Synthesis and Thermoreversible Gelation of Coil–Rod Copolymers with a Dendritic Polyethylene Core and Multiple Helical Poly(γ-benzyl-L-glutamate) Arms
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Materials
2.3. Synthesis of γ-Benzyl-L-glutamate-N-carboxyanhydride
2.4. Synthesis of Polar Comonomer HEA-TMS
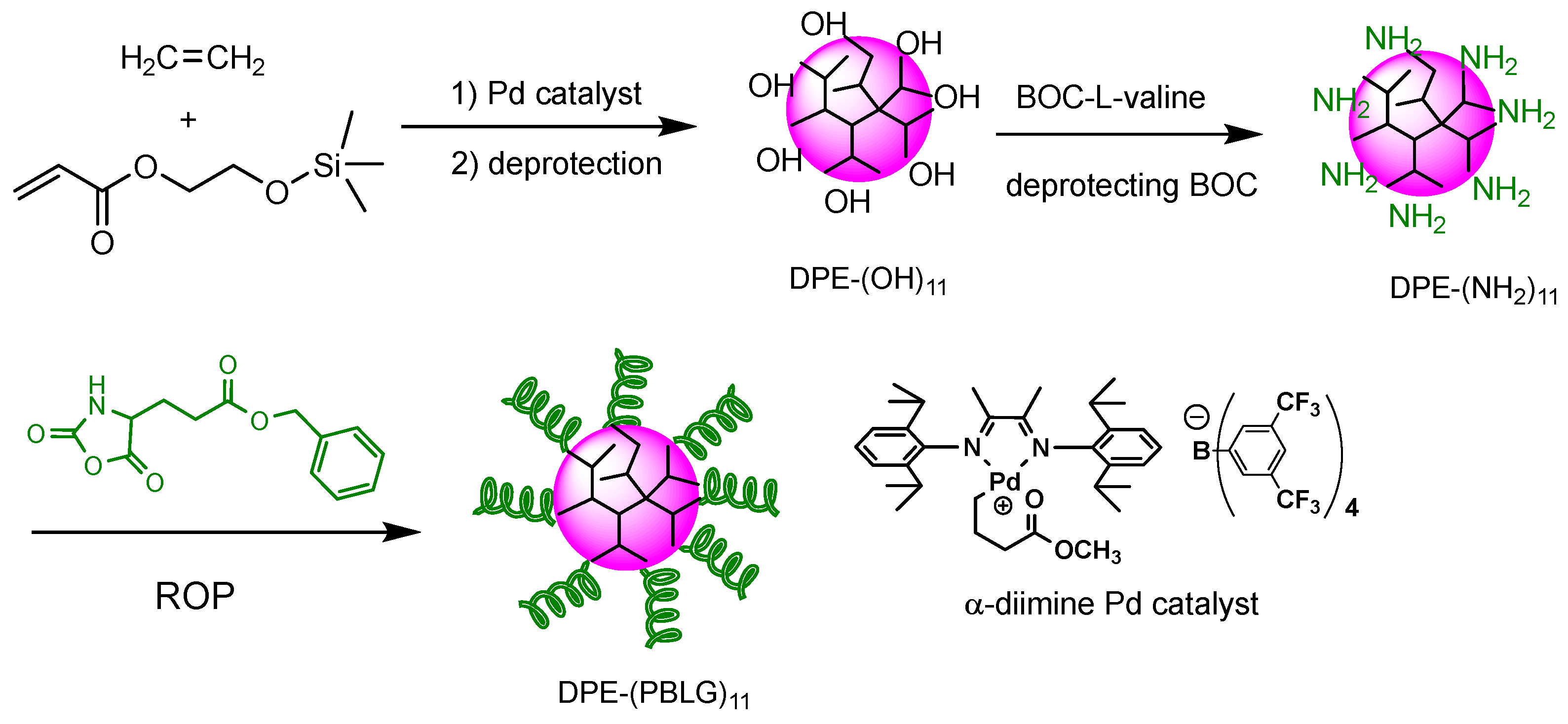
2.5. Synthesis of DPE-(OH)11 by Palladium-Catalyzed Copolymerization of Ethylene and HEA-TMS
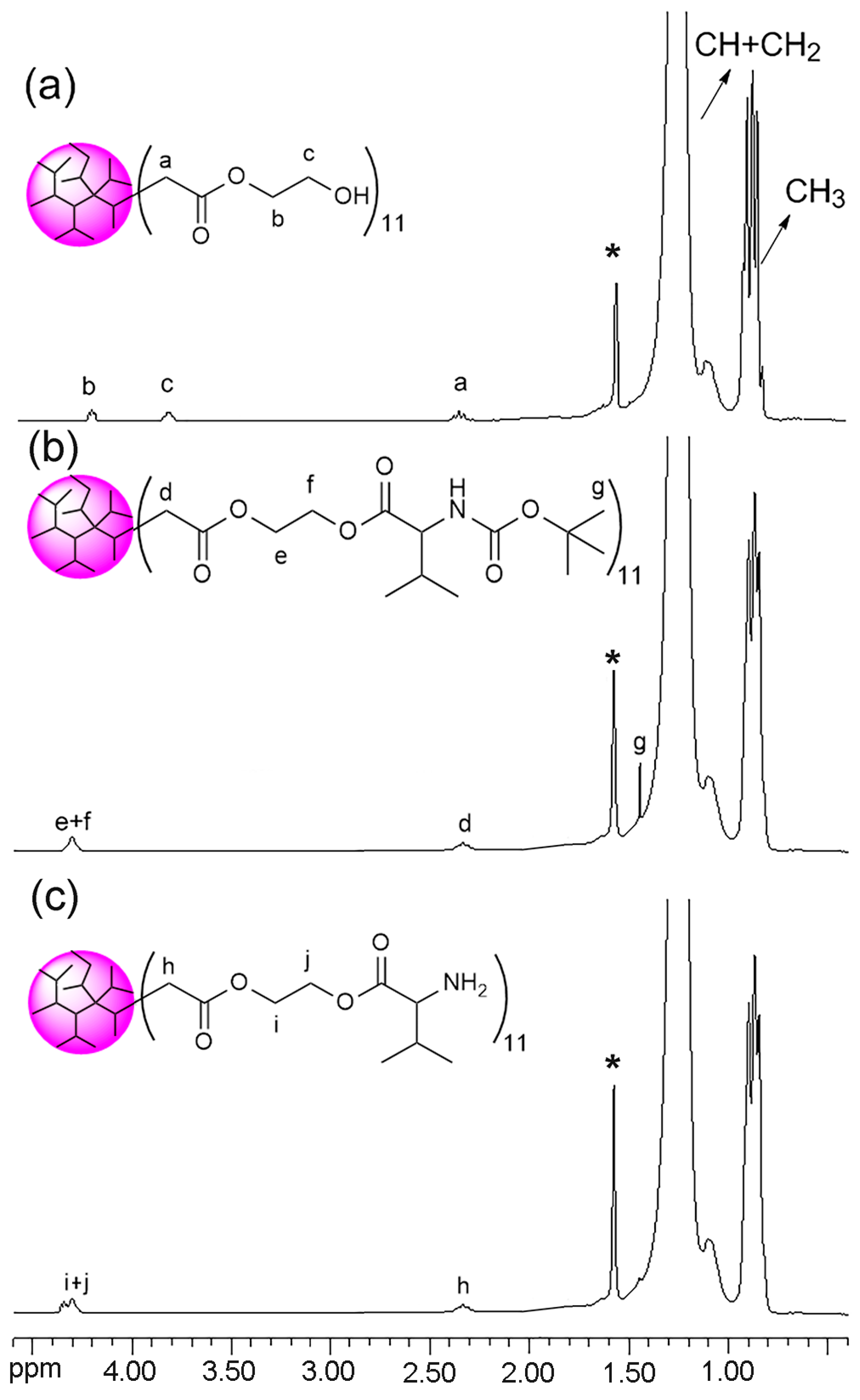
2.6. Synthesis of DPE-(NH2)11
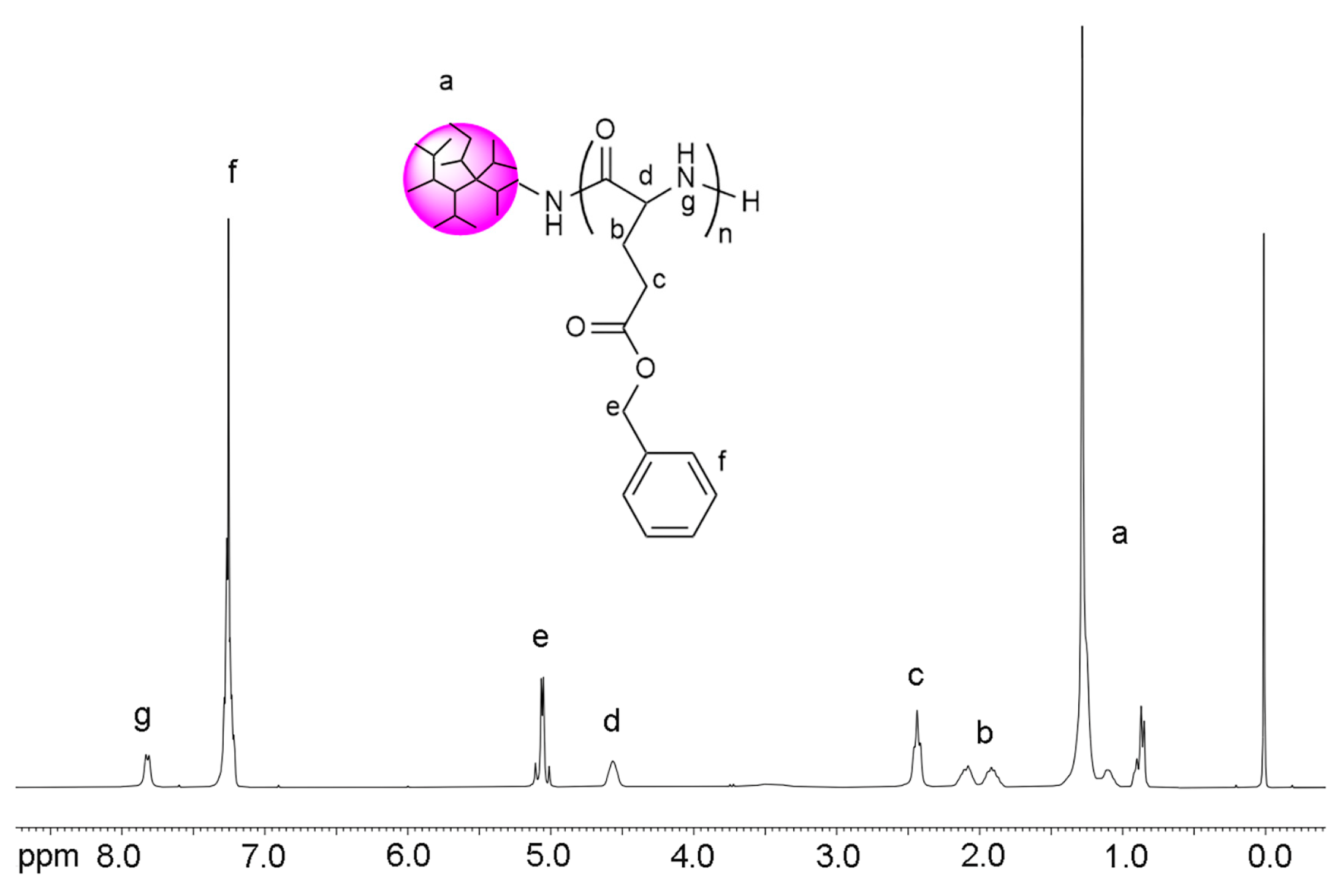
2.7. Synthesis of DPE-(PBLG)11 Copolymers
2.8. Gelation Experiment for DPE-(PBLG)11
2.9. Measurements
3. Results and Discussion
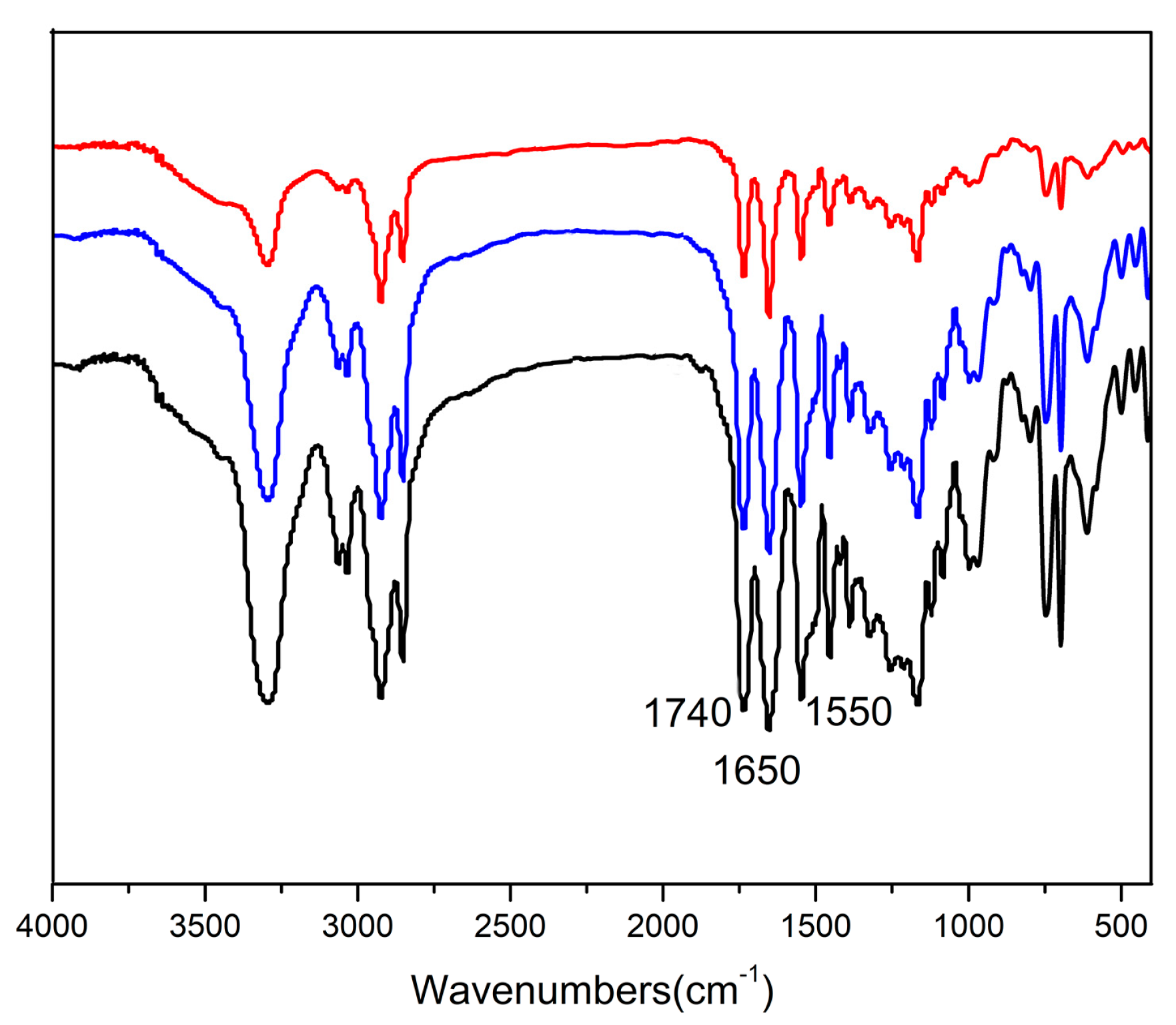
3.1. Synthesis and Characterization of DPE Tethered with Amine Groups
3.2. Synthesis and Characterization of DPE-(PBLG)n Copolymers
3.3. Gelation of DPE-(PBLG)11 Copolymer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures—Synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.S. Shape-persistent molecular architectures of nanoscale dimension. Acc. Chem. Res. 1997, 30, 402–413. [Google Scholar] [CrossRef]
- Liu, X.; Jin, X.; Ma, P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011, 10, 398–406. [Google Scholar] [CrossRef]
- Knoll, K.; Nießner, N. Styrolux+ and styroflex+− from transparent high impact polystyrene to new thermoplastic elastomers: Syntheses, applications and blends with other styrene based polymers. In Macromolecular Symposia; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 1998; Volume 132, pp. 231–243. [Google Scholar]
- Wang, J.; Ye, Z.; Zhu, S. Topology-engineered hyperbranched high-molecular-weight polyethylenes as lubricant viscosity-index improvers of high shear stability. Ind. Eng. Chem. Res. 2007, 46, 1174–1178. [Google Scholar] [CrossRef]
