Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cotton Removal with Phosphoric Acid Pretreatment on Different Textile Waste Blends
2.3. Cotton Removal from TC with Phosphoric Acid and Enzymatic Pretreatment on TC Fabrics
2.4. Melt Spinning and Characterization of rPET Fibers Prepared from the Remaining PET Fibers in TC Fabric
2.4.1. Differential Scanning Calorimetry (DSC)
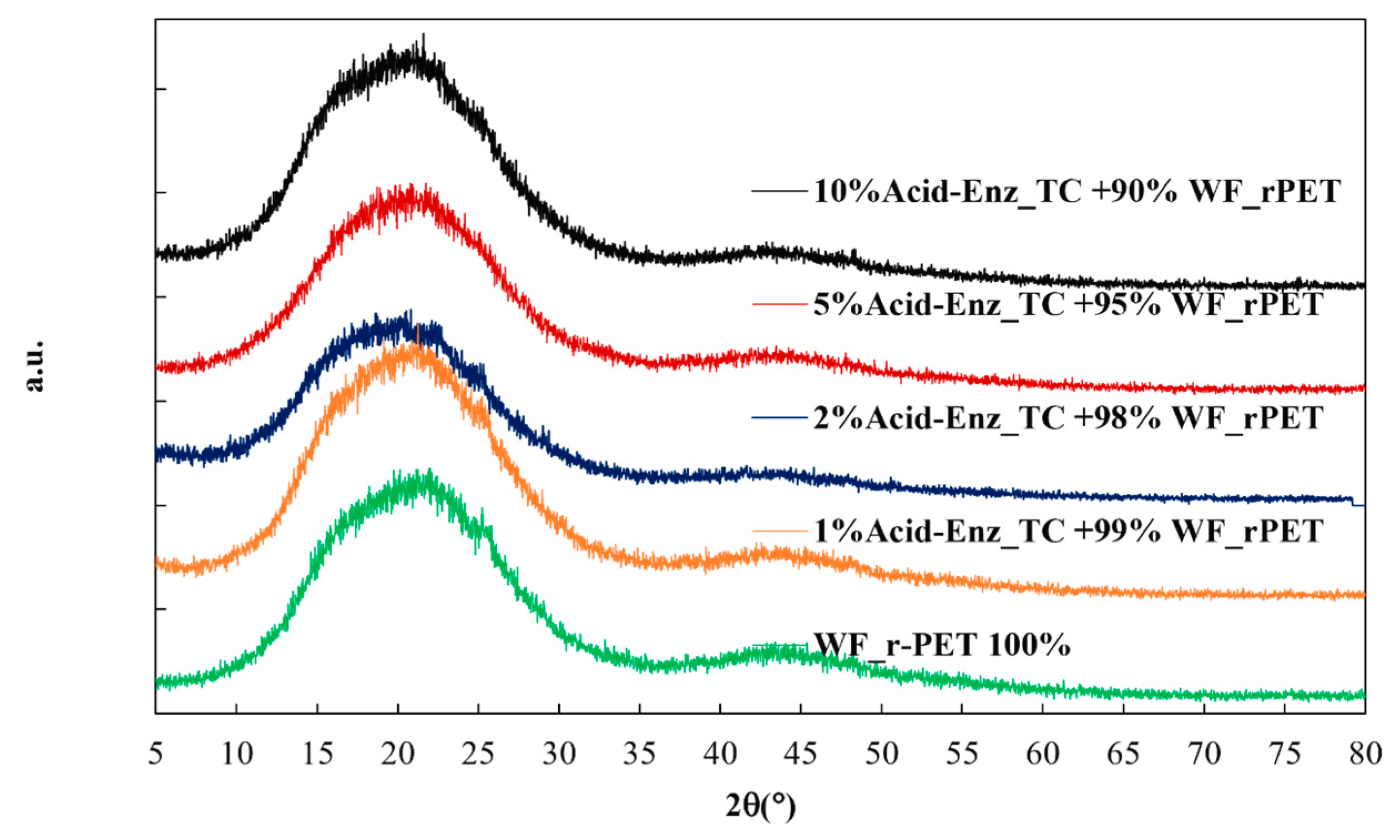
2.4.2. X-ray Diffraction Analysis (XRD)
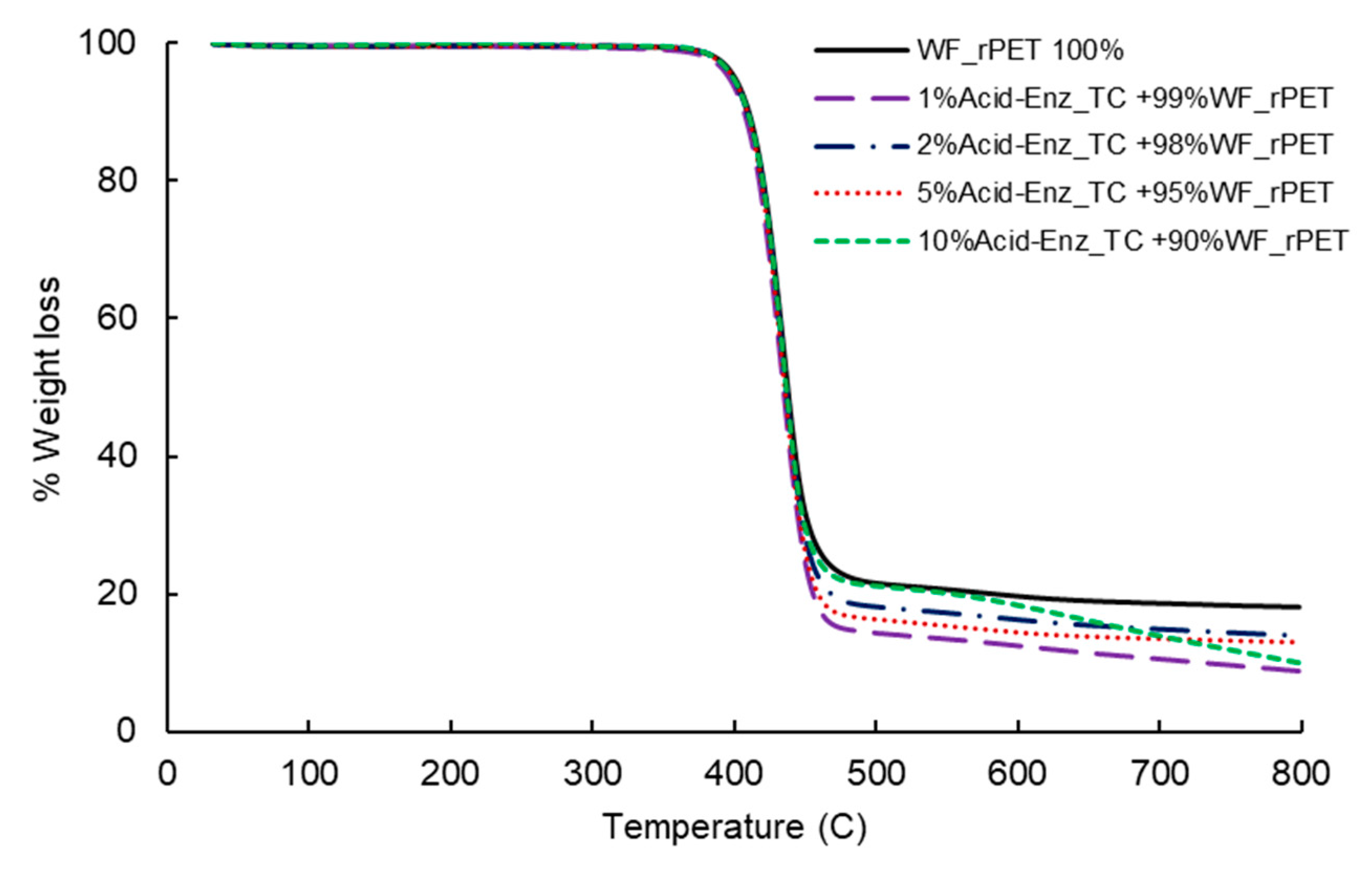
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Fiber Morphology under an Optical Microscope
2.4.5. Mechanical Properties
3. Results
3.1. Cotton Removal with Phosphoric Acid Pretreatment on Different Textile Waste Blends
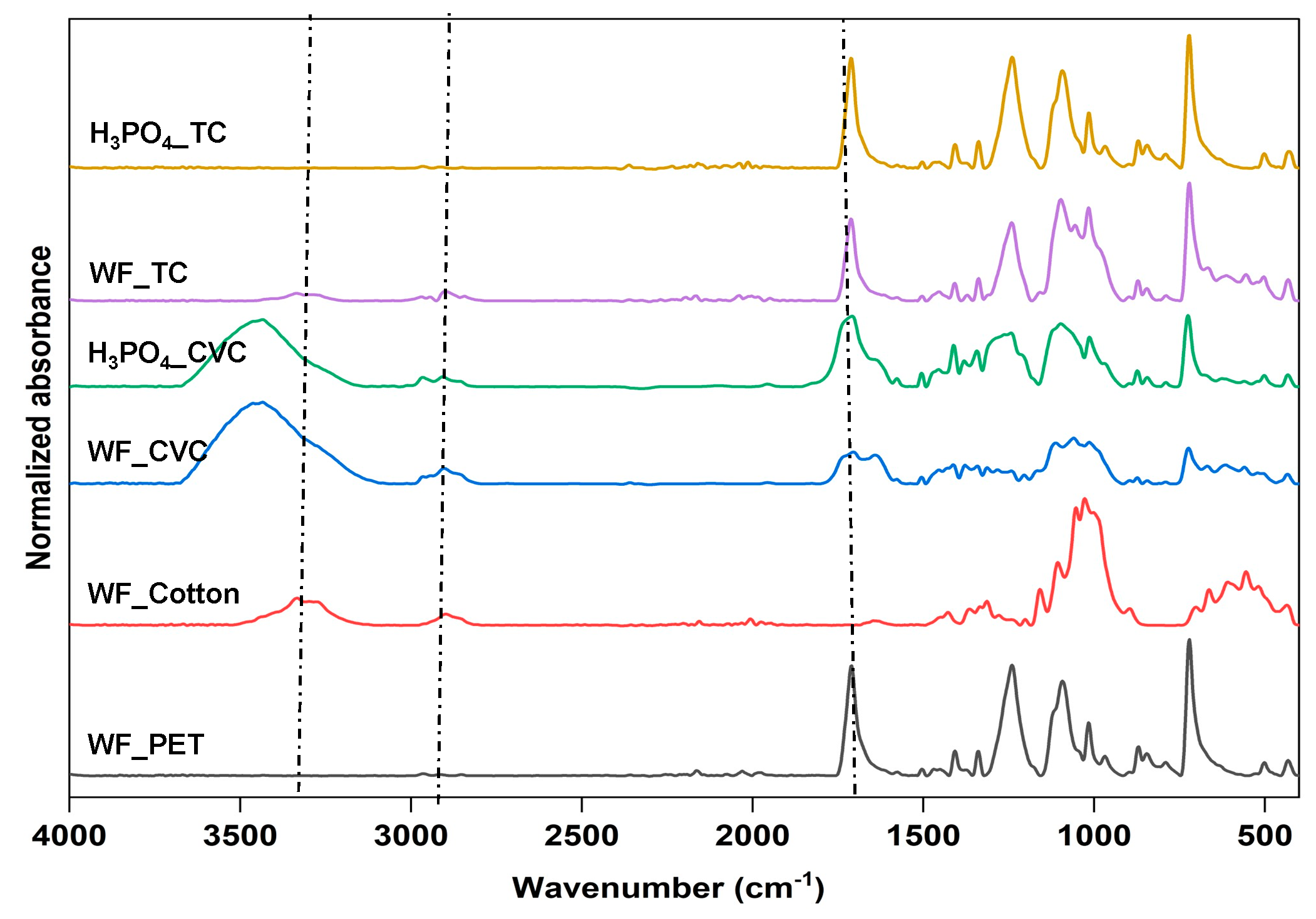
3.2. Cotton Removal from TC with Phosphoric Acid and Enzymatic Pretreatment on TC Fabrics
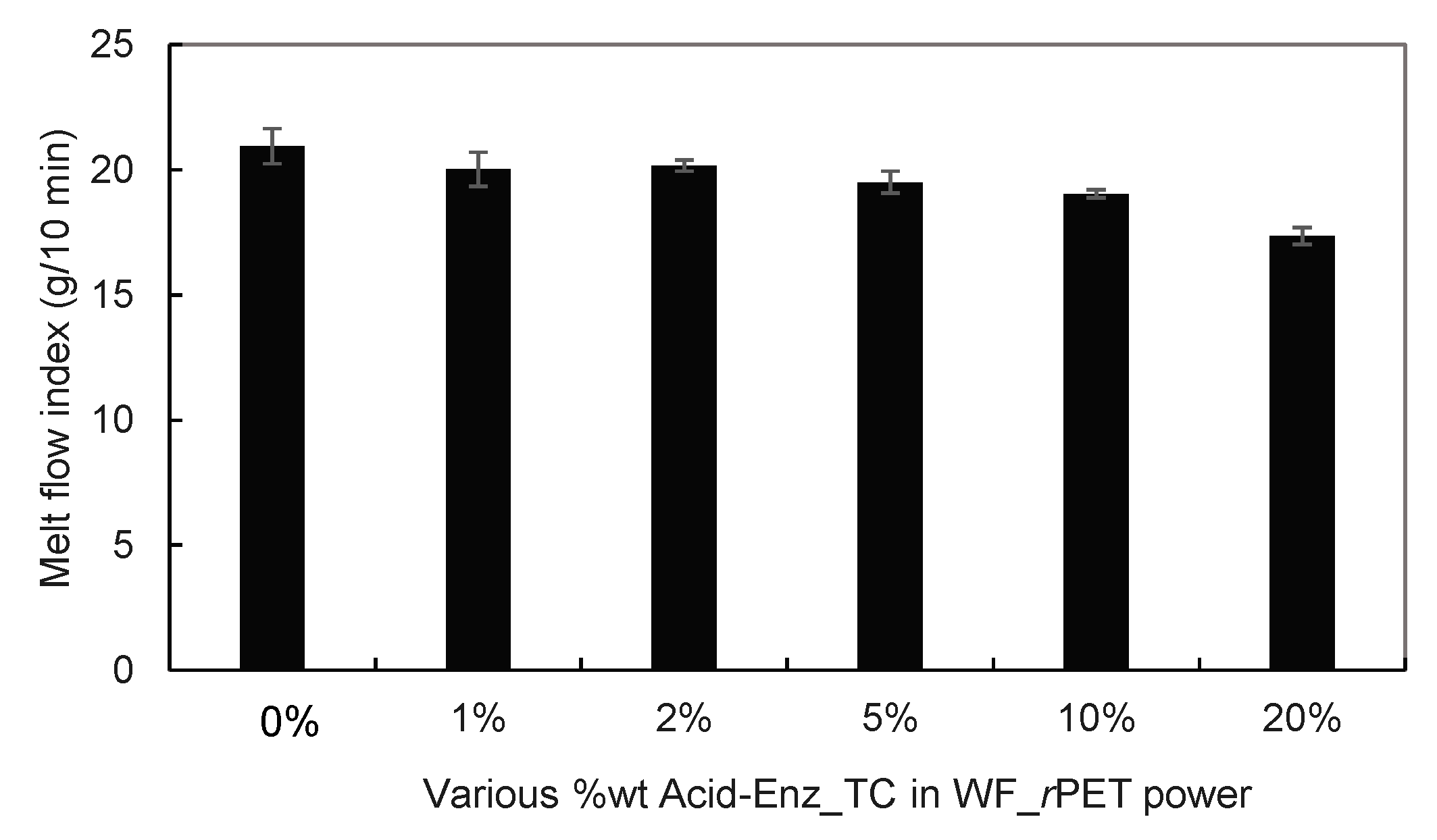
