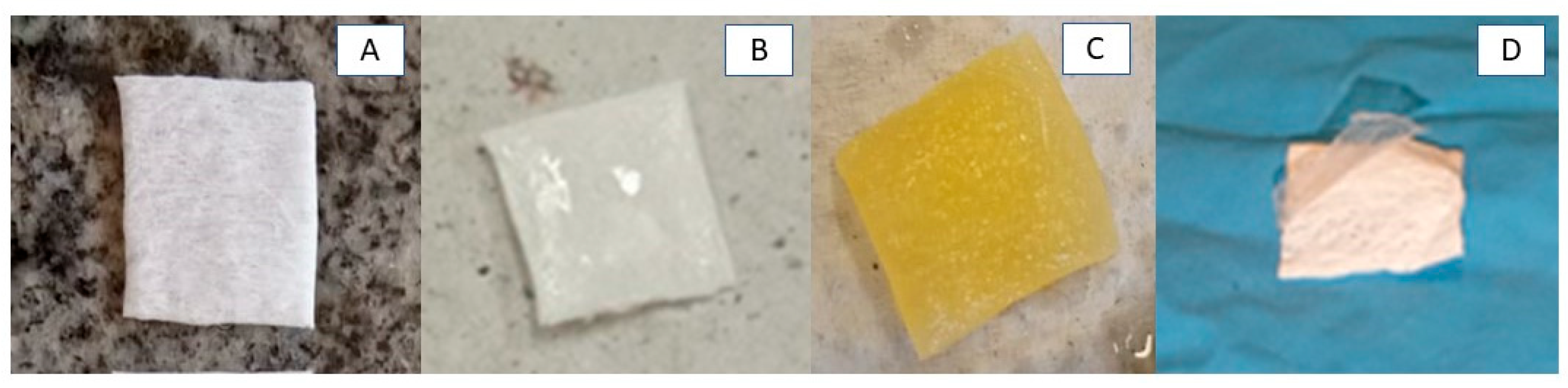
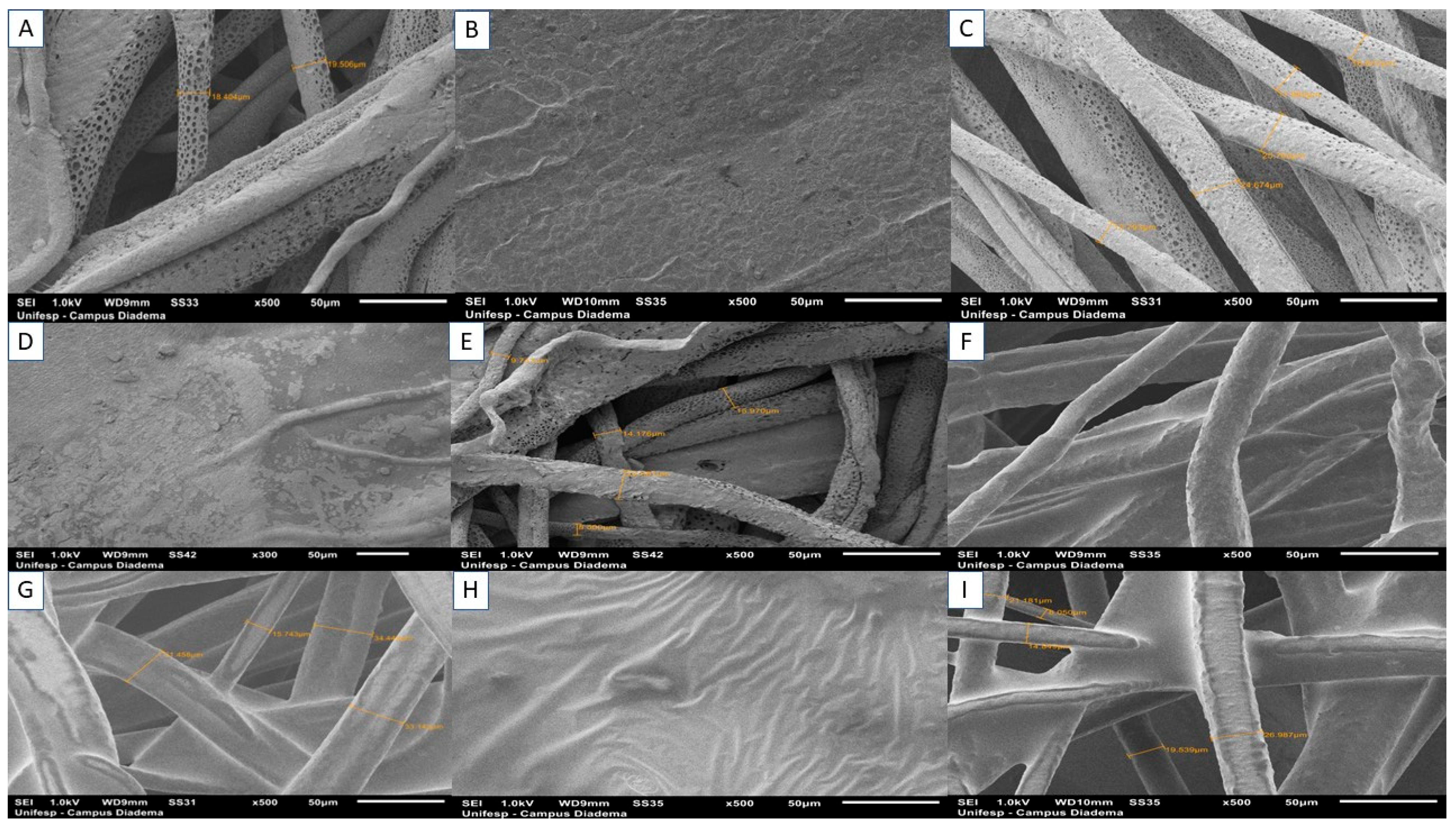
3.2. Production of RJS Scaffolds and Morphological Characteristics by SEM
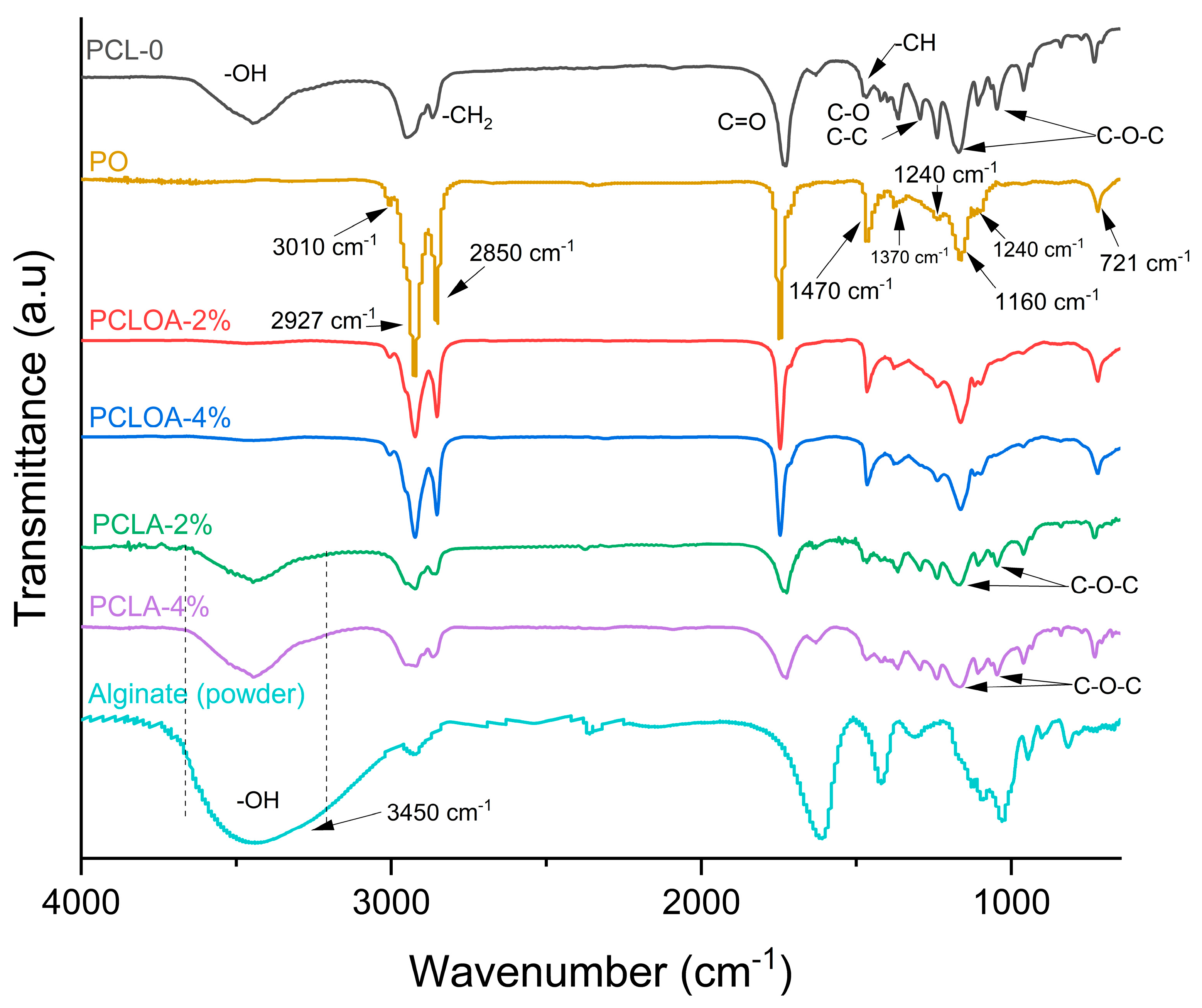
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
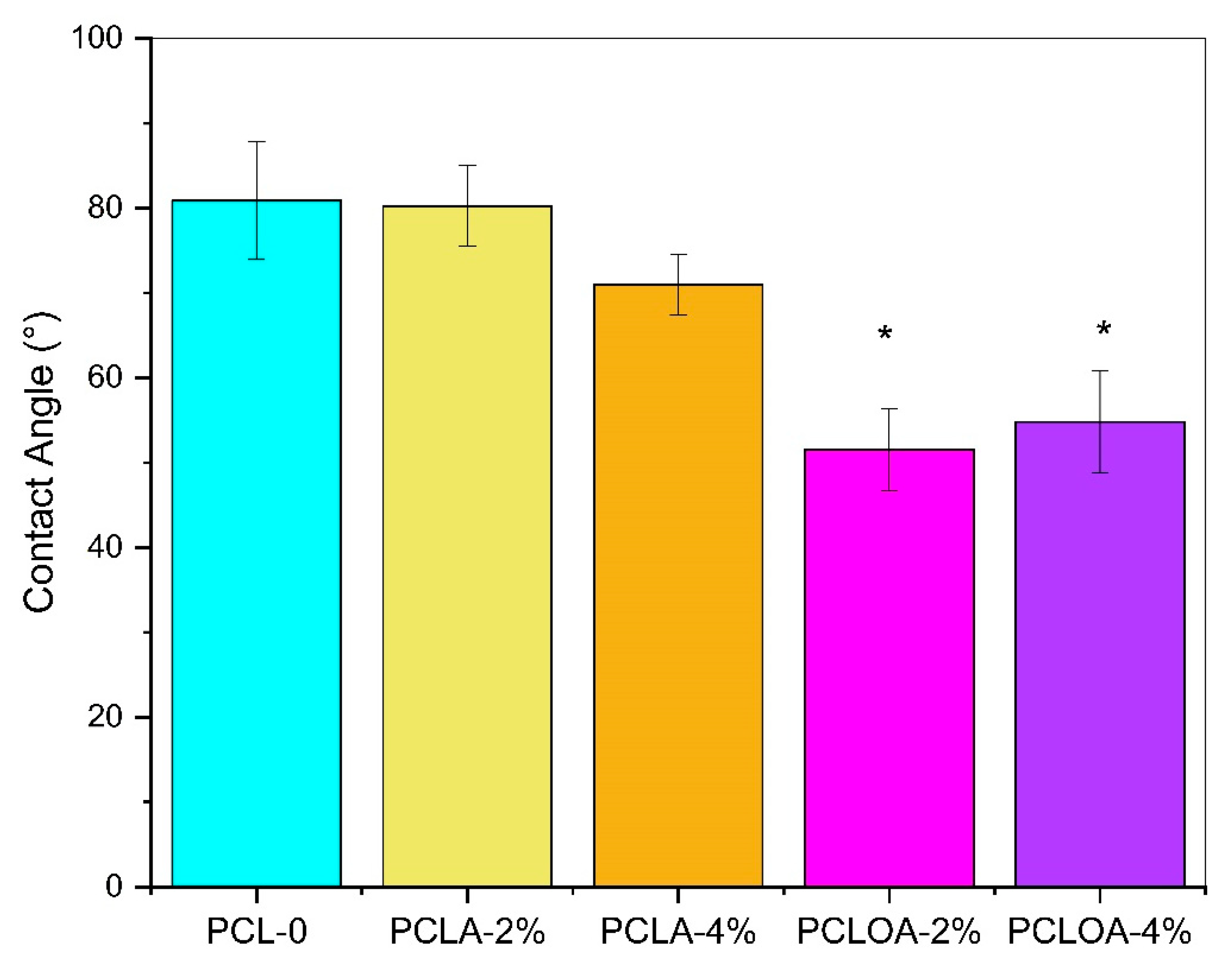
3.4. Contact Angle Assay
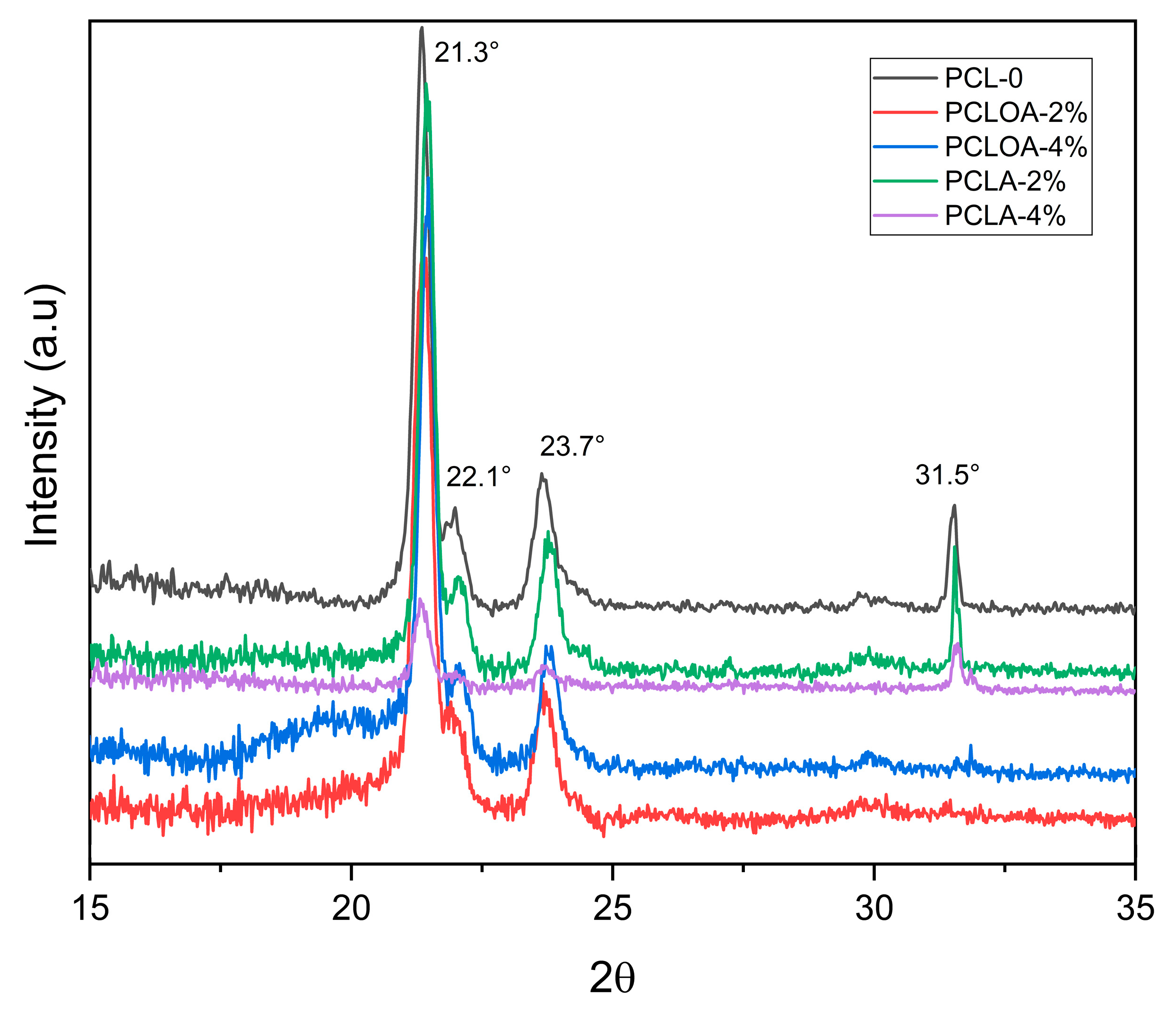
3.5. X-ray Diffraction (XRD)
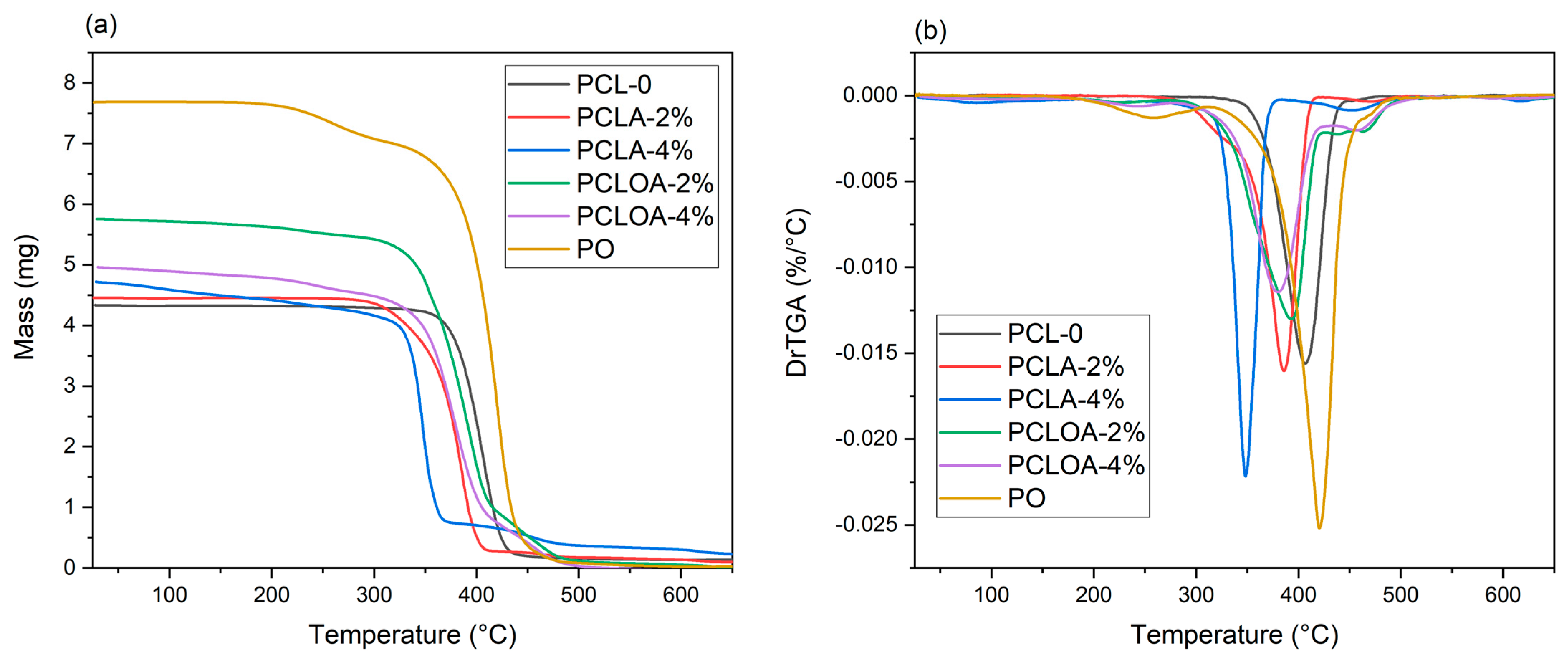
3.6. Thermogravimetric Analysis (TGA)
3.7. Cytotoxicity of RJS Scaffolds in 3T3 Fibroblast
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Cavelier, S.; Hannon, B.; Wei, M. Recent development in multizonal scaffolds for osteochondral regeneration. Bioact. Mater. 