Spherical CaCO3: Synthesis, Characterization, Surface Modification and Efficacy as a Reinforcing Filler in Natural Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Untreated CaCO3 Synthesis
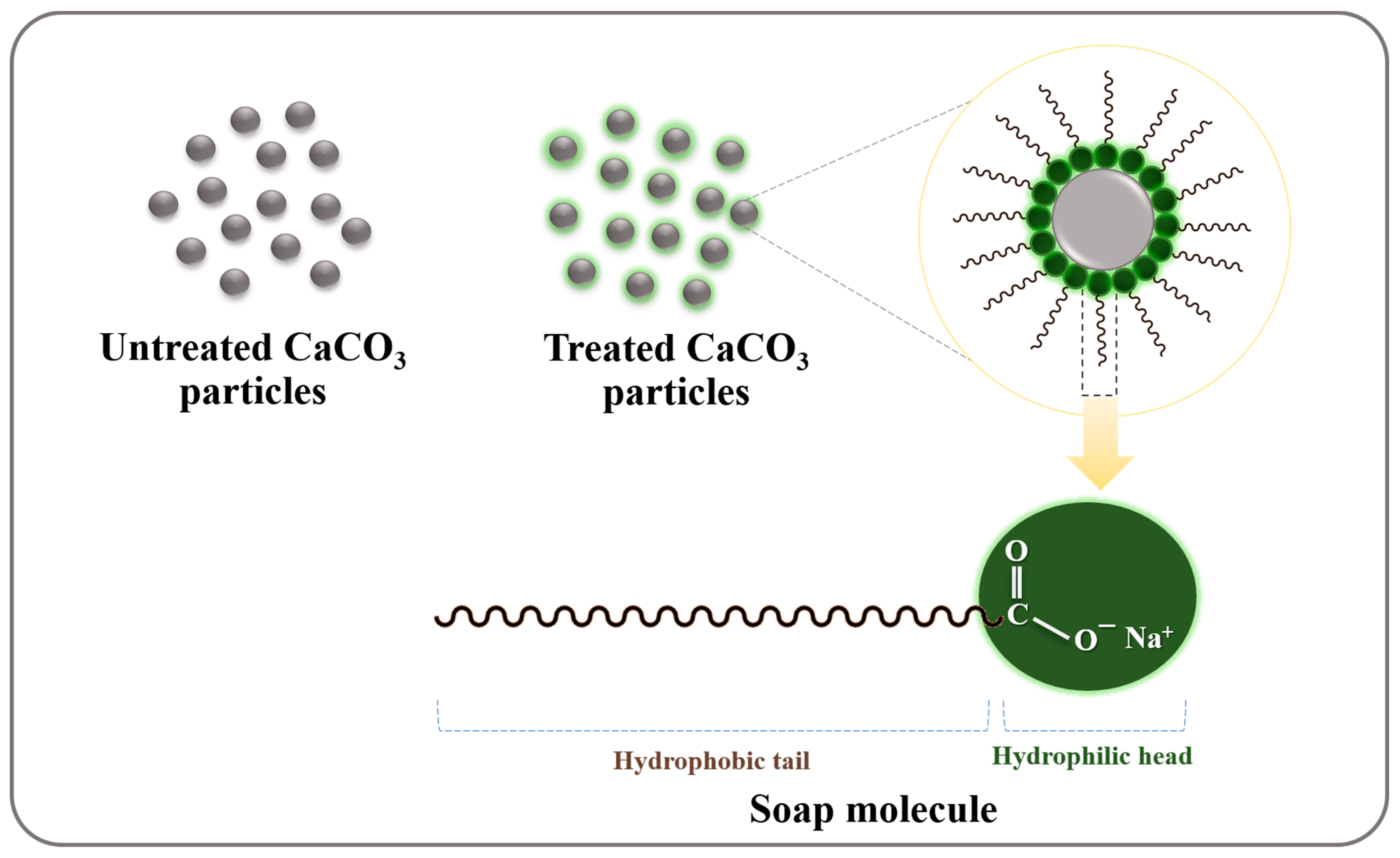
2.2.2. Olive Soap Preparation
2.2.3. Treated CaCO3 Synthesis
2.2.4. Preparation of NR/CaCO3 Composites
- NR/CaCO3 compound preparation
- NR/CaCO3 composite preparation
