Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Electrochemical Analysis
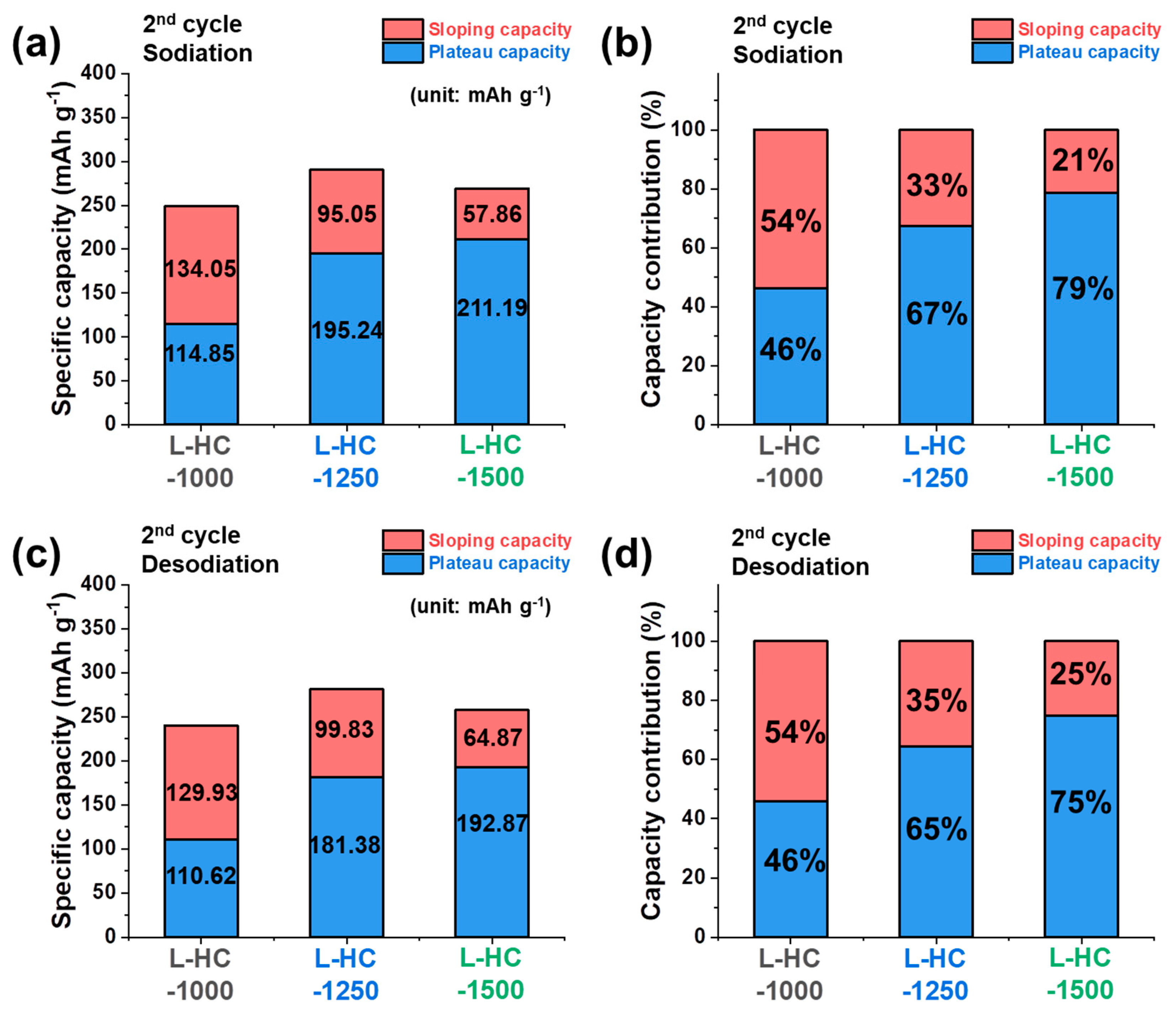
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Muhammad, S.; Jo, M.R.; Kim, H.; Song, K.; Agyeman, D.A.; Kim, Y.-I.; Yoon, W.-S.; Kang, Y.-M. In situ analyses for ion storage materials. Chem. Soc. Rev. 2016, 45, 5717–5770. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Abraham, K.M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternative to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent advances and prospects of cathode materials for sodium-ion batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef]
- Jo, M.R.; Kim, Y.; Yang, J.; Jeong, M.; Song, K.; Kim, Y.-I.; Lim, J.-M.; Cho, M.; Shin, J.-H.; Kim, Y.-M.; et al. Triggered reversible phase transformation between layered and spinel structure in manganese-based layered compounds. Nat. Commun. 2019, 10, 3385. [Google Scholar] [CrossRef]
- Berthelot, R.; Cartlier, D.; Delmas, C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater. 2010, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yao, Z.; Wang, Q.; Li, H.; Wang, J.; Liu, M.; Ganapathy, S.; Lu, Y.; Cabana, J.; Li, B.; et al. Revealing high Na-cntent P2-type layered oxides as advances sodium-ion cathodes. J. Am. Chem. Soc. 2020, 142, 5742–5750. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wang, R.Y.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W.; et al. Manganese-cobalt hexacyanoferrate cahotdes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Yang, J.; Han, D.-W.; Jo, M.R.; Song, K.; Kim, Y.-I.; Chou, S.-L.; Liu, H.-K.; Kang, Y.-M. Na3V2(PO4)3 particles partly embedded in carbon nanofibers with superb kinetics for ultra-high power sodium ion batteries. J. Mater. Chem. A 2015, 3, 1005–1009. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, S.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Lu, Y.C.; Ma, C.; Alvarado, J.; Kidera, T.; Dimov, N.; Meng, Y.S.; Okada, S. Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sources 2015, 284, 287–295. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; Chen, M.; Zhou, H. Monodispersed hierarchical Co3O4 spheres interwined with carbon nanotubes for use as anode materials in sodium-ion batteries. J. Mater. Chem. A 2014, 2, 13805–13809. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. Facile synthesis of nanostrucrued MnO2 as anode materials for sodium-ion batteries. ChemNanoMat 2016, 2, 196–200. [Google Scholar] [CrossRef]
- Yu, X.Y.; David Lou, X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.Y.; Sun, Y.K. Micro-intertexture carbon-free iron sulfides as advanced high tap density anodes for rechargeable batteries. ACS Appl. Mater. Interfaces 2017, 9, 39416–39426. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Wing-hei Lau, V.; Lee, G.-H.; Park, M.; Kang, Y.-M. Engineering solid electrolyte interphase on red phosphorus for long-term and high-capacity sodium storage. Chem. Mater. 2019, 32, 448–458. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett. 2013, 13, 5480–5484. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-principles study of alkali metal-graphite intercalation compounds. J. Power Sources 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, L.; Yu, M.-M.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow carbon nanospheres with superior rate capability for sodium-based batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Adair, K.R.; Sun, X.; Yu, Y. Carbon nanofiber-based nanostructures for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2017, 5, 13882–13906. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, B.; Du, Y.; Li, Y.; Zhou, X.; Dai, Z.; Bao, J. Fluorine-doped carbon particles derived from Lotus petioles as high-performance anode materials for sodium-ion batteries. J. Phys. Chem. C 2015, 119, 21336–21344. [Google Scholar] [CrossRef]