2023, 25, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zeng, Y.; Varghese, S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater. 2018, 78, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Pires, A.L.R.; Bierhalz, A.C.K.; Moraes, Â.M. Biomateriais: Tipos, aplicações e mercado. Quim. Nova 2015, 38, 957–971. [Google Scholar] [CrossRef]
- Dias, F.J.d.N.; Pinto, S.A.d.A.; dos Santos, A.R.; Mainardi, M.D.C.A.J.; Rischka, K.; Zavaglia, C.A.d.C. Resveratrol-loaded polycaprolactone scaffolds obtained by rotary jet spinning. Int. J. Polym. Anal. Charact. 2022, 27, 289–301. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (ε-caprolactone) fiber: An overview. J. Eng. Fiber. Fabr. 2014, 9, 74–90. [Google Scholar] [CrossRef]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef] [PubMed]
- Nešović, K.; Janković, A.; Radetić, T.; Vukašinović-Sekulić, M.; Kojić, V.; Živković, L.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Jarrah, R.M.; Potes, M.D.A.; Vitija, X.; Durrani, S.; Ghaith, A.K.; Mualem, W.; Zamanian, C.; Bhandarkar, A.R.; Bydon, M. Alginate hydrogels: A potential tissue engineering intervention for intervertebral disc degeneration. J. Clin. Neurosci. 2023, 113, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in adhesive hydrogels for tissue engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Montaseri, Z.; Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F. Composite silk fibroin hydrogel scaffolds for cartilage tissue regeneration. J. Drug Deliv. Sci. Technol. 2023, 79, 104018. [Google Scholar] [CrossRef]