2.3. Characterization of CaCO3 Fillers and NR/CaCO3 Composites
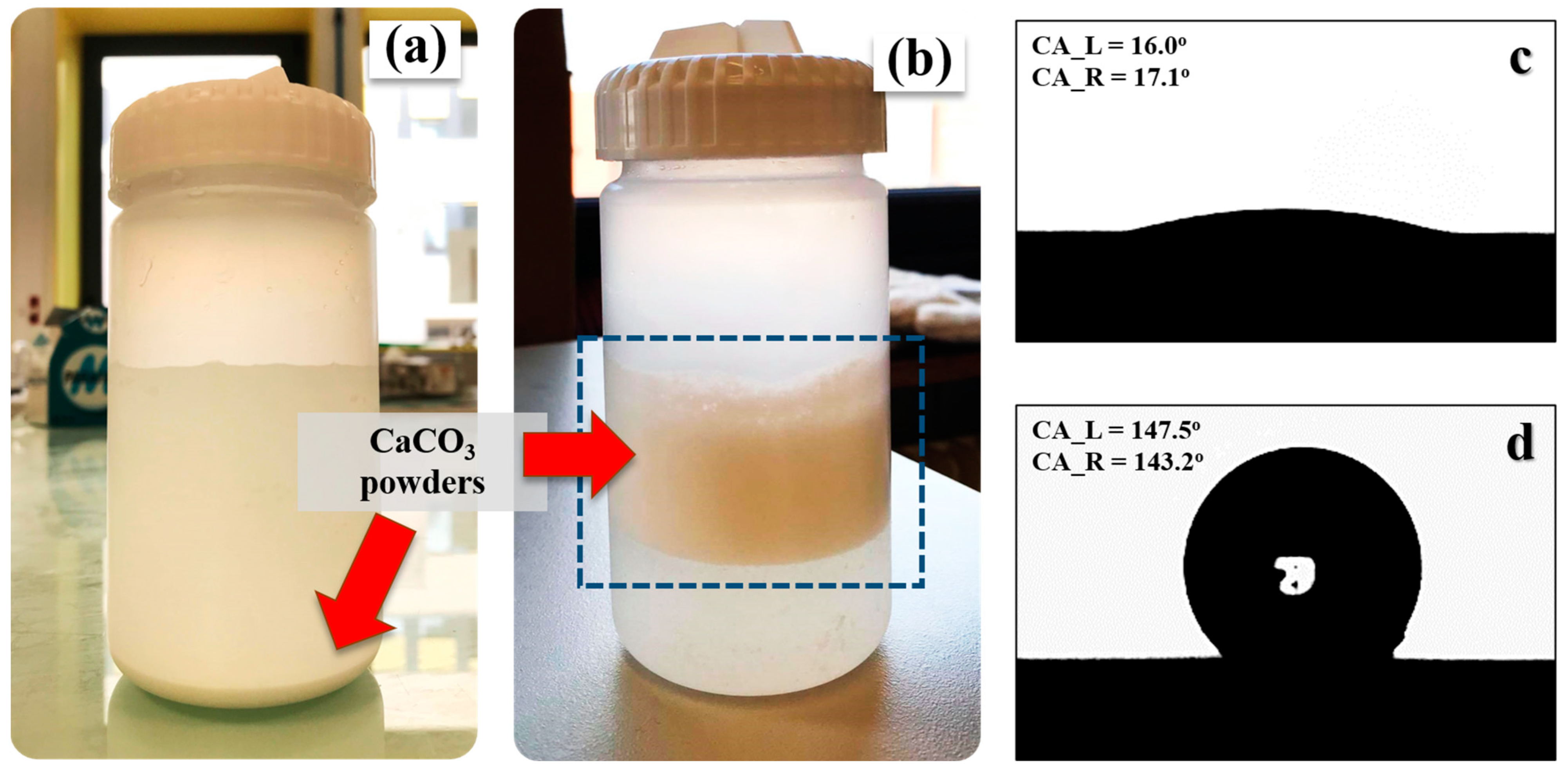
2.3.1. Optical Contact Angle
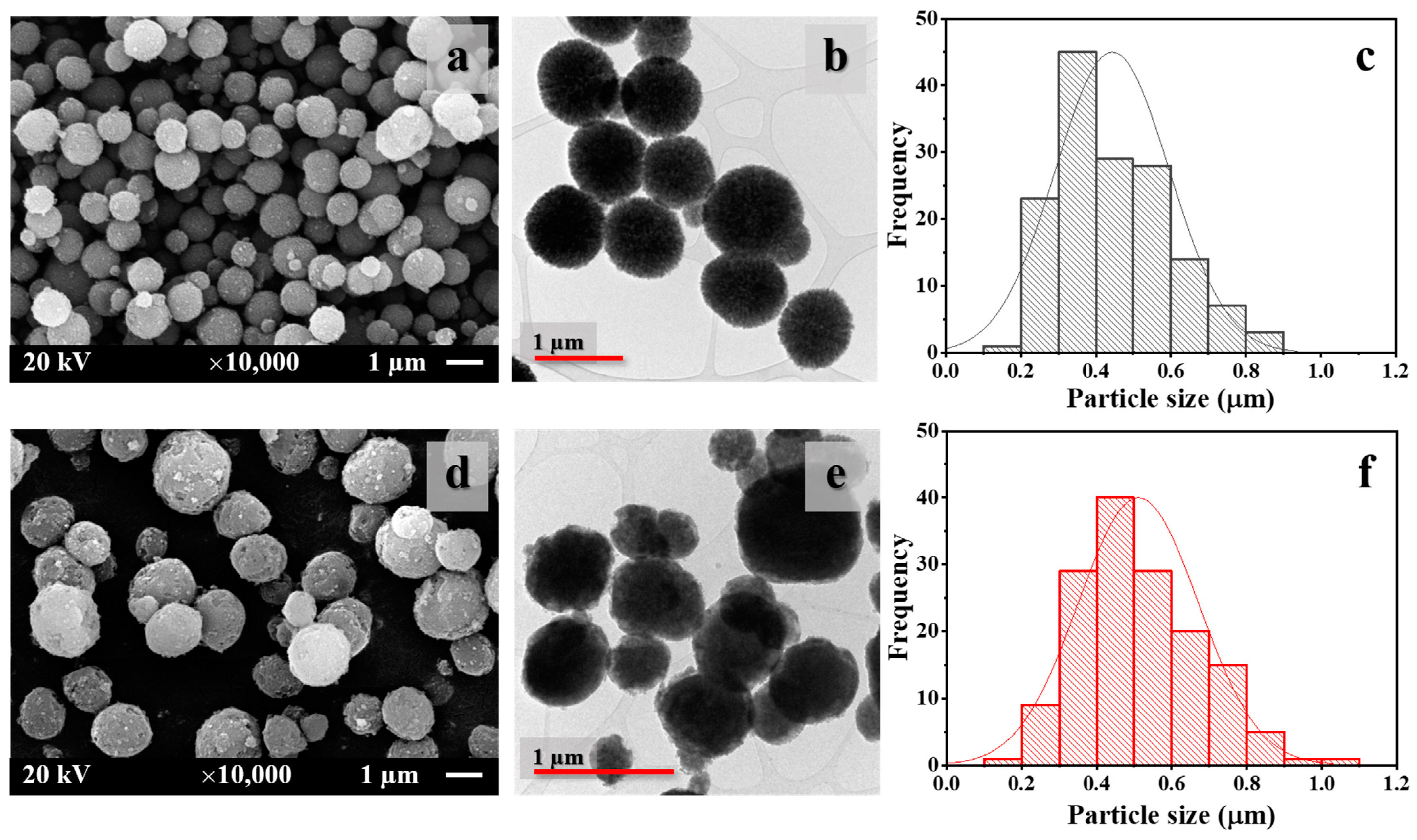
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Transmission