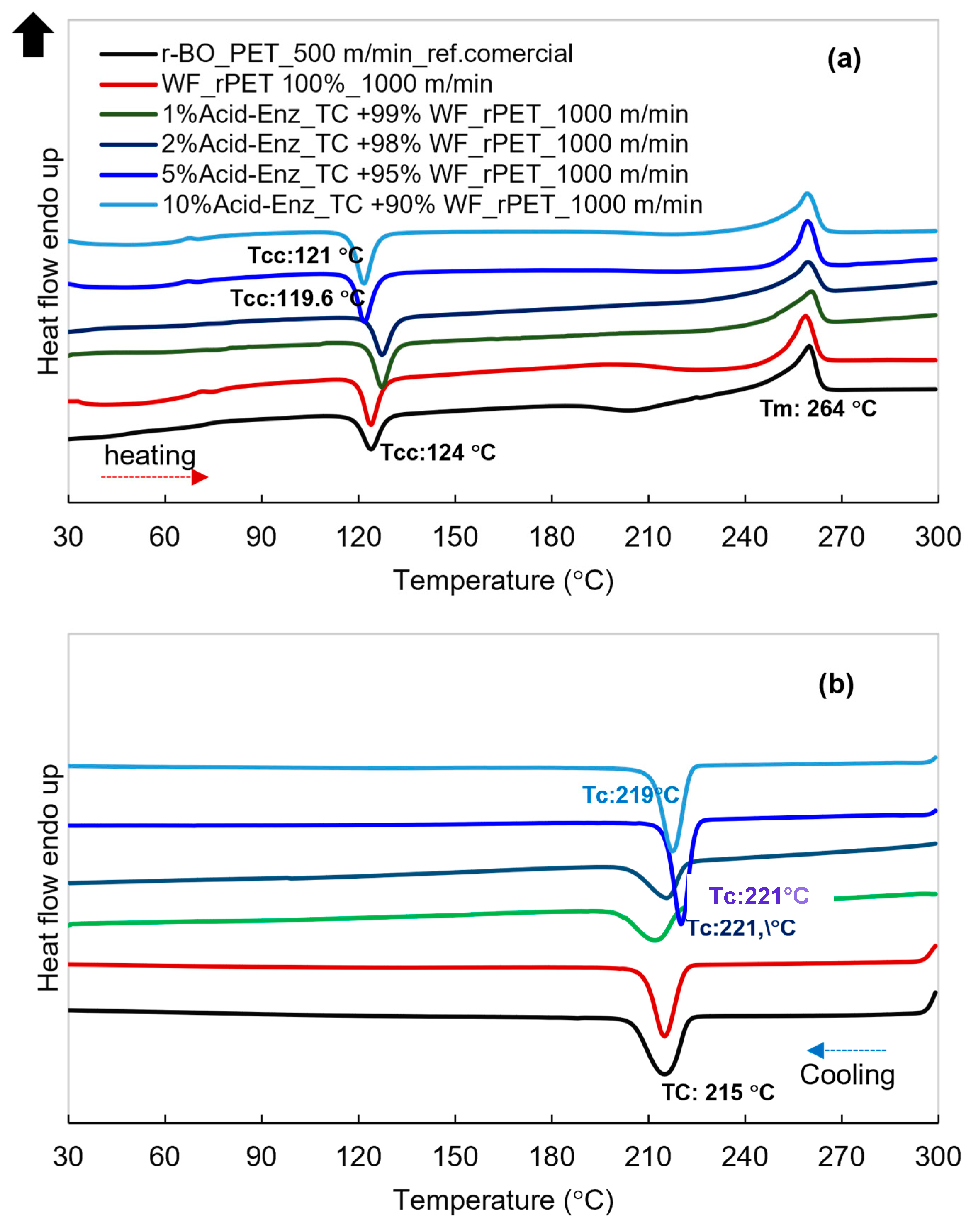
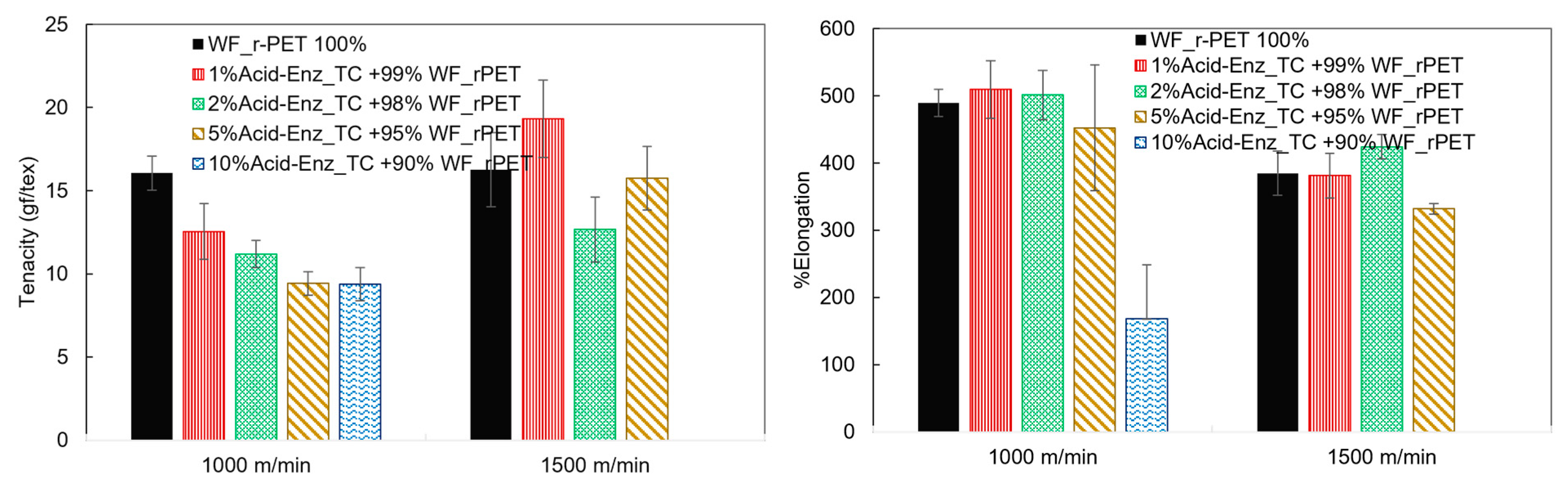
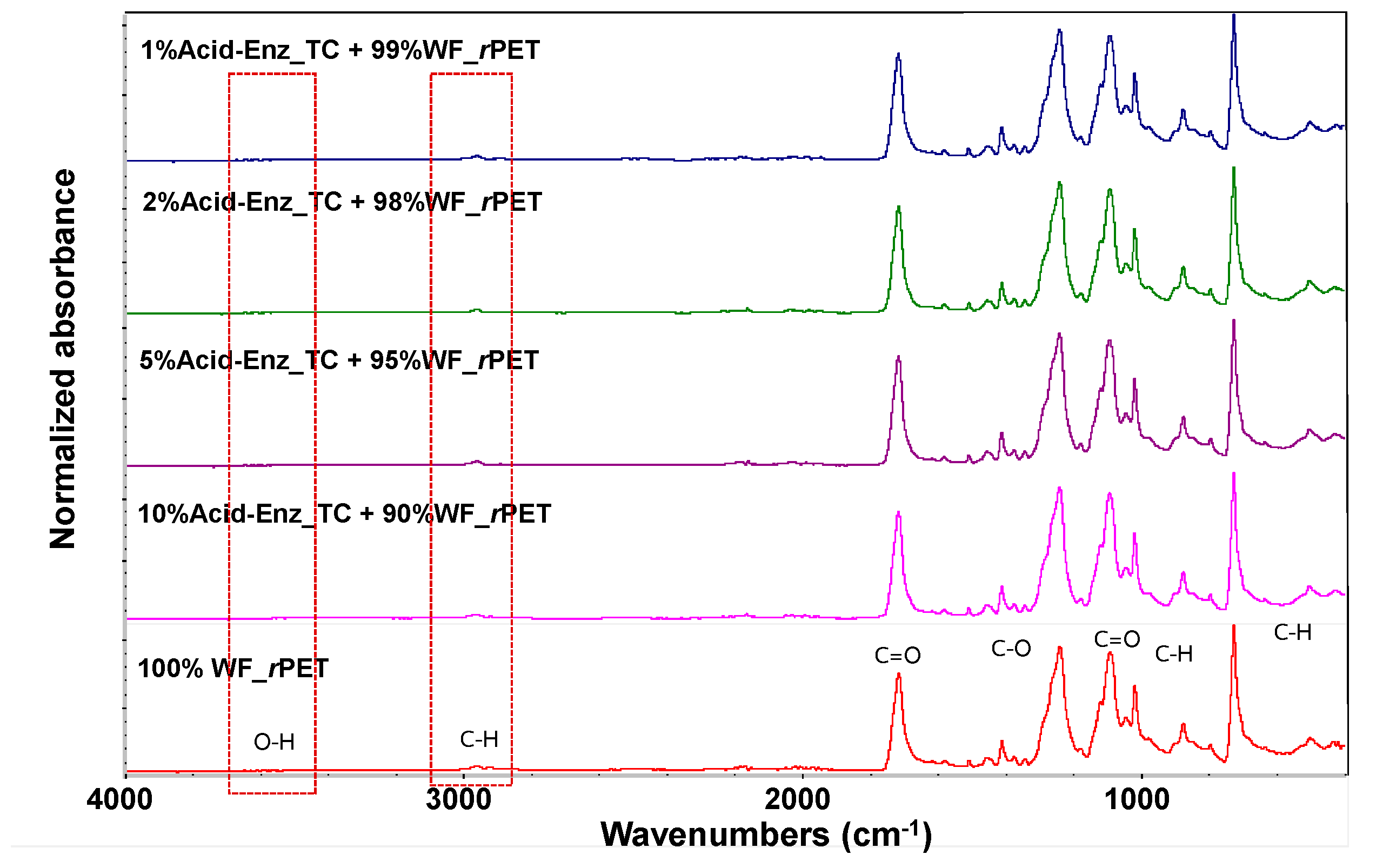
3.3. Melt Spinning and Characterization of rPET Fibers Prepared from Remaining PET Fibers in Treated TC Fabric
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Impact of Textile Production and Waste on the Environment (Infographics). Available online: https://www.europarl.europa.eu/news/en/headlines/society/20201208STO93327/the-impact-of-textile-production-and-waste-on-the-environment-infographics (accessed on 10 September 2023).
- CTR (Council for Textile Recycling). 2018. Available online: http://www.weardonaterecycle.org/ (accessed on 10 September 2023).
- Textile Exchange. Preferred Fiber & Materials Market Report 2022. 2022. Available online: https://textileexchange.org/app/uploads/2022/10/Textile-Exchange_PFMR_2022.pdf (accessed on 10 September 2023).