- Simmons, C.V.; Banov, F.; Banov, D. Use of a topical anhydrous silicone base containing fatty acids from pracaxi oil in a patient with a diabetic ulcer. SAGE Open Med. Case Rep. 2015, 3, 2050313X15589676. [Google Scholar] [CrossRef]
- Nobre Lamarão, M.L.; Ferreira, L.M.; Gyles Lynch, D.; Morais, L.R.; Silva-Júnior, J.O.; Ribeiro-Costa, R.M. Pentaclethra macroloba: A Review of the Biological, Pharmacological, Phytochemical, Cosmetic, Nutritional and Biofuel Potential of this Amazonian Plant. Plants 2023, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.O.; Coppede, J.S.; Fernandes, V.C.; Sant’Ana, C.D.; Ticli, F.K.; Mazzi, M.V.; Giglio, J.R.; Pereira, P.S.; Soares, A.M.; Sampaio, S.V. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba. J. Ethnopharmacol. 2005, 100, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xia, Y.; Wang, Y.; Zhao, X.; Xue, Z.; Quan, F.; Geng, C.; Zhao, Z. Preparation and property investigation of crosslinked alginate/silicon dioxide nanocomposite films. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.T.; Oliveira, J.A.R.; Do Nascimento, L.A.S.; Costa, C.E.F.; Filho, G.N.R.; Concha, V.O.C.; Passos, M.F. PCL/andiroba oil (Carapa guianensis aubl.) hybrid film for wound healing applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- ISO 5509:2000(E); Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids (2nd ed.). International Organization for Standardization: Geneva, Switzerland, 2000.