- Morgan, S.; Ye, Z.; Subramanian, R.; Zhu, S. Higher-molecular-weight hyperbranched polyethylenes containing crosslinking structures as lubricant viscosity-index improvers. Polym. Eng. Sci. 2010, 50, 911–918. [Google Scholar] [CrossRef]
- Johnson, L.K.; Meckin, S.; Brookhart, M. Copolymerization of ethylene and propylene with functionalized vinyl monomers by palladium(II) catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Meckin, S.; Johnson, L.K.; Wang, L.; Brookhart, M. Mechanistic studies of the palladium-catalyzed copolymerization of ethylene and α-olefins with methyl acrylate. J. Am. Chem. Soc. 1998, 120, 888–889. [Google Scholar] [CrossRef]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain walking: A new strategy to control polymer topology. Science 1999, 283, 2059–2062. [Google Scholar] [CrossRef]
- Guan, Z. Recent Progress of Catalytic polymerization for controlling polymer topology. Chem. Asian J. 2010, 5, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z. Control of polymer topology by chain-walking catalysts. Eur. J. 2002, 8, 3086–3092. [Google Scholar] [CrossRef]
- Ye, Z.; Li, S. Hyperbranched polyethylenes and functionalized polymers by chain walking polymerization with Pd-diimine catalysis. Macromol. React. Eng. 2010, 4, 319–332. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.S.; Guan, Z. Synthesis of functional olefin copolymers with controllable topologies using a chain-walking catalyst. J. Am. Chem. Soc. 2003, 125, 6697–6704. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guan, Z. Transition metal-catalyzed one-pot synthesis of water-soluble dendritic molecular nanocarriers. J. Am. Chem. Soc. 2004, 126, 2662–2663. [Google Scholar] [CrossRef]
