Improving Durability of Dye-Based Polarizing Films Using Novel Reactive Dyes as Dichroic Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Synthesis of Direct Dyes
2.2.1. Synthesis of Dye Intermediate (I1)
2.2.2. Synthesis of Direct Dyes (D1~D5)
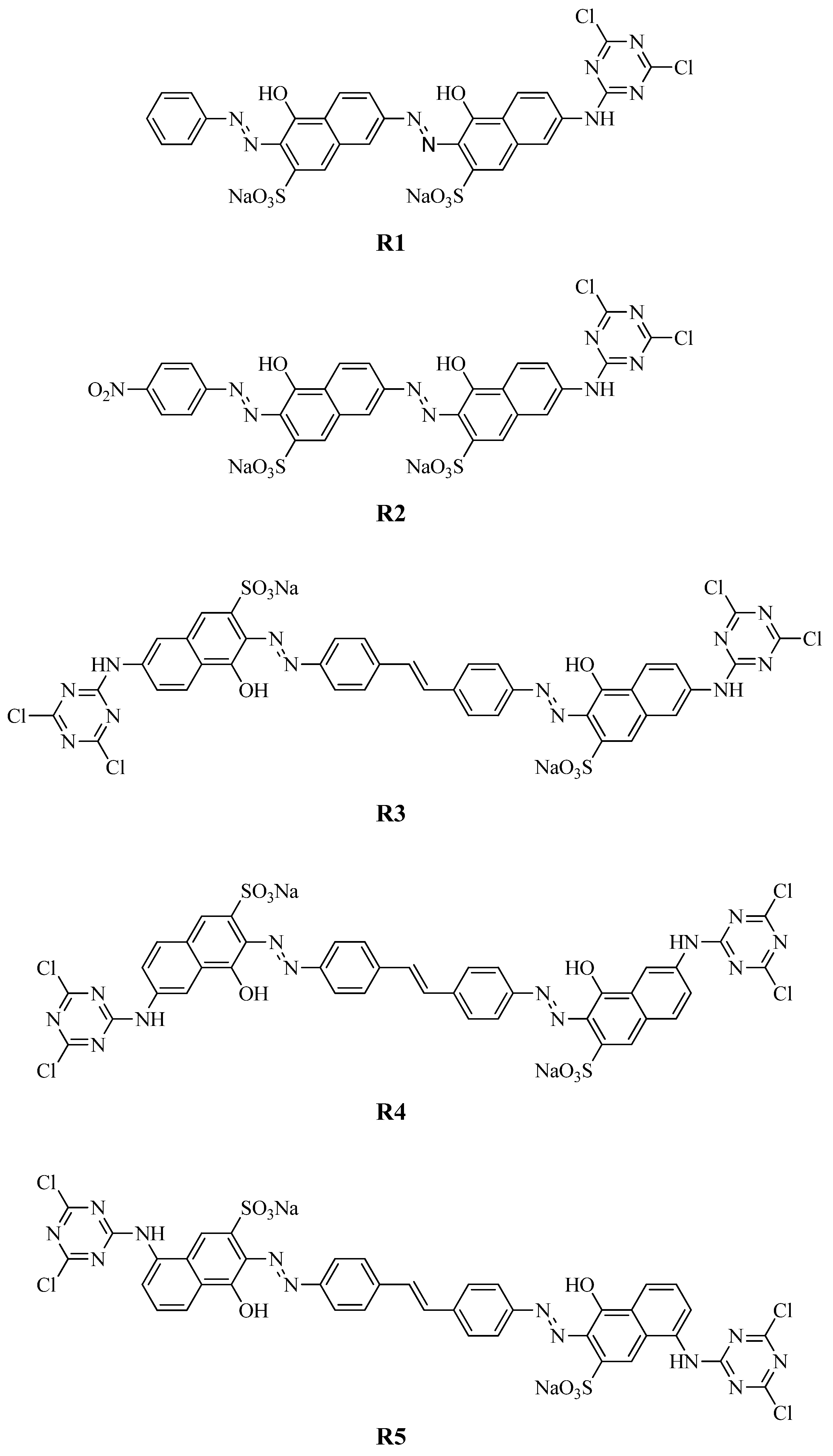
2.3. Synthesis of Reactive Dyes (R1~R5)
2.4. Preparation of Polarizing Films
2.5. Preparation of Polarizing Films
2.6. Molecular Orbital (MO) Calculation
2.7. Durability Measurement of Polarizing Films under High Temperature and Humidity
3. Results and Discussion
3.1. Synthesis and Spectral Properties of Direct and Reactive Dyes
3.2. Comparison Optical Properties between Direct Dye-Based PF and Reactive Dye-Based PF
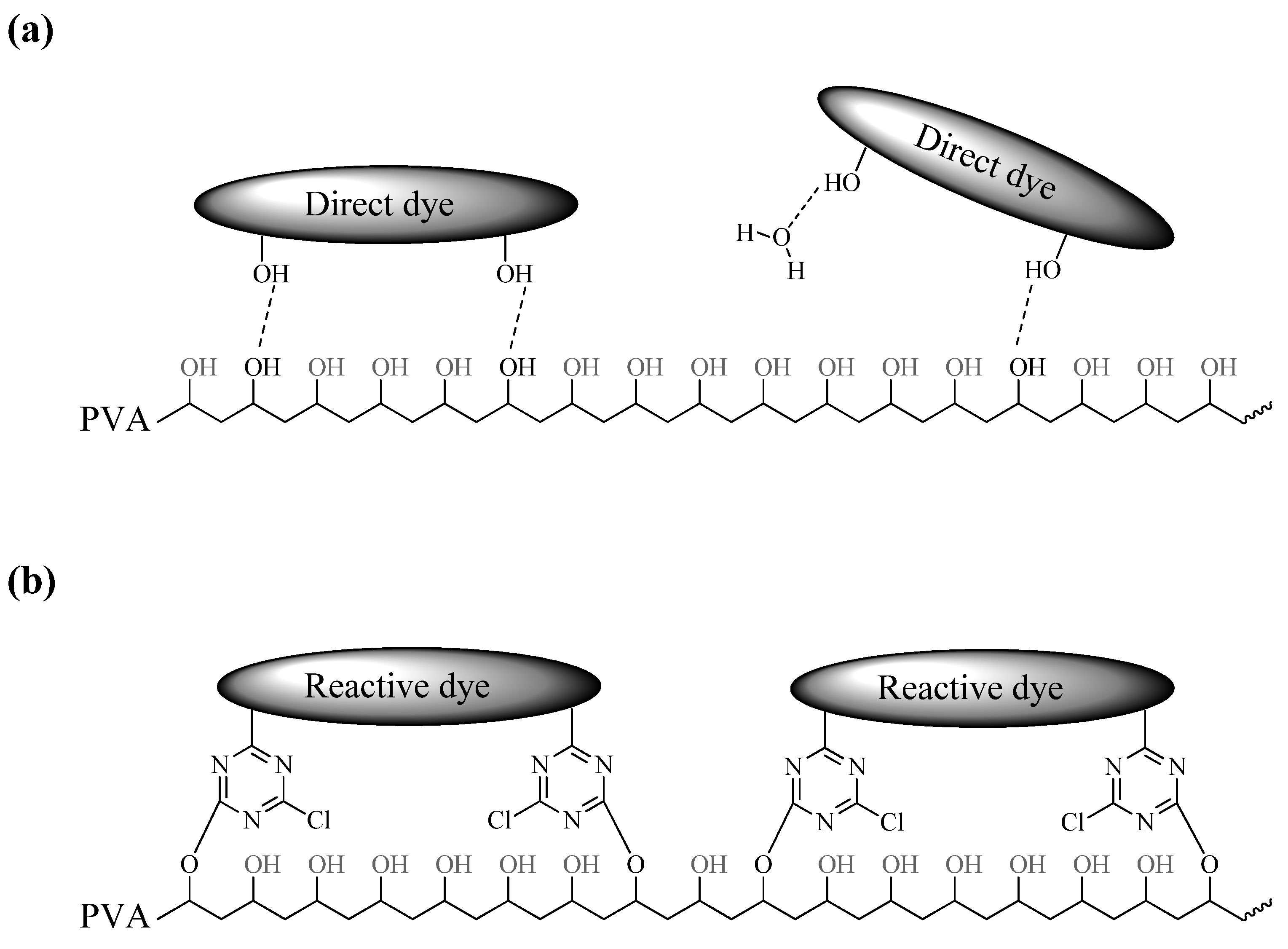
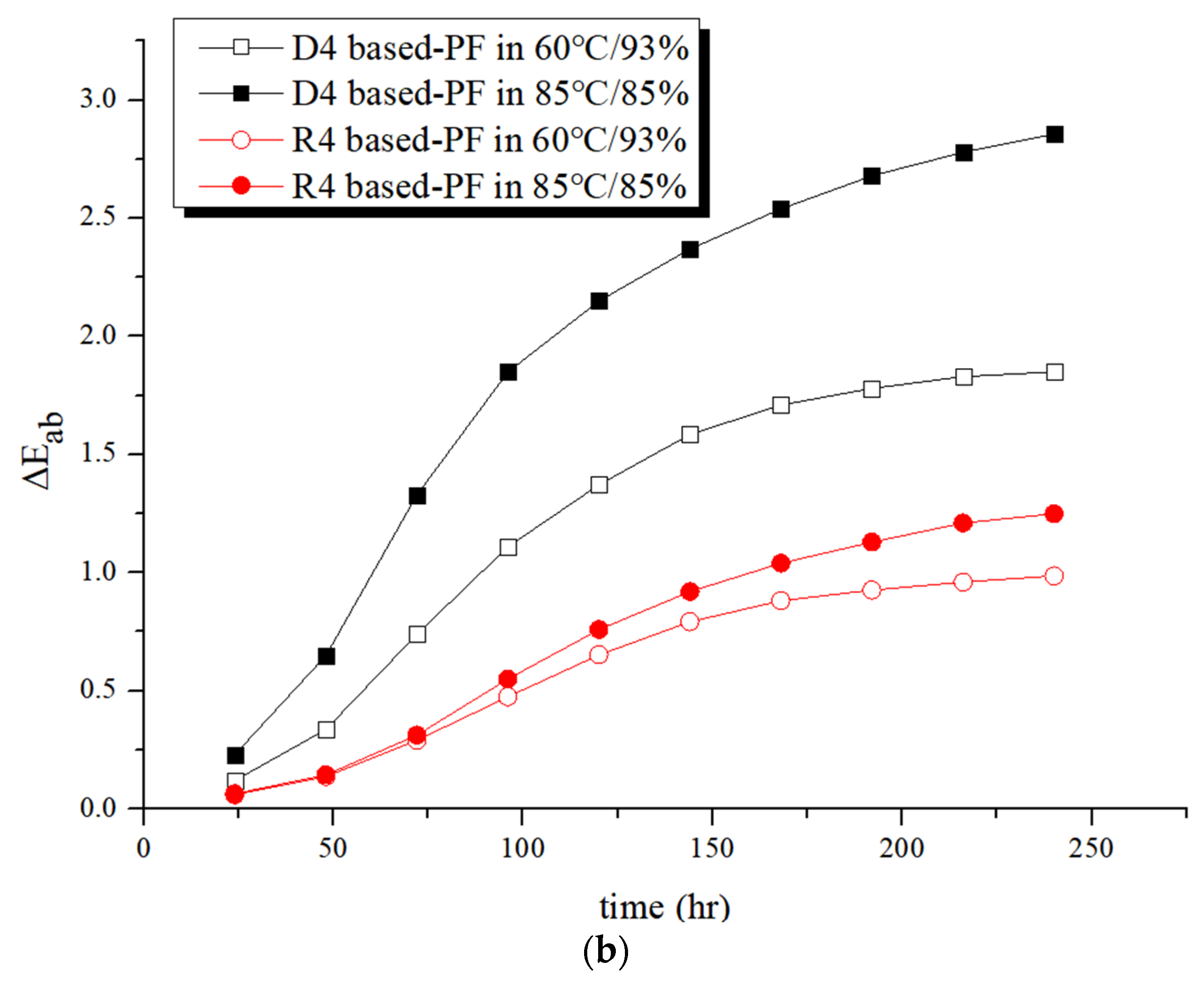
3.3. Durability of Polarizing Films under High Temperature and Humidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, S.E.; Hwang, I.S. Modeling of the optical anisotropy of a dye polarizer. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1363–1370. [Google Scholar] [CrossRef]
- Dirix, Y.; Tervoort, T.A.; Bastiaansen, C. Optical properties of oriented polymer/dye polarizers. 2. ultimate properties. Macromolecules 1997, 30, 2175–2177. [Google Scholar] [CrossRef]
- Takamiya, H.; Tanahashi, Y.; Matsuyama, T.; Tanigami, T.; Yamaura, K.; Matsuzawa, S. On the poly(vinyl alcohol)-iodine complexes. J. Appl. Polym. Sci. 1993, 50, 1807–1813. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol; John Wiley and Sons: London, UK, 1973; pp. 507–508. [Google Scholar]
