Room-Temperature NH3 Gas Surface Acoustic Wave (SAW) Sensors Based on Graphene/PPy Composite Films Decorated by Au Nanoparticles with ppb Detection Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
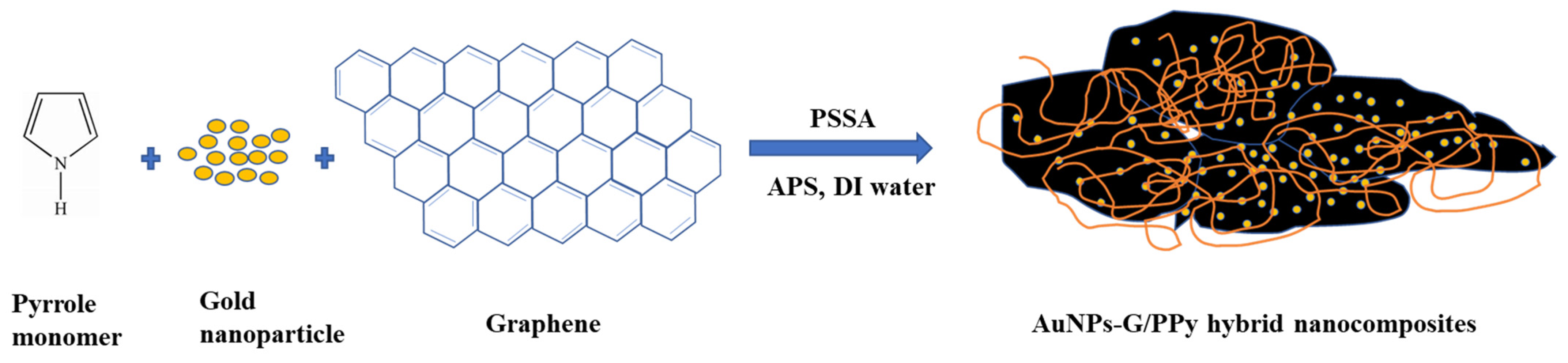
2.2. Preparation of AuNPs–G/PPy Hybrid Nanocomposite Film
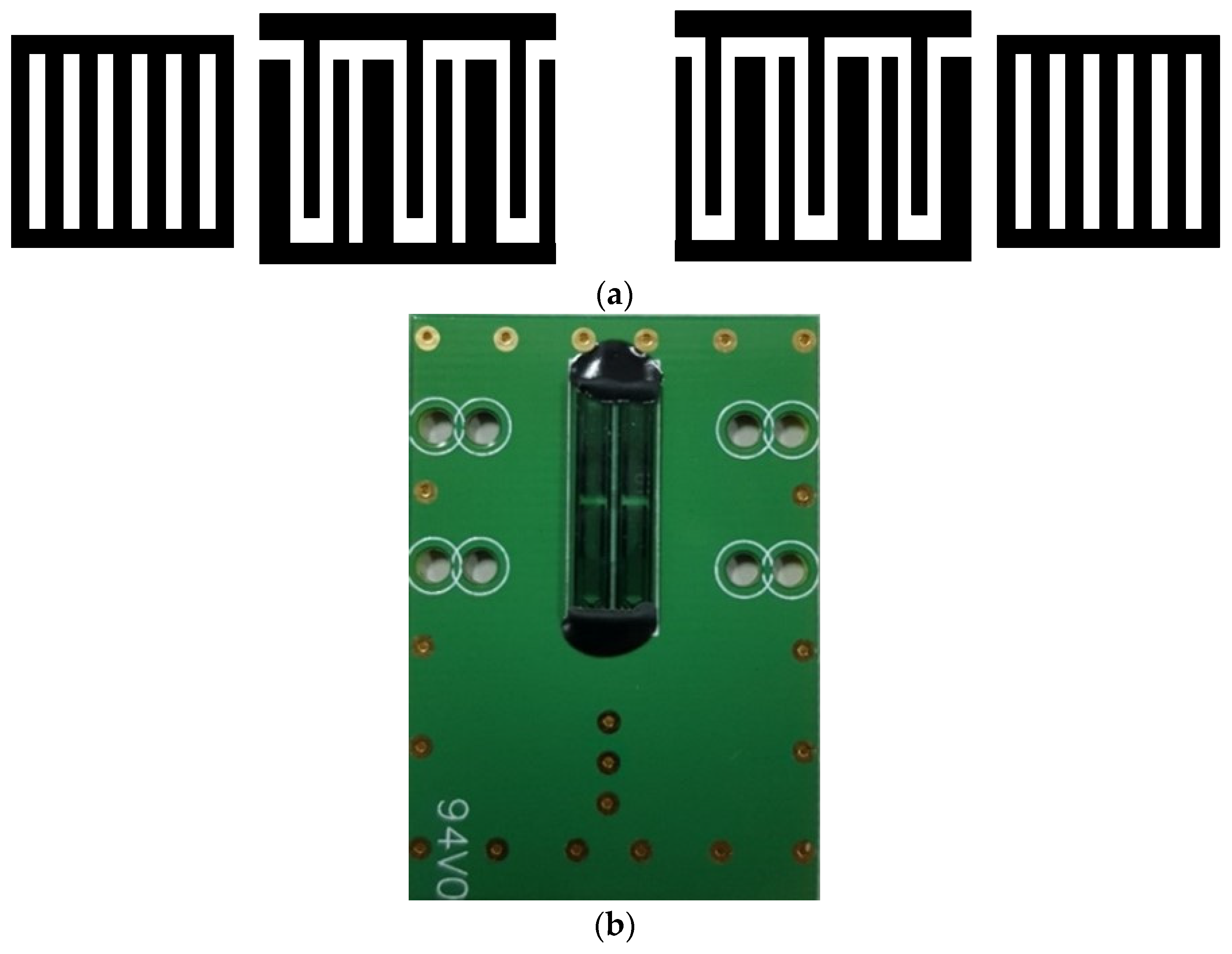
2.3. SAW Sensor Fabrication
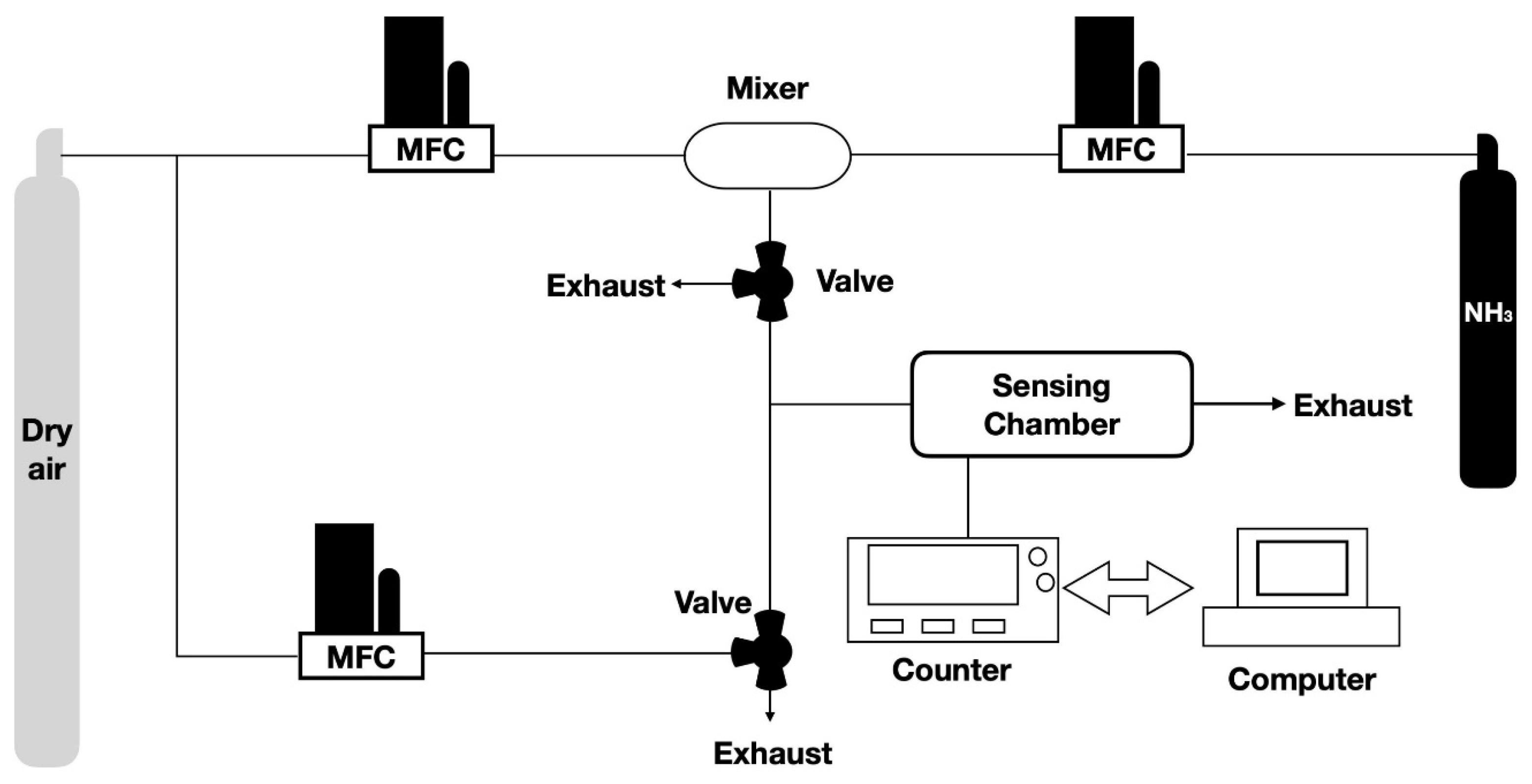
2.4. Gas Sensing Measurements
3. Results and Discussion
3.1. Material Analysis of the AuNPs–G/PPy Hybrid Nanocomposite
3.2. Gas Sensing Properties
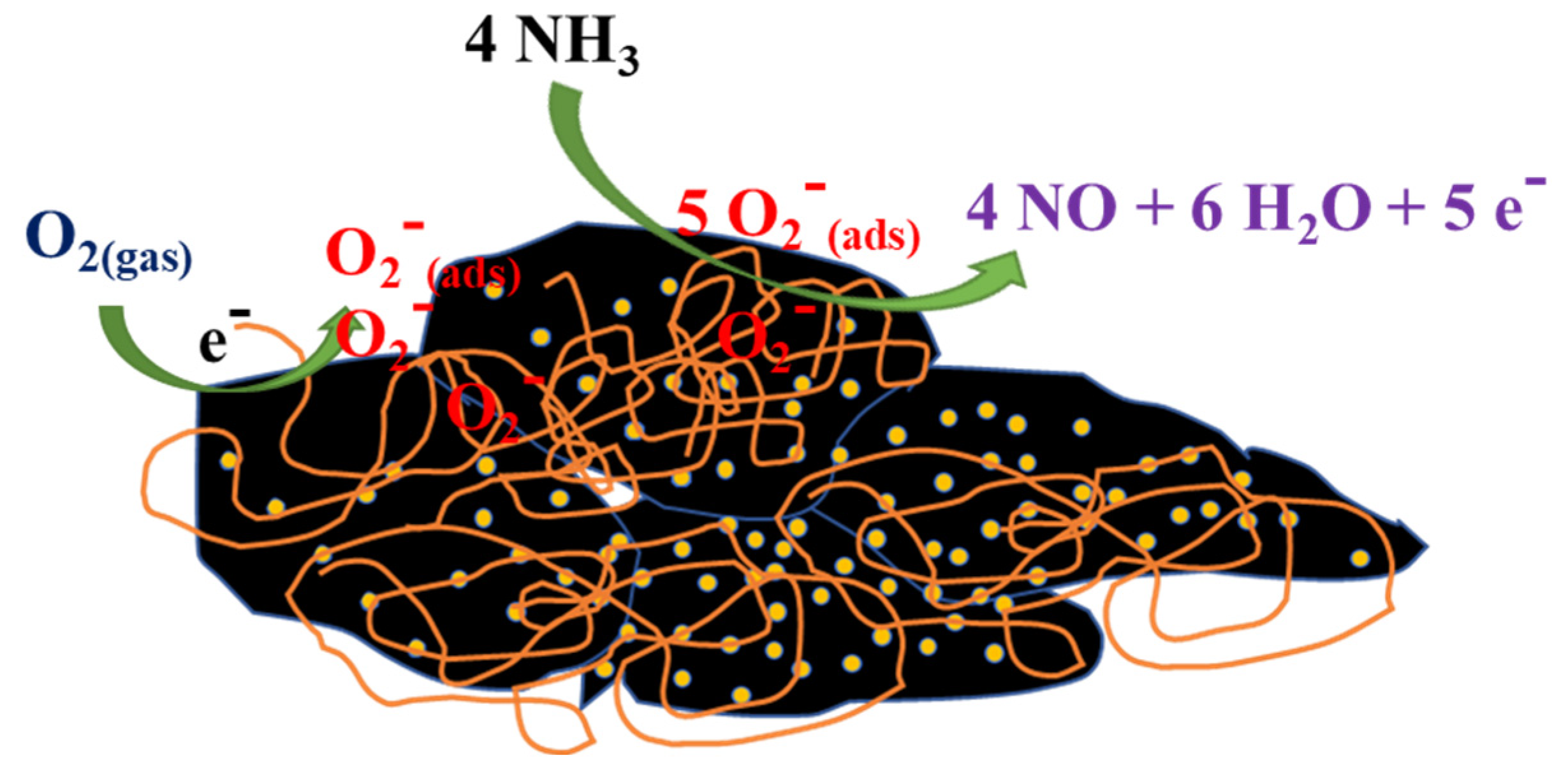
3.3. Mechanism of Gas Sensing
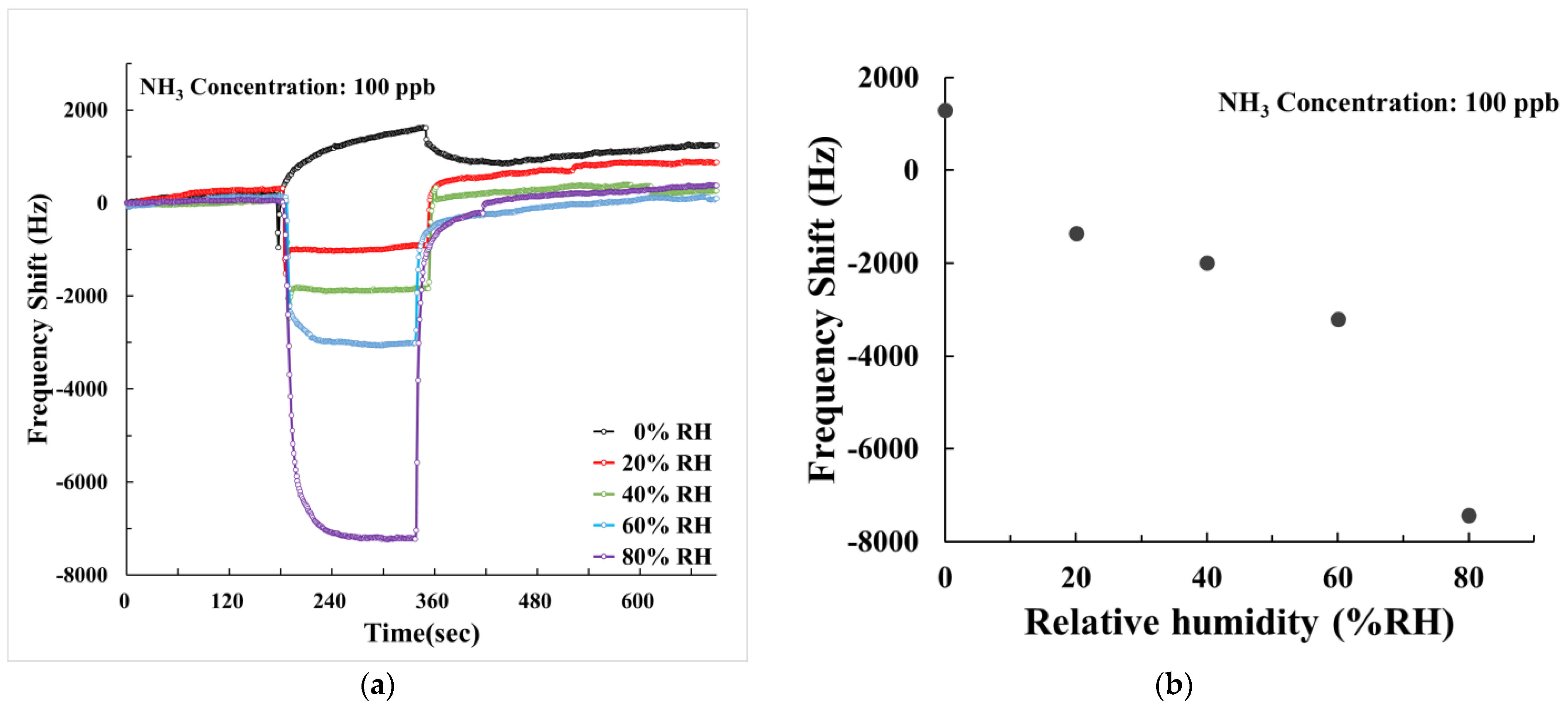
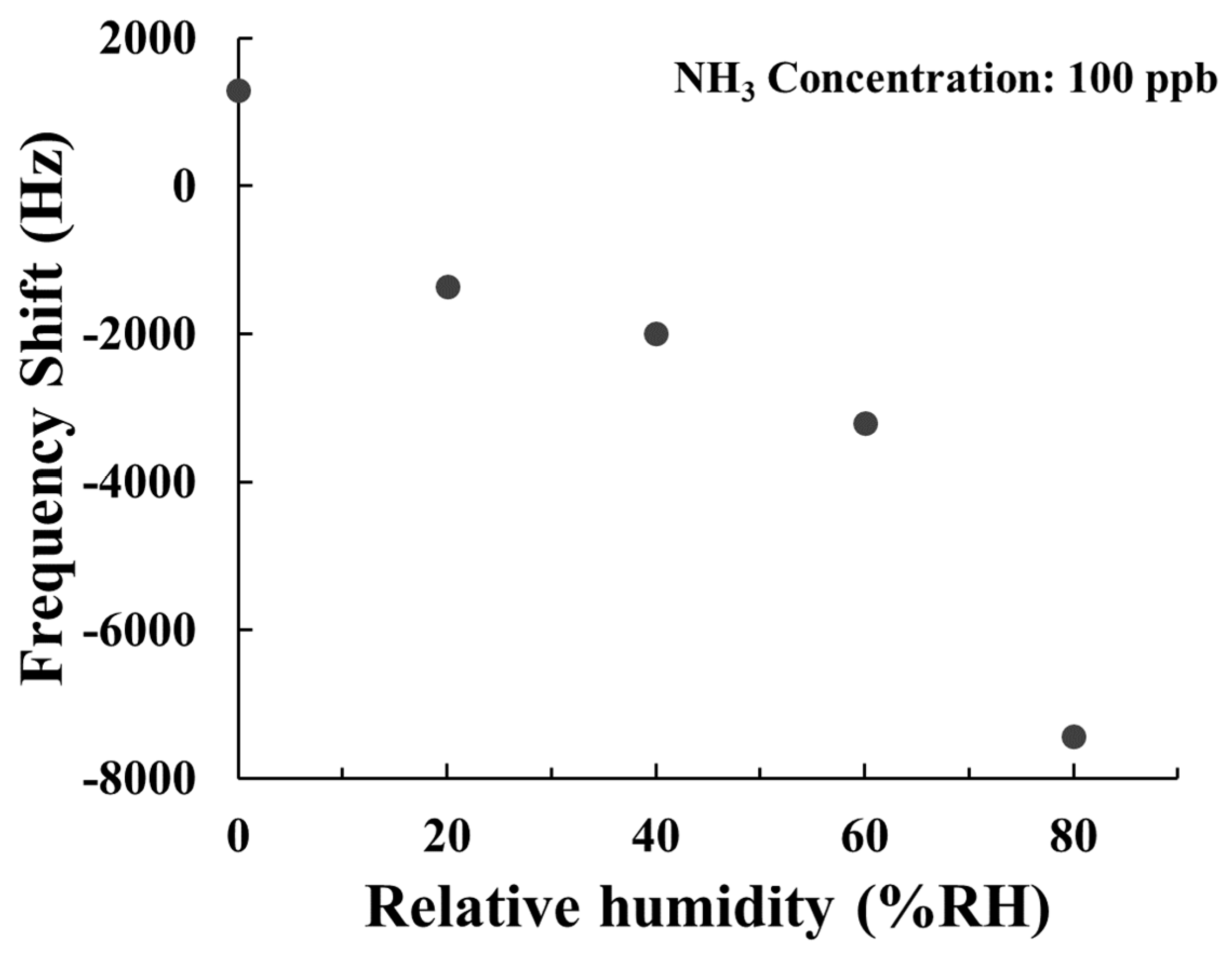
3.4. Humidity Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maity, A.; Ghosh, B. Fast response paper based visual color change gas sensor for efficient ammonia detection at room temperature. Sci. Rep. 2018, 8, 16851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xie, D.; Lin, S.; Zhang, W.; Yang, Y.; Zhang, R.; Shi, X.; Wang, H.; Zhang, Z.; Zu, X. Elastic loading enhanced NH3 sensing for surface acoustic wave sensor with highly porous nitrogen doped diamond like carbon film. Sens. Actuators B Chem. 2021, 344, 130175. [Google Scholar] [CrossRef]