- Popeney, C.S.; Lukowiak, M.C.; Böttcher, C.; Schade, B.; Welker, P.; Mangoldt, D.; Gunkel, G.; Guan, Z.; Haag, R. Tandem coordination, ring-opening, hyperbranched polymerization for the synthesis of water-soluble core–shell unimolecular transporters. ACS Macro Lett. 2012, 1, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Felgner, P.L.; Guan, Z. Efficient catalytic synthesis of dendritic polymers having internal fluorescence labels for bioconjugation. Biomacromolecules 2008, 9, 1745–1754. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, Z. Hyperbranched polyethylenes by chain walking polymerization: Synthesis, properties, functionalization, and applications. Polym. Chem. 2012, 3, 286–301. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Subramanian, R.; Ye, Z.; Lu, J.; Yu, Q. Chain walking ethylene copolymerization with an ATRP Inimer for one-pot synthesis of hyperbranched polyethylenes tethered with ATRP initiating sites. Macromol. Rapid Commun. 2007, 28, 2185–2191. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, Z.; Subramanian, R. S Synthesis of block copolymers of ethylene with styrene and n-butyl acrylate via a tandem strategy combining ethylene “living” polymerization catalyzed by a functionalized Pd−diimine catalyst with atom transfer radical polymerization. Macromolecules 2008, 41, 640–649. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, Q.; Su, J.; Wu, Q. Local polarity andmicroviscosity of the interior of dendritic polyethylene amphiphiles. Macromolecules 2011, 44, 6885–6890. [Google Scholar] [CrossRef]
- Gao, H.; Tang, Y.; Hu, Z.; Guan, Q.; Shi, X.; Zhu, F.; Wu, Q. Synthesis of amphiphilic copolymers with a dendritic polyethylene core and poly(ethylene oxide) arms and their self-assembled nanostructures. Polym. Chem. 2013, 4, 1107–1114. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. Synthesis of hyperbranched polyethylene amphiphiles by chain walking polymerization in tandem with raft polymerization and supramolecular self-assembly vesicles. Macromol. Rapid Commun. 