- Song, D.H.; Yoo, H.Y.; Lee, J.J.; Kim, J.P. Polarizing films based on oriented poly(vinyl alcohol)-dichroic dyes. Mol. Cryst. Liq. Cryst. 2006, 445, 355–360. [Google Scholar] [CrossRef]
- Song, D.H.; Yoo, H.Y.; Kim, J.P. Synthesis of stilbene-based azo dyes and application for dichroic materials in poly(vinyl alcohol) polarizing films. Dye. Pigment. 2007, 75, 727–731. [Google Scholar] [CrossRef]
- Chang, J.B.; Hwang, J.H.; Park, J.S.; Kim, J.P. The effect of dye structure on the dyeing and optical properties of dichroic dyes for PVA polarizing film. Dye. Pigment. 2011, 88, 366–371. [Google Scholar] [CrossRef]
- Chang, J.B.; Yuk, S.B.; Park, J.S.; Kim, J.P. Dichroic and spectral properties of anthraquinone-based azo dyes for PVA polarizing film. Dye. Pigment. 2012, 92, 737–744. [Google Scholar] [CrossRef]
- Sekar, N. Direct dyes. In Handbook of Textile and Industrial Dyeing; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2011; Volume 1, pp. 425–445. [Google Scholar]
- Daria, Ś.K.; Jolanta, W.G.; Piotr, S.; Jolanta, K. The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes. Molecules 2020, 25, 5435–5467. [Google Scholar]
- Song, D.H.; Kim, J.P. Effect of transition moments and orientational behavior of dichroic dyes on the optical anisotropy of poly(vinyl alcohol) polarizing films. Dye. Pigment. 2009, 80, 219–225. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, W.; Zhang, C.; Hossain, Y.; Oli, Z.; Pervez, N.; Sarker, S.; Hoque, I.; Cai, Y.; Naddeo, V. Combination of wet fixation and drying treatments to improve dye fixation onto spray-dyed cotton fabric. Sci. Rep. 2021, 11, 15403. [Google Scholar] [CrossRef]