- Rath, R.J.; Oveissi, F.; Shahrbabaki, Z.; Yun, J.; Naficy, S.; Dehghani, F.; Farajikhah, S. A paper-based sensor capable of differentiating ammonia and carbon dioxide gas. Mater. Today Commun. 2023, 35, 105895. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, X.; Ji, Y.; Qian, F.; Fan, J.; Wang, H.; Qiu, L.; Li, W.; Yang, H. High-temperature and flexible piezoelectric sensors for lamb-wave-based structural health monitoring. ACS Appl. Mater. Interfaces 2021, 13, 47764–47772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Q.; Zhang, X.; Wan, H.; Wang, P. Acoustic transducer and its applications in biosensors. In Handbook of Cell Biosensors; Thouand, G., Ed.; Springer: Cham, Switzerland, 2021; pp. 517–535. ISBN 978-3-319-47405-2. [Google Scholar]
- Jiang, C.; Chen, Y.; Cho, C. A three-dimensional finite element analysis model for SH-SAW torque sensors. Sensors 2019, 19, 4290. [Google Scholar] [CrossRef]
- Grabka, M.; Witkiewicz, Z.; Jasek, K.; Piwowarski, K. Acoustic wave sensors for detection of blister chemical warfare agents and their simulants. Sensors 2022, 22, 5607. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, J.; Kittmann, A.; Durdaut, P.; Spetzler, B.; Faupel, F.; Höft, M.; Quandt, E.; Gerken, M. Multi-mode love-wave SAW magnetic-field sensors. Sensors 2020, 20, 3421. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Hung, T.