2012, 33, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, J.; Zhang, W.; Ding, M.; Chen, X.; Wu, Q. Temperature-sensitive phase transition of dendritic polyethylene amphiphiles with core−shell architecture revealed by a Rayleigh scattering technique. Langmuir 2010, 26, 5801–5807. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Zhou, J.; Qi, M.; Shi, J.; Zhang, J. Study on high-temperature composite properties of fluorosilicone rubber with nano-Sb2O3. J. Appl. Polym. Sci. 2020, 137, 49302. [Google Scholar] [CrossRef]
- Song, J.; He, J.; Hu, J.; Ma, J.; Jiang, H.; Hu, S.; Ye, H.; Xu, L. A Universal strategy for producing fluorescent polymers based on designer hyperbranched polyethylene ternary copolymers. Macromolecules 2022, 55, 2085–2095. [Google Scholar] [CrossRef]
- Al-Sulami, A.; Ladelta, V.; Hadjichristidis, N. In-chain functionalized poly(ε-caprolactone): A valuable precursor towards the synthesis of 3-miktoarm star containing hyperbranched polyethylene. J. Polym. Sci. 2020, 58, 2764–2773. [Google Scholar] [CrossRef]
- Nunvářová, K.; Charvátová, B.; Šlouf, M.; Hermanová, S.; Merna, J. Synthesis of amphiphilic copolymers based on dendritic polyethylene grafted by polyhydroxyethylmethacrylate and polyhydroxypropylmethacrylate and their use for construction of nanoparticles. Eur. Polym. J. 2019, 115, 193–200. [Google Scholar] [CrossRef]
- Chécot, F.; Lecommandoux, S.; Gnanou, Y.; Klok, H.-A. Water-soluble stimuli-responsive vesicles from peptide-based diblock copolymers. Angew. Chem. Int. Ed. 2002, 41, 1339–1343. [Google Scholar] [CrossRef]
- Chécot, F.; Lecommandoux, S.; Klo, H.A.; Gnanou, Y. From supramolecular polymersomes to stimuli-responsive nano-capsules based on poly(diene-b-peptide) diblock copolymers. Eur. Phys. J. E 2003, 10, 25–35. [Google Scholar] [CrossRef]