- Morris, K.F.; Lewis, D.M.; Broadbent, P.J. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Color. Technol. 2008, 124, 186–194. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Z.; Lin, J.; Zhou, L.; Chen, G. Effect of KI Concentration in Correcting Tank on Optical Properties of PVA Polarizing Film. Polymers 2022, 14, 1413. [Google Scholar] [CrossRef]
- Dirix, Y.; Tervoort, T.A.; Bastiaansen, C. Optical properties of oriented polymer/dye polarizers. Macromolecules 1995, 28, 486–491. [Google Scholar] [CrossRef][Green Version]
- CAChe 6.1.8; Fujitsu Systems Business of America Inc.: Beaverton, OR, USA, 2023.
- Lye, J.; Freeman, H.S.; Hinks, D. Molecular modeling in dye chemistry: Studies involving two disperse dyes. Text. Res. J. 1999, 69, 583–590. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. Viewpoint 11-approaches to the global minimum problem. J. Mol. Struct. Theochem. 1993, 285, 157–168. [Google Scholar] [CrossRef]
- Ridley, J.E.; Zerner, M.C. An intermediate neglect of differential overlap technique for spectroscopy: Pyrrole and the azines. Theor. Chim. Acta 1973, 32, 111–134. [Google Scholar] [CrossRef]
- Kubo, Y.; Yoshida, K.; Adachi, M.; Nakamura, S.; Maeda, S. Experimental and theoretical study of near-infrared absorbing naphthoquinone methide dyes with a nonplanar geometry. J. Am. Chem. Soc. 1991, 113, 2868–2873. [Google Scholar] [CrossRef]
- Hanemann, T.; Böhm, M.C.; Haase, W. Calculation of UV/VIS absorption spectra of liquid crystals and dye molecules: An INDO MO approach. Liq. Cryst. 1992, 11, 917–927. [Google Scholar] [CrossRef]
- Lee, W.J. Synthesis and Application of Functional Dyes Based on the Thiazole System. Ph.D. Thesis, University of Leeds, Leeds, UK, 2002. [Google Scholar]