- Colocar a Referencia AOCS—American Oil Chemists Society. Official Methods and Recommended Practices of the AOCS, 5th ed.; AOCS: Champaign, IL, USA, 2004. [Google Scholar]
- Manhezi, A.C.; Bachion, M.M.; Pereira, Â.L. Utilização de ácidos graxos essenciais no tratamento de feridas. Rev. Bras. Enferm. 2008, 61, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, N.S.; Camponogara, C.; Cruz, L.; Oliveira, S.M. Oleic acid exhibits an expressive anti-inflammatory effect in croton oil-induced irritant contact dermatitis without the occurrence of toxicological effects in mice. J. Ethnopharmacol. 2021, 267, 113486. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Masner, M.; Lujea, N.; Bisbal, M.; Acosta, C.; Kunda, P. Linoleic and oleic acids enhance cell migration by altering the dynamics of microtubules and the remodeling of the actin cytoskeleton at the leading edge. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Alves, A.Q.; Da Silva, V.A.; Goés, A.J.S.; Silva, M.S.; De Oliveira, G.G.; Bastos, I.V.G.A.; De Castro Neto, A.G.; Alves, A.J. The fatty acid composition of vegetable oils and their potential use in wound care. Adv. Ski. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guidoni, M.; Figueira, M.M.; Ribeiro, G.P.; Lenz, D.; Grizotto, P.A.; de Melo Costa Pereira, T.; Scherer, R.; Bogusz, S.; Fronza, M. Development and evaluation of a vegetable oil blend formulation for cutaneous wound healing. Arch. Dermatol. Res. 2019, 311, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Gonçalves, C.B.; Ferreira, S.R.S.; Block, J.M. Composition, thermal behavior and antioxidant activity of pracaxi (Pentaclethra macroloba) seed oil obtained by supercritical CO2. Biocatal. Agric. Biotechnol. 2020, 24, 101521. [Google Scholar] [CrossRef]
- Morguette, A.E.B.; Bigotto, B.G.; Varella, R.d.L.; Andriani, G.M.; Spoladori, L.F.d.A.; Pereira, P.M.L.; de Andrade, F.G.; Lancheros, C.A.C.; Nakamura, C.V.; Arakawa, N.S.; et al. Hydrogel Containing Oleoresin From Copaifera officinalis Presents Antibacterial Activity Against Streptococcus agalactiae. Fontiers Microbiol. 2019, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Merchiers, J.; Meurs, W.; Deferme, W.; Peeters, R.; Buntinx, M.; Reddy, N.K. Influence of polymer concentration and nozzle material on centrifugal fiber spinning. Polymers 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.; Alavarse, A.C.; da Vinci, M.A.; Bonvent, J.J. The Synergistic Effect of Polymer Composition, Solvent Volatility, and Collector Distance on Pullulan and PVA Fiber Production by Rotary Jet Spinning. Fibers Polym. 2021, 22, 942–956. [Google Scholar] [CrossRef]
- Modolo, L.P.; França, W.R.; Simbara, M.M.O.; Malmonge, S.M.; Arnaldo , R.S., Jr. Dense, porous, and fibrous scaffolds composed of PHBV, PCL, and their 75:25 blend: An in vitro morphological and cytochemical characterization. Int. J. Polym. Anal. Charact. 2023, 28, 73–87. [Google Scholar] [CrossRef]
- Altun, E.; Ahmed, J.; Onur, M.; Harker, A.; Edirisinghe, M. The effect of solvent and pressure on polycaprolactone solutions for particle and fibre formation. Eur. Polym. J. 2022, 173, 111300. [Google Scholar] [CrossRef]
- Thakare, V.G.; Joshi, P.A.; Godse, R.R.; Bhatkar, V.B.; Wadegaokar, P.A. Fabrication of polycaprolactone/zirconia nanofiber scaffolds using electrospinning technique. J. Polym. Res. 2017, 24, 232. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Effect of ZnO Nanoparticles on the In Vitro Degradation of Electrospun Polycaprolactone Membranes in Simulated Body Fluid. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 28–37. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Li, C.; Cao, J.; He, E.; Wu, X.; Wang, F.; Wang, L. Facile preparation PCL/modified nano ZnO organic-inorganic composite and its application in antibacterial materials. J. Polym. Res. 2020, 27, 78. [Google Scholar] [CrossRef]