- Kukula, H.; Schlaad, H.; Antonietti, M.; Főrster, S. The formation of polymer vesicles or “peptosomes” by polybutadiene-block-poly(L-glutamate)s in dilute aqueous solution. J. Am. Chem. Soc. 2002, 124, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Sigel, R.; Losik, M.; Schlaad, H. pH responsiveness of block copolymer vesicles with a polypeptide corona. Langmuir 2007, 23, 7196–7199. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, K.E.; Ahn, S.; Venkatachalam, G.; Savin, D.A. rod-sphere transition in polybutadiene−poly(L-lysine) block copolymer assemblies. Langmuir 2007, 23, 2851–2856. [Google Scholar] [CrossRef]
- Chécot, F.; Brûlet, A.; Oberdisse, J.; Gnanou, Y.; Mondain-Monval, O.; Lecommandoux, S. Structure of polypeptide-based diblock copolymers in solution: stimuli-responsive vesicles and micelles. Langmuir 2005, 21, 4308–4315. [Google Scholar] [CrossRef]
- Gao, H.; Li, G.; Hu, Z.; Xiao, Z.; Liang, G.; Wu, Q. Synthesis of amphiphilic polyethylene-b-poly(L-glutamate) block copolymers with vastly different solubilities and their stimuli-responsive polymeric micelles in aqueous solution. Polymer 2014, 55, 4593–4600. [Google Scholar] [CrossRef]
- Pei, L.; Ma, H.; Jiang, Y.; Zheng, H.; Gao, H. Amphiphilic polyethylene-b-poly(L-lysine) block copolymer: Synthesis, self-assembly, and responsivity. Int. J. Mol. Sci. 2023, 24, 5495. [Google Scholar] [CrossRef]
- Gao, H.; Hu, Z.; Guan, Q.; Liu, Y.; Zhu, F.; Wu, Q. Synthesis and thermoreversible gelation of coil-helical polyethylene-block-poly(γ-benzyl-L-glutamate) diblock copolymer. Polymer 2013, 54, 4923–4929. [Google Scholar] [CrossRef]
- Tadmor, R.; Khalfin, R.L.; Cohen, Y. Reversible gelation in isotropic solutions of the helical polypeptide poly(γ-benzyl-L-glutamate): kinetics and formation mechanism of the fibrillar network. Langmuir 2002, 18, 7146–7150. [Google Scholar] [CrossRef]