- Simone, V.; Boulineau, A.; Geyer, A.; Rouchon, D.; Simonin, L.; Martiner, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Hongshuai, H.; Xiaoqing, Q.; Weifeng, W.; Yun, Z.; Xiaobo, J. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard carbon anodes: Fundamental understanding and commercial perspetives for Na-ion batteries beyond Li-ion and K-ion counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Kim, J.-B.; Lee, G.-H.; Wing-hei Lau, V.; Zhang, J.; Zou, F.; Chen, M.; Zhou, L.; Nam, K.-Y.; Kang, Y.-M. Microstructural investigation into Na-ion storage behaviors of cellulose-based hard carbons for Na-ion batteries. J. Phys. Chem. C 2021, 125, 14559–14566. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Lee, G.-H.; Hwang, T.; Kim, J.-B.; Yang, J.; Zou, F.; Cho, M.; Kang, Y.-M. Origin of enhanced reversible Na ion storage in hard carbon anodes through p-type molecular doping. J. Mater. Chem. A 2022, 10, 16506–16513. [Google Scholar] [CrossRef]
- Luo, W.; Bommier, C.; Jian, Z.; Li, X.; Carter, R.; Vail, S.; Lu, Y.; Lee, J.-J.; Ji, X. Low-Surface-Area Hard Carbon Anode for Na-Ion Batteries via Graphene Oxide as a Dehydration Agent. ACS Appl. Mater. Interfaces 2015, 7, 2626–2631. [Google Scholar] [CrossRef]
- Hong, J.; Qie, L.; Zeng, R.; Yi, Z.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.-J.; Zhang, W.-X.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple biowaste derived hard carbon as a powerful anode material for Na ion batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Shen, F.; Luo, W.; Dai, J.; Yao, Y.; Zhu, M.; Hitz, E.; Tang, Y.; Chen, Y.; Sprenkle, V.L.; Li, X.; et al. Ultra-thick, low-totuosity, and mesoporous wood carbon anode for high-performance sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600377. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Titirici, M.-M.; Chen, L.; Huang, X. Hard carbon microtubes made from renewable cotton as high-performance anode materials for sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Sagues, W.J.; Yang, J.; Moroe, N.; Han, S.-D.; Vinzant, T.; Yung, M.; Jameel, H.; Nimlos, M.; Park, S. A simple method for producing bio-based anode materials for lithium-ion batteries. Green Chem. 2020, 22, 7093–7108. [Google Scholar] [CrossRef]
- Yoon, D.; Hwang, J.; Chang, W.; Kim, J. Carbon with expanded and well-developed graphene planes derived directly from condensed lignin as a high-performance anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 569–581. [Google Scholar] [CrossRef]
- Brown, T.R.; Brown, R.C. A review of cellulosic biofuel commercial-scale projects in the United States. Biofuel Bioprod. Biorefin. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Madhu, R.; Periasamy, A.P.; Schlee, P.; Herou, S.; Titirici, M.-M. Lignin: A sustainable precursor for nanostructured carbon materials for supercapacitors. Carbon 2023, 207, 172–197. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, X.; Xiong, W.; Wang, H.; Ji, X.; Kong, F.; Yang, G.; Xu, J. Converting amorphous kraft lignin to hollow carbon shell frameworks as electrode materials for lithium-ion batteries and supercapacitors. Ind. Crops Prod. 2021, 174, 114184. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Li, L.; An, B.; Lahiri, A.; Wang, P.; Fang, Y. Doublet of D and 2D bands in graphene deposited with Ag nanoparticles by surface enhanced Raman spectroscopy. Carbon 2013, 65, 359–364. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Karacan, I.; Erzurunmluoglu, L. Formation of Non-graphitizing carbon fibers prepared from poly(p-phenylene terephthalamide) precursor fibers. Fibers Polym. 2015, 16, 961–974. [Google Scholar] [CrossRef]
- Qiu, Z.; Cui, Y.; Wang, D.; Wang, Y.; Hu, H.; Li, X.; Cai, T.; Gao, X.; Hu, H.; Wu, M.; et al. Dual carbon Li-ion capacitor with high energy density and ultralong cycling life at a wide voltage window. Sci. China. Mater. 2022, 65, 2373–2384. [Google Scholar] [CrossRef]
- Kim, J.; Yamada, Y.; Suzuki, Y.; Ciston, J.; Sato, S. Pyrolysis of Epoxidized Fullerenes Analyzed by Spectroscopies. J. Phys. Chem. C 2014, 118, 7076–7084. [Google Scholar] [CrossRef]
- Beda, A.; Taberna, P.-L.; Simon, P.; Ghimbeu, C.M. Hard carbons derived from green phenolic resins for Na-ion batteries. Carbon 2018, 139, 248–257. [Google Scholar] [CrossRef]
- Gnaraj, J.S.; Thompson, R.W.; Iaconatti, S.N.; DiCarlo, J.F.; Abraham, K.M. Formation and growth of surface films on graphitic anode materials for Li-ion batteries. Electrochem. Solid-State. Lett. 2005, 8, A128–A132. [Google Scholar] [CrossRef]
- Chen, M.; Luo, F.; Liao, Y.; Liu, C.; Xu, D.; Wang, Z.; Liu, Q.; Wang, D.; Ye, Y.; Li, S.; et al. Hard carbon derived for lignin with robust and low-potential sodium ion storage. J. Electroanal. Chem. 2022, 919, 116526. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. A superior low-cost amorphous carbon anode made from pitch and lignin for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 96–104. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Tan, H.; Zhang, B. Advanced lignin-derived hard carbon for Na-ion batteries and a comparison with Li and K ion storage. Carbon 2020, 157, 316–323. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Zhang, B.; Yuso, A.M.; Rety, B.; Tarascon, J.-M. Valorizing low cost and renewable lignin as hard carbon for Na-ion batteries: Impact of lignin grade. Carbon 2019, 153, 634–647. [Google Scholar] [CrossRef]





| Sample | IF/IG | ID/IG | I2D/IG |
|---|---|---|---|
| L-HC-1000 | 0.42 | 1.26 | 0.19 |
| L-HC-1250 | 0.38 | 1.13 | 0.15 |
| L-HC-1500 | 0.17 | 1.13 | 0.53 |
| Graphene reference | 0.01 | 0.04 | 0.47 |
| Sample | Vacancy, C-H | Sp2C | Sp3C | C-O/C=O | Total |
|---|---|---|---|---|---|
| L-HC-1000 | 10.8 | 66.5 | 17.7 | 5.0 | 100 |
| L-HC-1250 | 8.4 | 70.7 | 16.9 | 4.0 | 100 |
| L-HC-1500 | 6.3 | 77.0 | 13.3 | 3.4 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Han, S.-D.; Koo, B.; Lee, S.-H.; Yang, J. Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery. Polymers 2023, 15, 4373. https://doi.org/10.3390/polym15224373
Kim J, Han S-D, Koo B, Lee S-H, Yang J. Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery. Polymers. 2023; 15(22):4373. https://doi.org/10.3390/polym15224373
Chicago/Turabian StyleKim, Jungpil, Sang-Don Han, Bonwook Koo, Sang-Hyun Lee, and Junghoon Yang. 2023. "Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery" Polymers 15, no. 22: 4373. https://doi.org/10.3390/polym15224373
APA StyleKim, J., Han, S.-D., Koo, B., Lee, S.-H., & Yang, J. (2023). Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery. Polymers, 15(22), 4373. https://doi.org/10.3390/polym15224373