-T.; Chung, M.-H.; Lin, G.-S.; Shen, C.-Y. Piezoelectric microsensor for selective detection of low concentrations of ammonia. Solid State Electron. 2021, 186, 108191. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Miu, D.; Viespe, C. Surface acoustic wave sensors for ammonia detection at room temperature based on SnO2/Co3O4 bilayers. J. Sens. 2019, 2019, 8203810. [Google Scholar] [CrossRef]
- Qiu, J.; Xia, X.; Hu, Z.; Zhou, S.; Wang, Y.; Wang, Y.; Zhang, R.; Li, J.; Zhou, Y. Molecular ammonia sensing of PEDOT:PSS/Nitrogen doped MXene Ti3C2Tx composite film at room temperature. Nanotechnology 2022, 33, 065501. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-T.; Chung, M.-H.; Wu, J.-Y.; Shen, C.-Y. A room-temperature surface acoustic wave ammonia sensors based on rGO/DPP2T-TT composite films. Sensors 2022, 22, 5280. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Hybrid polyaniline-WO3 flexible sensor: A room temperature competence towards NH3 gas. Sens. Actuators B Chem. 2019, 288, 279–288. [Google Scholar] [CrossRef]
- Bai, S.; Tian, Y.; Cui, M.; Sun, J.; Tian, Y.; Luo, R.; Chen, A.; Li, D. Polyaniline@ SnO2 heterojunction loading on flexible PET thin film for detection of NH3 at room temperature. Sens. Actuators B Chem. 2016, 226, 40–547. [Google Scholar] [CrossRef]