- You, Y.; Chen, Y.; Hua, C.; Dong, C. Synthesis and thermoreversible gelation of dendron-like polypeptide/linear poly(ε-caprolactone)/dendron-like polypeptide triblock copolymers. J. Polym. Sci. Pol. Chem. 2010, 48, 709–718. [Google Scholar] [CrossRef]
- Kim, Y.T.; Winnik, M.A.; Manners, I. Synthesis and self-assembly of dendritic-helical block copolypeptides. Soft Matter 2006, 2, 957–965. [Google Scholar] [CrossRef]
- Smith, D.K.; Hirst, A.R.; Love, C.S.; Hardy, J.G.; Brignell, S.V.; Huang, B. Self-assembly using dendritic building blocks—Towards controllable nanomaterials. Prog. Polym. Sci. 2005, 30, 220–293. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, C.; Vandermeulen, G.W.M.; Rider, D.A.; Kim, C.; Winnik, M.A.; Manners, I. Gelation of helical polypeptide–random coil diblock copolymers by a nanoribbon mechanism. Angew. Chem. Int. Ed. 2005, 44, 7964–7968. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, A.; Mantion, A.; McAloney, R.; Huber, A.; Falkenhagen, J.; Goh, C.M.; Thünemann, A.F.; Winnik, M.A.; Menzel, H. Elucidation of the structure of poly(γ-benzyl-L-glutamate) nanofibers and gel networks in a helicogenic solvent. Colloid. Polym. Sci. 2013, 291, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Vacogne, C.D.; Schopferer, M.; Schlaad, H. Physical gelation of α-helical copolypeptides. Biomacromolecules 2016, 17, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Schlaad, H. Synthesis of nearly monodisperse polystyrene–polypeptideblock copolymersviapolymerisation of N-carboxyanhydrides. Chem. Commun. 2003, 23, 2944–2955. [Google Scholar] [CrossRef]
- Dimitrov, I.; Kukula, H.; Cölfen, H.; Schlaad, H. Advances in the synthesis and characterization of polypeptide-based hybrid block copolymers. Macromol. Symp. 2004, 215, 383–393. [Google Scholar] [CrossRef]