Electron Microscopy (TEM)
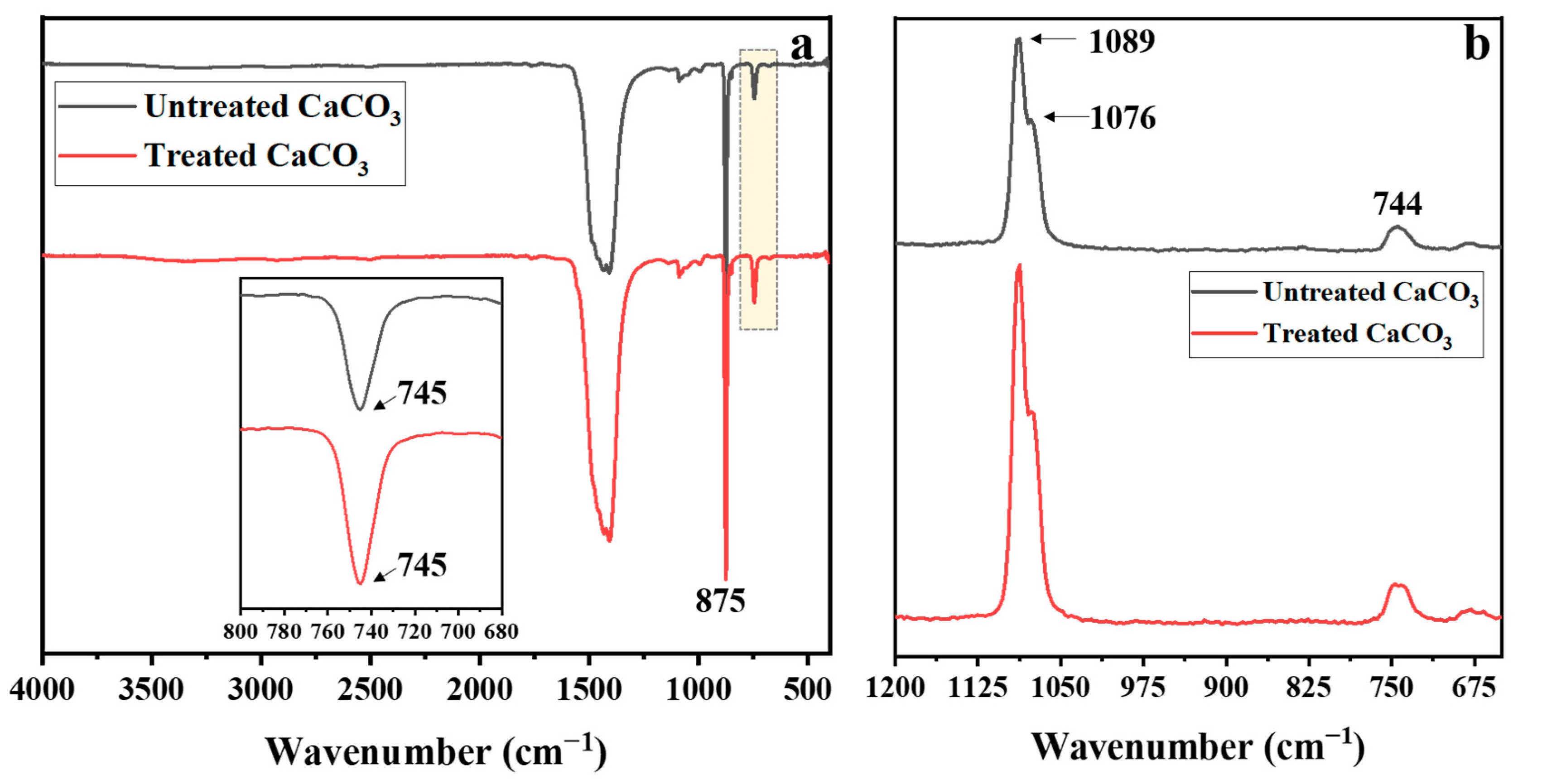
2.3.4. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.5. Raman Spectroscopy
2.3.6. X-ray Diffraction (XRD) Analysis