- Jie, X.; Zeng, D.; Zhang, J.; Xu, K.; Wu, J.; Zhu, B.; Xie, C. Graphene-wrapped WO3 nanospheres with room-temperature NO2 sensing induced by interface charge transfer. Sens. Actuators B Chem. 2015, 220, 201–209. [Google Scholar] [CrossRef]
- Wang, T.; Hao, J.; Zheng, S.; Sun, Q.; Zheng, D.; Wang, Y. Highly sensitive and rapidly responding room-temperature NO2 gas sensors based on WO3 nanorods/sulfonated graphene nanocomposites. Nano Res. 2018, 11, 791–803. [Google Scholar] [CrossRef]
- Tang, X.; Lahem, D.; Raskin, J.P.; Gérard, P. A fast and room-temperature operation ammonia sensor based on compound of graphene with polypyrrole. IEEE Sens. J. 2018, 18, 9088–9096. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.P.; Reckinger, N.; Yan, Y.; André, N.; Lahem, D.; Debliquy, M. Enhanced gas detection by altering gate voltage polarity of polypyrrole/graphene field-effect transistor sensor. Chemosensors 2022, 10, 467. [Google Scholar] [CrossRef]
- Hung, T.-T.; Chung, M.-H.; Chiu, J.-J.; Yang, M.-W.; Tien, T.-N.; Shen, C.-Y. Poly(4-styrenesulfonic acid) doped polypyrrole/tungsten oxide/reduced graphene oxide nanocomposite films based surface acoustic wave sensors for NO sensing behavior. Org. Electron. 2021, 88, 106006. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Patil, S.L.; Raut, B.T.; Godse, P.R.; Sen, S.; Patil, V.B. Polypyrrole thin film: Room temperature ammonia gas sensor. IEEE Sens. J. 2011, 11, 137–2141. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene-based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.; He, H.; Cai, Z.; Gao, N.; He, C.; He, Y. Highly sensitive nitrite sensor based on AuNPs/rGO nanocomposites modified graphene electrochemical transistors. Biosens. Bioelectron. 2019, 146, 111751. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Lu, C.; Su, P. One-pot synthesis of AuNPs/rGO/WO3 nanocomposite for simultaneously sensing hydroquinone and catechol. Mater. Chem. Phys. 2018, 215, 293–298. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y. Preparation of polypyrrole-coated silver nanoparticles by one-step UV-induced polymerization. Mater. Lett. 2005, 59, 2484–2487. [Google Scholar] [CrossRef]
- Wajahat, M.; Kim, J.H.; Ahn, J.; Lee, S.; Bae, J.; Pyo, J.; Seol, S.K. 3D printing of Fe3O4 functionalized graphene-polymer (FGP) composite microarchitectures. Carbon 2020, 167, 278–284. [Google Scholar] [CrossRef]
- Sharma, P.; Darabdhara, G.; Reddy, T.M.; Borah, A.; Bezboruah, P.; Gogoi, P.; Hussain, N.; Sengupta, P.; Das, M.R. Synthesis, characterization and catalytic application of Au NPs-reduced graphene oxide composites material: An eco-friendly approach. Catal. Commun. 2013, 40, 139–144. [Google Scholar] [CrossRef]