- Hellaye, M.L.; Fortin, N.; Guilloteau, J.; Soum, A.; Lecommandoux, S.; Guillaume, S.M. Biodegradable polycarbonate-b-polypeptide and polyester-b-polypeptide block copolymers: Synthesis and nanoparticle formation towards biomaterials. Biomacromolecules 2008, 9, 1924–1933. [Google Scholar] [CrossRef]
- Klok, H.-A.; Langenwalter, J.F.; Lecommandoux, S. Self-assembly of peptide-based diblock oligomers. Macromolecules 2000, 33, 7819–7826. [Google Scholar] [CrossRef]
- Lecommandoux, S.; Achard, M.-F.; Langenwalter, J.F.; Klok, H.-A. Self-Assembly of Rod−Coil Diblock Oligomers Based on α-Helical Peptides. Macromolecules 2001, 34, 9100–9111. [Google Scholar] [CrossRef]
- Yu, S.M.; Conticello, V.P.; Zhang, G.; Kayser, C.; Fournier, M.J.; Mason, T.L.; Tirrell, D.A. Smectic ordering in solutions and films of a rod-like polymer owing to monodispersity of chain length. Nature 1997, 389, 167–170. [Google Scholar] [CrossRef]
- Kuo, S.; Lee, H.; Huang, W.; Jeong, K.; Chang, F. Solid state and solution self-assembly of helical polypeptides tethered to polyhedral oligomeric silsesquioxanes. Macromolecules 2009, 42, 1619–1626. [Google Scholar] [CrossRef]
- Kim, K.; Park, C.; Kim, C.; Winnik, M.A.; Manners, I. Self-assembly of dendron-helical polypeptidecopolymers: Organogels and lyotropic liquid crystals. Chem. Commun. 2006, 13, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Sakellariou, G. Synthesis of well-defined polypeptide-based materials via the ring-opening polymerization of α-amino acid N-carboxyanhydrides. Chem. Rev. 2009, 109, 5528–5578. [Google Scholar] [CrossRef]
- Palui, G.; Garai, A.; Nanda, J.; Nandi, A.K.; Banerjee, A. Organogels from Different Self-Assembling New Dendritic Peptides: Morphology, Reheology, and Structural Investigations. J. Phys. Chem. B 2010, 114, 1249–1256. [Google Scholar] [CrossRef]
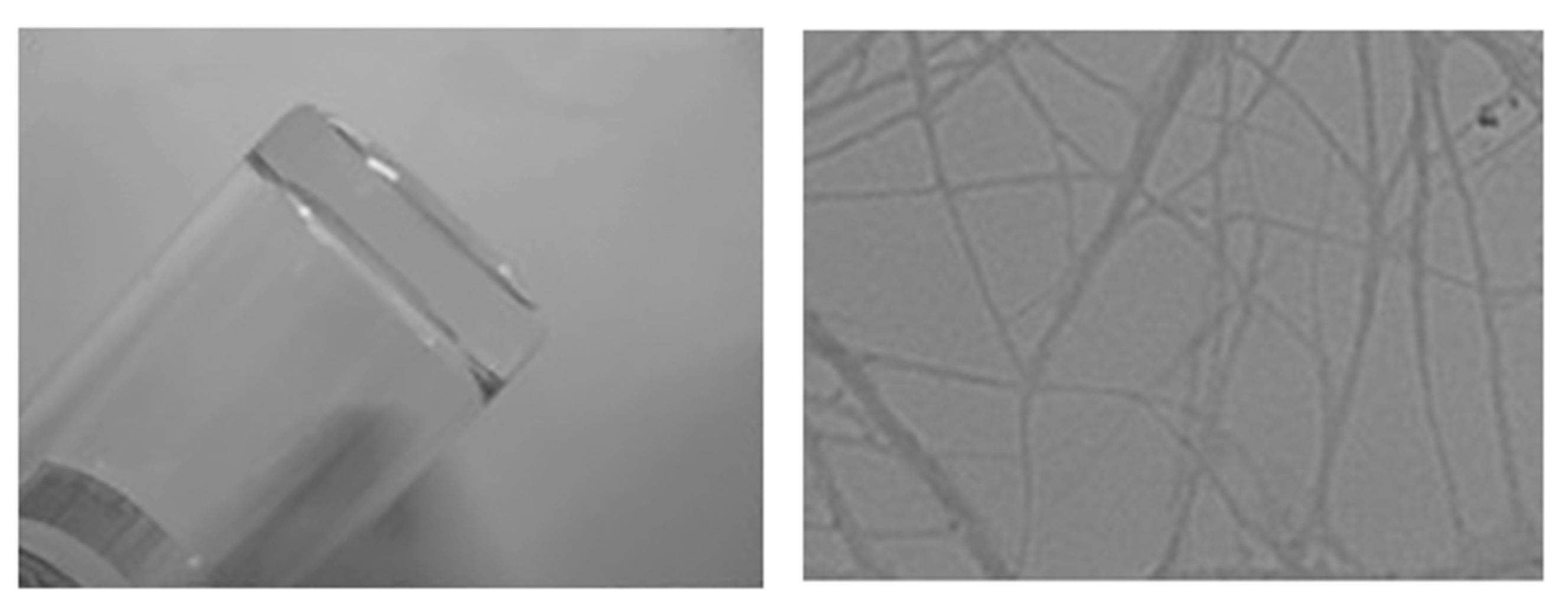