- Elephantech. Polyester Textile Decolorization Technology “Neochromato Process”. 2023. Available online: https://info.elephantech.co.jp/en/neochromato (accessed on 10 September 2023).
- Wang, S.; Salmon, S. Progress toward circularity of polyester and cotton textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukosiute, S.I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z.; Zhang, M.; Zhang, B.; Dai, J. Separation and characterization of waste cotton/polyester blend fabric with hydrothermal method. Fibers Polym. 2018, 19, 742–750. [Google Scholar] [CrossRef]
- Boondaeng, A.; Keabpimai, J.; Srichola, P.; Vaithanomsat, P.; Trakunjae, C.; Niyomvong, N. Optimization of textile waste blends of cotton and PET by enzymatic hydrolysis with reusable chemical pretreatment. Polymers 2023, 15, 1964. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Lin, L.; Liu, S.; Ouyang, P. Effect of phosphoric acid pretreatment on enzymatic hydrolysis of microcrystalline cellulose. Biotechnol. Adv. 2010, 28, 613–619. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Biores. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- ASTM D1238; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- Roungpaisan, N.; Srisawat, N.; Rungruangkitkrai, N.; Chartvivatpornchai, N.; Boonyarit, J.; Kittikorn, T.; Chollakup, R. Effect of recycling PET fabric and bottle grade on r-PET fiber structure. Polymers 2023, 15, 2330. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobileamorphous rigid amorphous fractions in the performance of recycled poly(ethyleneterephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- ASTM D3822; Standard Test Method for Tensile Properties of Single Textile Fibers. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- Łojewska, J.; Mi’skowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl. Clay Sci. 2007, 35, 194–200. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2015, 3, 1632–1640. [Google Scholar] [CrossRef]
- Abraham, E.; Deepe, B.; Pothen, L.A.; Cintill, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; de Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Biores. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef]
- Siriorn, I.N.A.; Munchumart, B.; Natanicha, B.; Nut, S. Viscosity improvement of recycled poly (ethylene terephthalate) from waste bottles by adding antioxidants and chain-extender. E3S Web Conf. 2021, 302, 02019. [Google Scholar] [CrossRef]
- Adeakin, O.A.S. Structural crystalline development of polyester fibre pretreated with organic solvents using XRD. Int. J. Eng. Res. 2018, V7, 239–242. [Google Scholar] [CrossRef]
- Demirel, B.; Yaraș, A.; Elçiçek, H. Crystallization behavior of PET materials. BAÜ Fen. Bil. Enst. Derg. Cilt. 2011, 13, 26–35. [Google Scholar]
- Sattler, M.; Schweizer, H. Fibers 5. Polyester Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Baghaei, B.; Compiet, S.; Skrifvars, M. Mechanical properties of all-cellulose composites from end-of-life textiles. J. Polym. Res. 2020, 27, 260. [Google Scholar] [CrossRef]
- Chinchillas-Chinchillas, M.J.; Orozco-Carmona, V.M.; Clemente, G.A.B.; Jorge, L.A.-S.; Jasso-Ramos, S.; Luis, E.J.-R.; Andrés, C.-B. Synthesis of recycled poly(ethylene terephthalate)/polyacrylonitrile/styrene composite nanofibers by electrospinning and their mechanical properties evaluation. J. Polym. Environ. 2019, 27, 659–669. [Google Scholar] [CrossRef]





| Parameter | Setting |
|---|---|
| Orifice configuration | Round (0.32 mm) |