- Umar, M.F.; Nasar, A. Reduced graphene oxide/polypyrrole/nitrate reductase deposited glassy carbon electrode (GCE/rGO/PPy/NR): Biosensor for the detection of nitrate in wastewater. Appl. Water Sci. 2018, 8, 211. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglier, G. (Eds.) Solid State Gas Sensing; Springer: Berlin/Heidelberg, Germany, 2009; p. 289. ISBN 978-0-387-09664-3. [Google Scholar]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef]
- Kearney, D.J.; Hubbard, T.; Putnam, D. Breath ammonia measurement in helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 2523–2530. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Lee, C.T.; Wang, Y.S. High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. J. Alloys Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Andò, B.; Baglio, S.; Castorina, S.; Graziani, S.; Tondepu, S.V.G.; Petralia, S.; Messina, M.A.; Maugeri, L.; Neri, G.; Ferlazzo, A. A capacitive sensor, exploiting a YSZ functional layer, for ammonia detection. IEEE Trans. Instrum. Meas. 2022, 71, 9505811. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Sun, J.; Shu, X.; Tian, Y.; Tong, Z.; Bai, S.; Luo, R.; Li, D.; Liu, C.C. Facile preparation of polypyrrole-reduced graphene oxide hybrid for enhancing NH3 sensing at room temperature. Sens. Actuators B Chem. 2017, 241, 658–664. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloys Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]




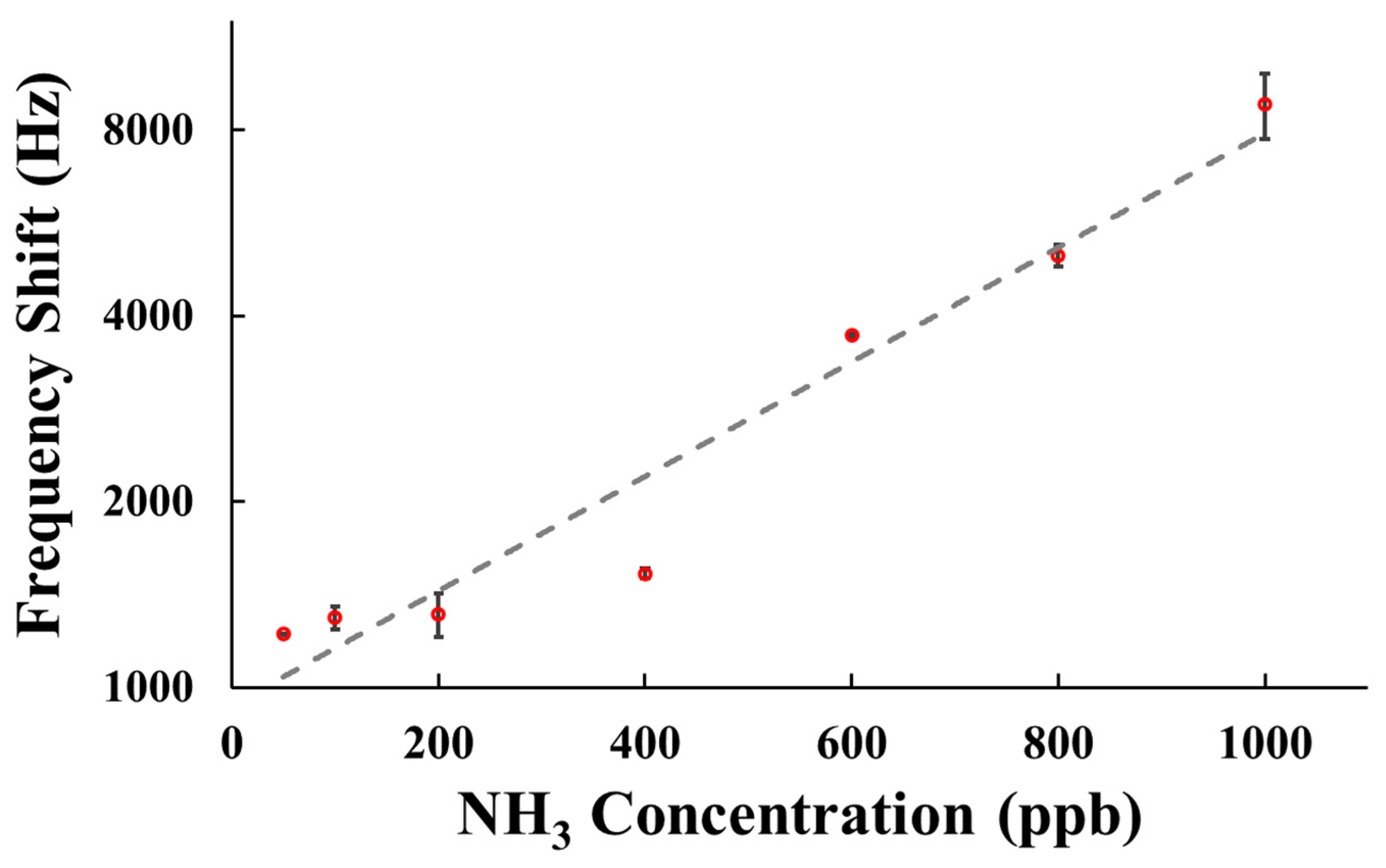

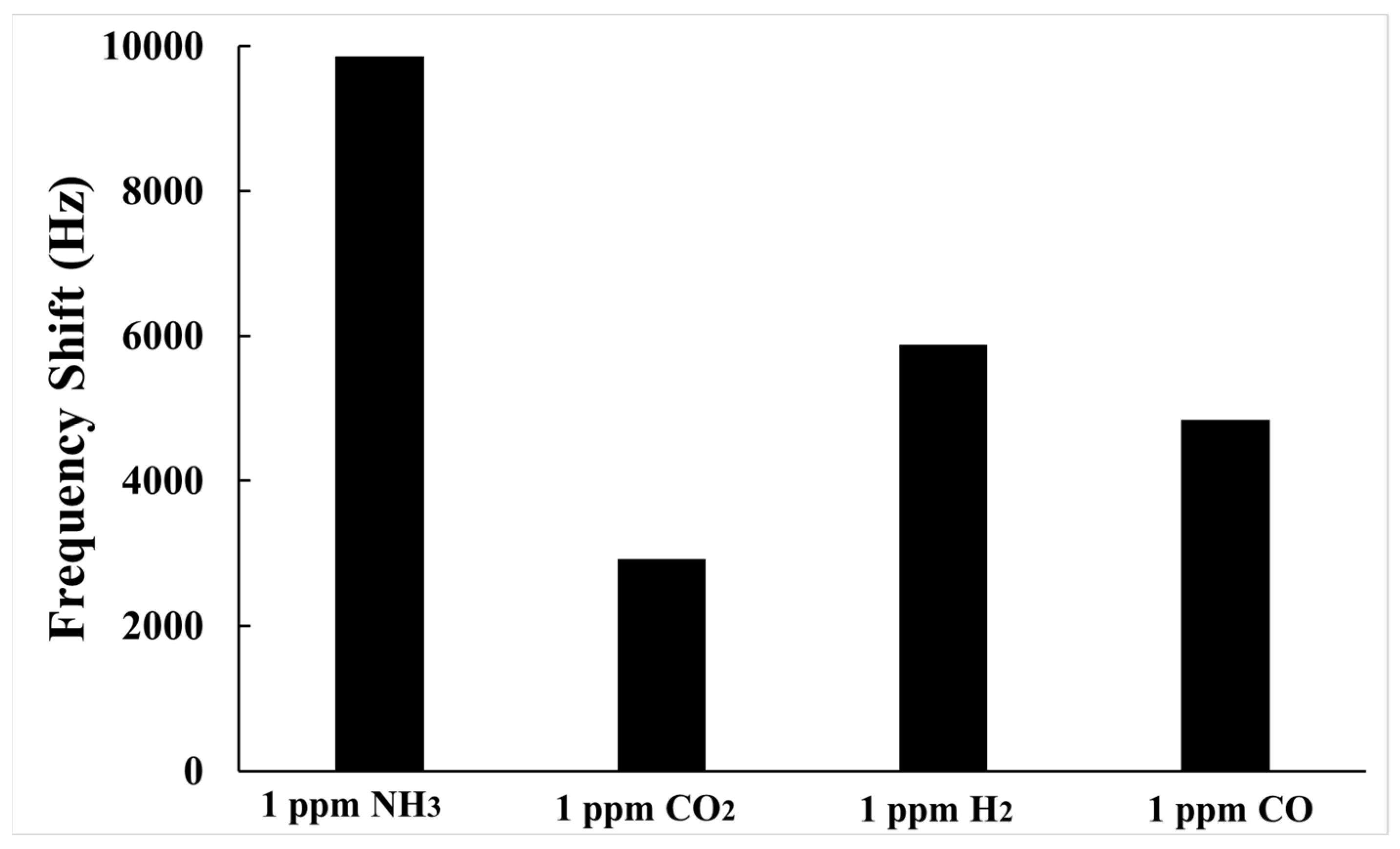
| NH3 Concentration (ppb) | 50 | 100 | 200 | 400 | 600 | 800 | 1000 |
|---|---|---|---|---|---|---|---|
| Frequency shift (Hz) | 1222 | 1353 | 1208 | 1559 | 3692 | 4808 | 9867 |
| Response time (s) | 128 | 125 | 120 | 118 | 98 | 76 | 61 |
| Recovery time (s) | 140 | 120 | 122 | 120 | 87 | 77 | 56 |
| Time (Day) | 1 | 10 | 20 | 30 |
|---|---|---|---|---|
| Frequency shift (Hz) | 1273 | 1222 | 1219 | 563 |
| Sensing Film | Sensitivity | Detection Limit (LOD) | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|
| SnO2/Co3O4 | 3.33 Hz/ppm | 9 ppm | 100–120 s | 30–50 s | [11] |
| PANI/WO3 | 121% to 100 ppm | 1 ppm | 32 s | 388 s | [14] |
| PANI/SnO2 | 29 to 100 ppm | ≥1.8 ppm | 31 s | - | [15] |
| PANI/HNTs | 257.14% (50 ppm) | 10 ppb | 158 s | 162 s | [33] |
| PANI–rGO | 13% (15 ppm) | 0.3 ppm | 96 s | 22.1 min | [34] |
| Yttrium Stabilized Zirconium (YSZ) | 3.53 × 10−14 F/μM | 2.5 ppb | - | - | [35] |
| PPy/G | 1.7% to 1 ppm | 1 ppm | 2 min | 5 min | [18] |
| PPy | 12% to 20 ppm | 5 ppm | 20 s | 20 min | [21] |