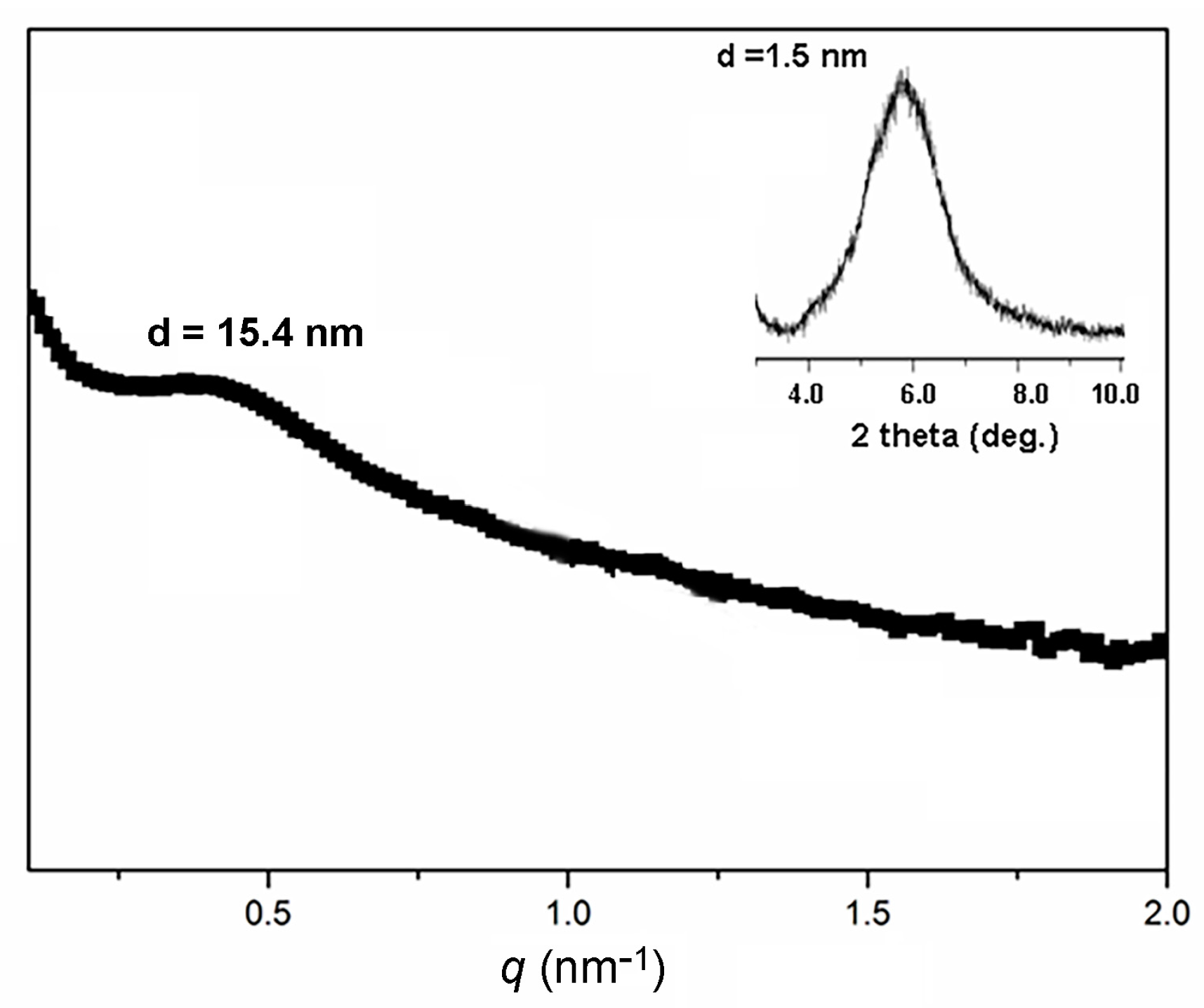
| Entry | Polymer b | NCA/DPE b | Conversion (%) | Mn-NMRc (kg/mol) | Mn-PBLGc (kg/mol) | DP d |
|---|---|---|---|---|---|---|
| 1 | DPE-(NH2)11 | 0 | – | 95.0 | – | – |
| 2 | DPE-(PBLG23)11 | 400 | 63.2 | 161.0 | 6.0 | 23 |
| 3 | DPE-(PBLG31)11 | 550 | 62.0 | 181.9 | 7.9 | 31 |
| 4 | DPE-(PBLG45)11 | 800 | 61.9 | 224.8 | 11.8 | 45 |
| Entry | Copolymer | NPBLG | Cgel a (wt%) | Tgel a (°C) |
|---|---|---|---|---|
| 1 | DPE-(PBLG23)11 | 23 | 10.5 | 65 |
| 2 | DPE-(PBLG31)11 | 31 | 9.4 | 68 |
| 3 | DPE-(PBLG45)11 | 45 | 8.7 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Liu, D.; Wei, X.; Song, J.; Xiao, Q.; Du, K.; Shi, X.; Gao, H. Synthesis and Thermoreversible Gelation of Coil–Rod Copolymers with a Dendritic Polyethylene Core and Multiple Helical Poly(γ-benzyl-L-glutamate) Arms. Polymers 2023, 15, 4351. https://doi.org/10.3390/polym15224351
Lu Y, Liu D, Wei X, Song J, Xiao Q, Du K, Shi X, Gao H. Synthesis and Thermoreversible Gelation of Coil–Rod Copolymers with a Dendritic Polyethylene Core and Multiple Helical Poly(γ-benzyl-L-glutamate) Arms. Polymers. 2023; 15(22):4351. https://doi.org/10.3390/polym15224351
Chicago/Turabian StyleLu, Yuliang, Dongtao Liu, Xinjie Wei, Jiming Song, Qiaogang Xiao, Kezheng Du, Xinbo Shi, and Haiyang Gao. 2023. "Synthesis and Thermoreversible Gelation of Coil–Rod Copolymers with a Dendritic Polyethylene Core and Multiple Helical Poly(γ-benzyl-L-glutamate) Arms" Polymers 15, no. 22: 4351. https://doi.org/10.3390/polym15224351
APA StyleLu, Y., Liu, D., Wei, X., Song, J., Xiao, Q., Du, K., Shi, X., & Gao, H. (2023). Synthesis and Thermoreversible Gelation of Coil–Rod Copolymers with a Dendritic Polyethylene Core and Multiple Helical Poly(γ-benzyl-L-glutamate) Arms. Polymers, 15(22), 4351. https://doi.org/10.3390/polym15224351