- Aziz, M.S.A.; Salama, H.E.; Sabaa, M.W. Biobased alginate/castor oil edible films for active food packaging. LWT Food Sci. Technol. 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Rashtchian, M.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Simorgh, S. Fabricating alginate/poly(caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr. Polym. 2020, 233, 115873. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer Alginate-Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef]
- Fernandes, R.S.; De Moura, M.R.; Aouada, F.A. Otimização da síntese de hidrogéis nanocompósitos intercalados para possível aplicação na área médica. Quim. Nova 2017, 40, 60–67. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Chen, C.A.; Martinez, J.; Tran, P.; Komvopoulos, K. Novel Electrospun Polycaprolactone/Calcium Alginate Scaffolds for Skin Tissue Engineering. Materials 2023, 16, 136. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhou, L.; Bi, Z.; Shi, M.; Wang, D. Fabrication of a novel Three-Dimensional porous PCL / PLA tissue engineering scaffold with high connectivity for endothelial cell migration. Eur. Polym. J. 2021, 161, 110834. [Google Scholar] [CrossRef]
- Bahú, J.O.; De Andrade, L.R.M.; Crivellin, S.; Khouri, N.G.; Sousa, S.O.; Fernandes, L.M.I.; Souza, S.D.A.; Concha, L.S.C.; Schiavon, M.I.R.B.; Benites, C.I.; et al. Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings. Pharmaceutics 2022, 14, 2500. [Google Scholar] [CrossRef]
- Paranhos, S.B.; Ferreira, S.; Augusto, C.; Canelas, D.A.; Patr, S.; Passos, M.F.; Emmerson, C.; Clay, A.; Monteiro, S.N. Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment. Polymers 2022, 14, 35. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Li, C. Fabrication of a bionic asymmetric wettable Cu-doped chitosan-laponite-PCL wound dressing with rapid healing and antibacterial effect. Biomed. Mater. 2022, 17, 055008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, H.; Zhang, Y.; Zhang, S.; Yang, X.; Wei, Y.; Huang, D.; Lian, X. Fabrication and characterization of double-layer asymmetric dressing through electrostatic spinning and 3D printing for skin wound repair. Mater. Des. 2022, 218, 1–15. [Google Scholar] [CrossRef]
- Yu, B.; He, C.; Wang, W.; Ren, Y.; Yang, J.; Guo, S.; Zheng, Y.; Shi, X. Asymmetric Wettable Composite Wound Dressing Prepared by Electrospinning with Bioinspired Micropatterning Enhances Diabetic Wound Healing. ACS Appl. Bio Mater. 2020, 3, 5383–5394. [Google Scholar] [CrossRef]
- Rochina, J.R.; Vidaurre, A.; Cortazár, I.C.; Lebourg, M. Effects of hydroxyapatite filler on long-term hydrolytic degradation of PLLA/PCL porous scaffolds. Polym. Degrad. Stab. 2015, 119, 121–131. [Google Scholar] [CrossRef]
- Costa, M.N.F.; dos, S.; Muniz, M.Â.P.; Negrão, C.A.B.; da Costa, C.E.F.; Lamarão, M.L.N.; Morais, L.; Júnior, J.O.C.S.; Costa, R.M.R. Characterization of Pentaclethra macroloba oil. J. Therm. Anal. Calorim. 2013, 115, 2269–2275. [Google Scholar] [CrossRef]