| AuNPs–G/PPy | 8 Hz/ppb | 3 ppb | 128 s | 140 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-Y.; Hung, T.-T.; Chuang, Y.-W.; Lai, S.-K.; Tai, C.-M. Room-Temperature NH3 Gas Surface Acoustic Wave (SAW) Sensors Based on Graphene/PPy Composite Films Decorated by Au Nanoparticles with ppb Detection Ability. Polymers 2023, 15, 4353. https://doi.org/10.3390/polym15224353
Shen C-Y, Hung T-T, Chuang Y-W, Lai S-K, Tai C-M. Room-Temperature NH3 Gas Surface Acoustic Wave (SAW) Sensors Based on Graphene/PPy Composite Films Decorated by Au Nanoparticles with ppb Detection Ability. Polymers. 2023; 15(22):4353. https://doi.org/10.3390/polym15224353
Chicago/Turabian StyleShen, Chi-Yen, Tien-Tsan Hung, Yao-Wei Chuang, Shao-Kai Lai, and Chi-Ming Tai. 2023. "Room-Temperature NH3 Gas Surface Acoustic Wave (SAW) Sensors Based on Graphene/PPy Composite Films Decorated by Au Nanoparticles with ppb Detection Ability" Polymers 15, no. 22: 4353. https://doi.org/10.3390/polym15224353
APA StyleShen, C.-Y., Hung, T.-T., Chuang, Y.-W., Lai, S.-K., & Tai, C.-M. (2023). Room-Temperature NH3 Gas Surface Acoustic Wave (SAW) Sensors Based on Graphene/PPy Composite Films Decorated by Au Nanoparticles with ppb Detection Ability. Polymers, 15(22), 4353. https://doi.org/10.3390/polym15224353