| Spinning temperature (°C) | 245/255/260/255–260 |
| Through rate (g/hole/min) | 0.24 |
| Take-up speed (m/min) | 1000 and 1500 |
| Sample Name | Winding Speed (m/min) | |
|---|---|---|
| 1000 | 1500 | |
| rPET_1000 | / | / |
| 1% Acid-Enz_TC + 99% rPET | / | / |
| 2% Acid-Enz_TC + 98% rPET | / | / |
| 5% Acid-Enz_TC + 95% rPET | / | / |
| 10% Acid-Enz_TC + 90% rPET | / | Δ |
| 20% Acid-Enz_TC + 80% rPET | X | X |
| Sample Name | Diameter (Micron) |
|---|---|
| 100% WF_rPET | 16.1 ± 0.92 |
| 1% Acid-Enz_TC + 99% WF_rPET | 18.8 ± 0.99 |
| 2% Acid-Enz_TC + 98% WF_rPET | 20.8 ± 0.74 |
| 5% Acid-Enz_TC + 95% WF_rPET | 21.2 ± 0.79 |
| 10% Acid-Enz_TC + 90% WF_rPET | 21.5 ± 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roungpaisan, N.; Srisawat, N.; Rungruangkitkrai, N.; Chartvivatpornchai, N.; Boonyarit, J.; Kittikorn, T.; Chollakup, R. Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric. Polymers 2023, 15, 4364. https://doi.org/10.3390/polym15224364
Roungpaisan N, Srisawat N, Rungruangkitkrai N, Chartvivatpornchai N, Boonyarit J, Kittikorn T, Chollakup R. Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric. Polymers. 2023; 15(22):4364. https://doi.org/10.3390/polym15224364
Chicago/Turabian StyleRoungpaisan, Nanjaporn, Natee Srisawat, Nattadon Rungruangkitkrai, Nawarat Chartvivatpornchai, Jirachaya Boonyarit, Thorsak Kittikorn, and Rungsima Chollakup. 2023. "Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric" Polymers 15, no. 22: 4364. https://doi.org/10.3390/polym15224364
APA StyleRoungpaisan, N., Srisawat, N., Rungruangkitkrai, N., Chartvivatpornchai, N., Boonyarit, J., Kittikorn, T., & Chollakup, R. (2023). Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric. Polymers, 15(22), 4364. https://doi.org/10.3390/polym15224364