- Rescignano, N.; Hernandez, R.; Lopez, L.D.; Calvillo, I.; Kenny, J.M.; Mijangos, C. Preparation of alginate hydrogels containing silver nanoparticles: A facile approach for antibacterial applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Raddatz, L.; Lavrentieva, A.; Pepelanova, I.; Bahnemann, J.; Geier, D.; Becker, T.; Scheper, T.; Beutel, S. Development and Application of an Additively Manufactured Calcium Chloride Nebulizer for Alginate 3D-Bioprinting Purposes. J. Funct. Biomater. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Aguero, L.; Alpdagtas, S.; Ilhan, E.; Zaldivar-Silva, D.; Gunduz, O. Functional role of crosslinking in alginate scaffold for drug delivery and tissue engineering: A review. Eur. Polym. J. 2021, 160, 110807. [Google Scholar] [CrossRef]


| Physicochemical Data | Units | Values * |
|---|---|---|
| Density | 25 °C g/mL | 0.9173 |
| Acidity level | mg NaOH/g | <10.0 |
| Iodine index | gI2/100 g | 50–77 |
| Peroxide content | 10 meq O2/Kg | <10.0 |
| Melting point | °C | 18.5 |
| Unsaponifiable matter | % | <1.5 |
| Saponification index | mg KOH/g1 | 175–188 |
| Sample Name | Composition * |
|---|---|
| PCL—0 | PCL 20% (w/v) |
| PCLA—2% | PCL 20% (w/v)/alginate (2% w/v) |
| PCLA—4% | PCL 20% (w/v)/alginate (4% w/v) |
| PCLA—6% | PCL 20% (w/v)/alginate (6% w/v) |
| PCLOA—2% | PCL 20% (w/v)/alginate (2% w/v), immersed in pracaxi oil |
| PCLOA—4% | PCL 20% (w/v)/alginate (4% w/v), immersed in pracaxi oil |
| PCLOA—6% | PCL 20% (w/v)/alginate (6% w/v), immersed in pracaxi oil |
| Nomenclature | Composition (%) | Chain |
|---|---|---|
| Lauric acid | 0.77 | C12:0 |
| Myristic acid | 0.72 | C14:0 |
| Palmitic acid | 2.42 | C16:0 |
| Margaric acid | 1.44 | C17:0 |
| Stearic acid | 3.15 | C18:0 |
| Oleic acid | 53.68 | C18:2 (ω-9) |
| Linoleic acid | 12.61 | C18:2 (ω-6) |
| Nonadecanoic Acid | 0.14 | C19:0 |
| Arachidic Acid | 1.34 | C20:0 |
| Behenic acid | 13.74 | C22:0 |
| Tricosanoic Acid | 0.12 | C23:0 |
| Lignoceric acid | 9.82 | C24:0 |
| Samples | Tonset (°C) | Tdmax (°C) | Total Weight Loss (%) | Residue (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1° Stage | 2° Stage | 3° Stage | 1° Stage | 2° Stage | 3° Stage | |||
| PCL—0 | 210.29 | 406.9 | 95.16 | 4.84 | ||||
| PCLA—2% | 238.59 | 424.60 | 385.77 | 472.28 | 96.41 | 3.59 | ||
| PCLA—4% | 55.15 | 248.49 | 385.05 | 85.9 | 348.13 | 451.59 | 91.74 | 8.26 |
| PO | 190.62 | 317.08 | 258.07 | 420.31 | 98.31 | 1.69 | ||
| PCLOA—2% | 197.57 | 276.66 | 426.38 | 225.65 | 393.46 | 439.03 | 97.40 | 2.60 |
| PCLOA—4% | 177.12 | 284.84 | 436.59 | 242.78 | 379.06 | 460.56 | 98.21 | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, T.d.P.d.L.; Canelas, C.A.d.A.; Dutra, J.d.C.F.; Rodrigues, A.P.D.; Brígida, R.T.S.S.; Concha, V.O.C.; da Costa, F.A.M.; Passos, M.F. Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests. Polymers 2023, 15, 4403. https://doi.org/10.3390/polym15224403
Lima TdPdL, Canelas CAdA, Dutra JdCF, Rodrigues APD, Brígida RTSS, Concha VOC, da Costa FAM, Passos MF. Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests. Polymers. 2023; 15(22):4403. https://doi.org/10.3390/polym15224403
Chicago/Turabian StyleLima, Tainara de Paula de Lima, Caio Augusto de Almeida Canelas, Joyce da Cruz Ferraz Dutra, Ana Paula Drummond Rodrigues, Rebecca Thereza Silva Santa Brígida, Viktor Oswaldo Cárdenas Concha, Fernando Augusto Miranda da Costa, and Marcele Fonseca Passos. 2023. "Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests" Polymers 15, no. 22: 4403. https://doi.org/10.3390/polym15224403
APA StyleLima, T. d. P. d. L., Canelas, C. A. d. A., Dutra, J. d. C. F., Rodrigues, A. P. D., Brígida, R. T. S. S., Concha, V. O. C., da Costa, F. A. M., & Passos, M. F. (2023). Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests. Polymers, 15(22), 4403. https://doi.org/10.3390/polym15224403