- White, D.L.; Taylor, G.N. New absorptive mode reflective liquid-crystal display device. J. Appl. Phys. 1974, 45, 4718–4723. [Google Scholar] [CrossRef]
- Seki, H.; Uchida, T.; Shibata, Y. Dichroic dyes for guest-host liquid-crystal cells. Mol. Cryst. Liq. Cryst. 1986, 138, 349–365. [Google Scholar] [CrossRef]
- Griffiths, J.; Feng, K. The influence of intramolecular hydrogen bonding on the order parameter and photostability properties of dichroic azo dyes in a nematic liquid crystal host. J. Mater. Chem. 1999, 9, 2333–2338. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, N.; Andoh, N.; Funabiki, K.; Shibata, K.; Muramatsu, H.; Ishigure, Y.; Kohyama, E.; Abe, Y.; Kaneko, M. Synthesis and properties of novel dichroic disazo dyes containing the tetrafluoro-p-phenylene moiety for guest-host liquid crystal displays. Chem. Mater. 1998, 10, 1921–1930. [Google Scholar] [CrossRef]
- Sadlej, J. Semi-Empirical Method of Quantum Chemistry; Ellis Horwood: Chichester, UK, 1985; pp. 93–101. [Google Scholar]
- Kim, Y.D.; Kim, J.P.; Kwon, O.S.; Cho, I.H. The synthesis and application of thermally stable dyes for ink-jet printed LCD color filters. Dye. Pigment. 2009, 81, 45–52. [Google Scholar] [CrossRef]
- Kim, Y.D.; Cho, J.H.; Park, J.R.; Choi, J.H.; Yoon, C.; Kim, J.P. Synthesis, application and investigation of structure–thermal stability relationships of thermally stable water-soluble azo naphthalene dyes for LCD red color filters. Dye. Pigment. 2011, 89, 1–8. [Google Scholar] [CrossRef]





| Dye | λmax (nm) | εmax (Lmol−1cm−1) | Dye | λmax (nm) | εmax (Lmol−1cm−1) |
|---|---|---|---|---|---|
| D1 | 544 | 62,000 | R1 | 544 | 52,000 |
| D2 [7] | 548 | 83,000 | R2 | 548 | 64,000 |
| D3 [7] | 542 | 88,000 | R3 | 524 | 57,000 |
| D4 [7] | 576 | 40,000 | R4 | 570 | 35,000 |
| D5 [7] | 592 | 54,000 | R5 | 568 | 36,000 |
| Dye | R | S (ST) | SM | β (°) | θ (°) |
|---|---|---|---|---|---|
| D1 | 11.8 | 0.78 | 0.79 | 2.71 | 22.2 |
| R1 | 10.0 | 0.75 | 0.75 | 1.68 | 24.0 |
| D2 [7] | 12.2 | 0.79 | 0.79 | 1.79 | 21.9 |
| R2 | 10.4 | 0.76 | 0.76 | 0.90 | 23.6 |
| D3 [7] | 25.2 | 0.89 | 0.89 | 2.30 | 15.6 |
| R3 | 20.7 | 0.87 | 0.88 | 4.48 | 16.7 |
| D4 [7] | 22.5 | 0.88 | 0.88 | 0.22 | 16.6 |
| R4 | 19.0 | 0.86 | 0.86 | 0.83 | 18.0 |
| D5 [7] | 19.7 | 0.86 | 0.86 | 3.21 | 17.5 |
| R5 | 12.1 | 0.79 | 0.79 | 0.94 | 22.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.D.; Lee, J.U.; Lee, J.J. Improving Durability of Dye-Based Polarizing Films Using Novel Reactive Dyes as Dichroic Materials. Polymers 2023, 15, 4365. https://doi.org/10.3390/polym15224365
Kim YD, Lee JU, Lee JJ. Improving Durability of Dye-Based Polarizing Films Using Novel Reactive Dyes as Dichroic Materials. Polymers. 2023; 15(22):4365. https://doi.org/10.3390/polym15224365
Chicago/Turabian StyleKim, Young Do, Jea Uk Lee, and Jung Jin Lee. 2023. "Improving Durability of Dye-Based Polarizing Films Using Novel Reactive Dyes as Dichroic Materials" Polymers 15, no. 22: 4365. https://doi.org/10.3390/polym15224365
APA StyleKim, Y. D., Lee, J. U., & Lee, J. J. (2023). Improving Durability of Dye-Based Polarizing Films Using Novel Reactive Dyes as Dichroic Materials. Polymers, 15(22), 4365. https://doi.org/10.3390/polym15224365