2.3.7. Thermogravimetric Analysis (TGA)
2.3.8. Brunauer–Emmett–Teller (BET) Analysis
2.4. Mechanical Properties of NR/CaCO3 Composites
2.4.1. Tensile Properties
2.4.2. Hardness (Shore A)
2.4.3. Statistical Analysis
3. Results and Discussion
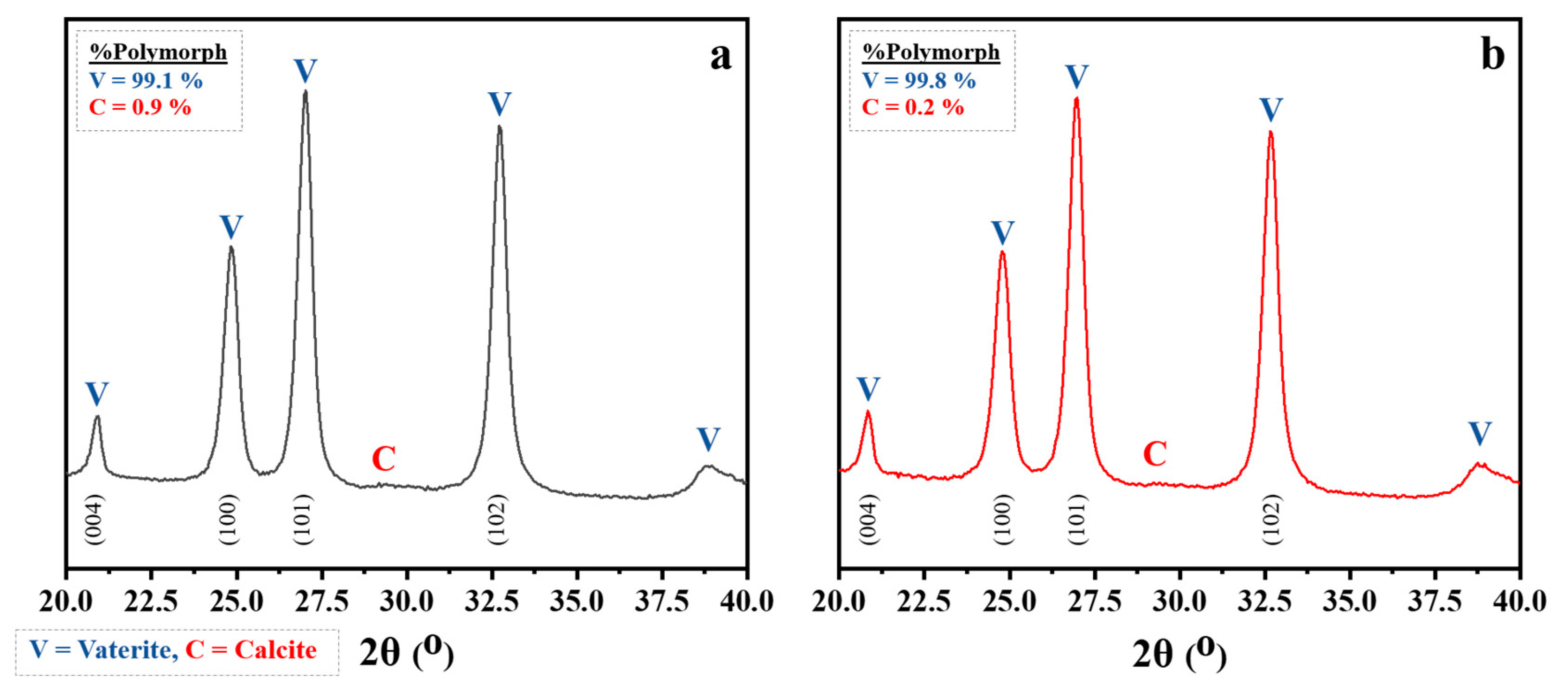
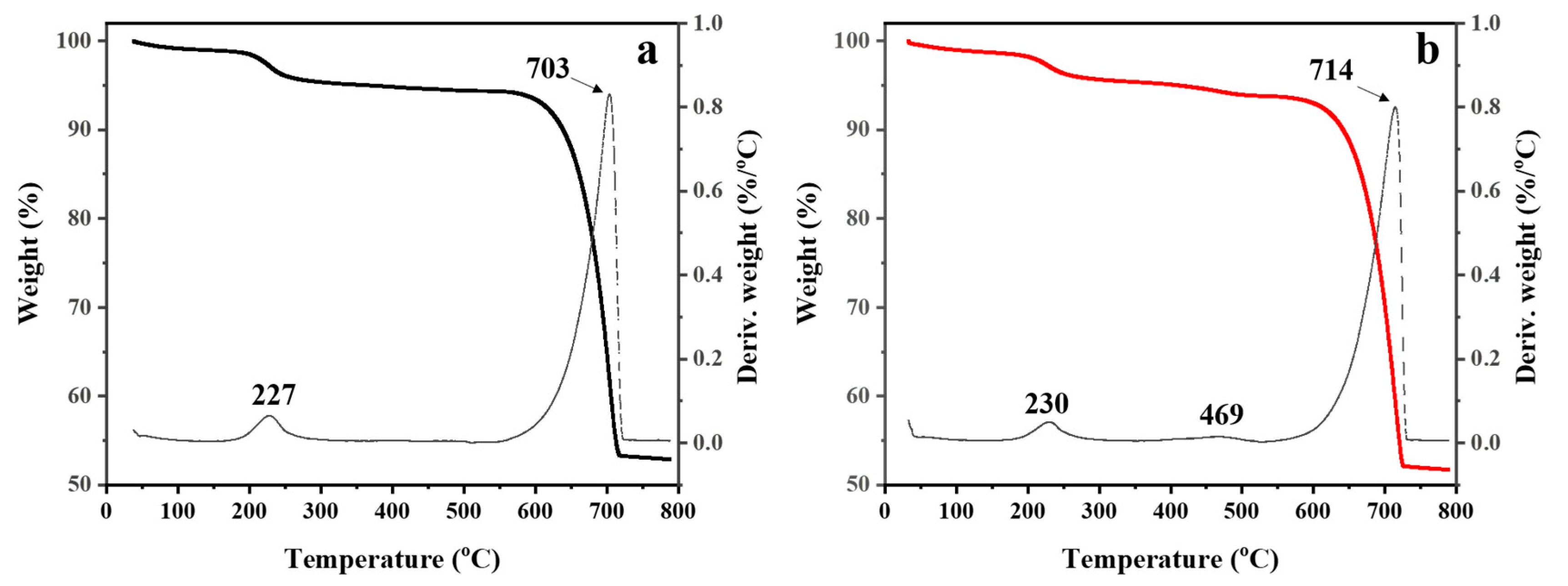
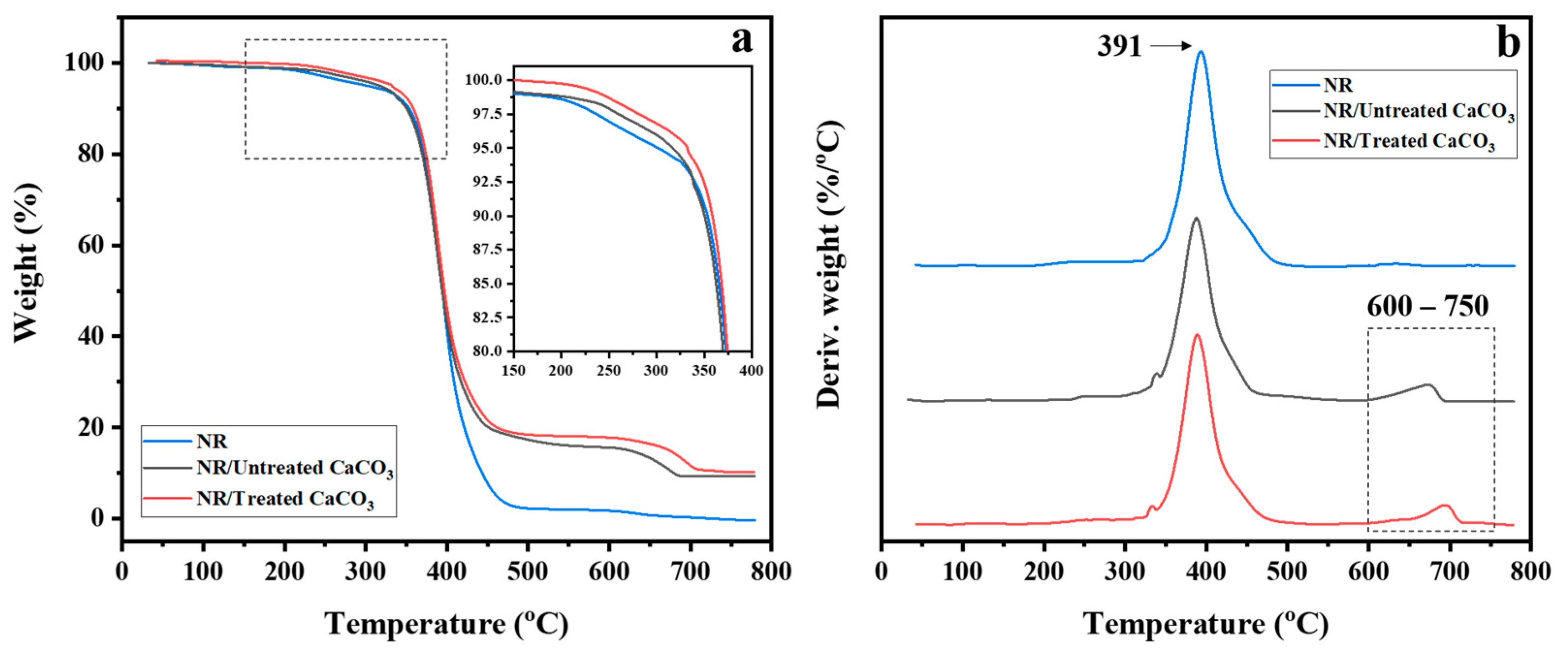
3.1. Untreated and Treated CaCO3 Powders
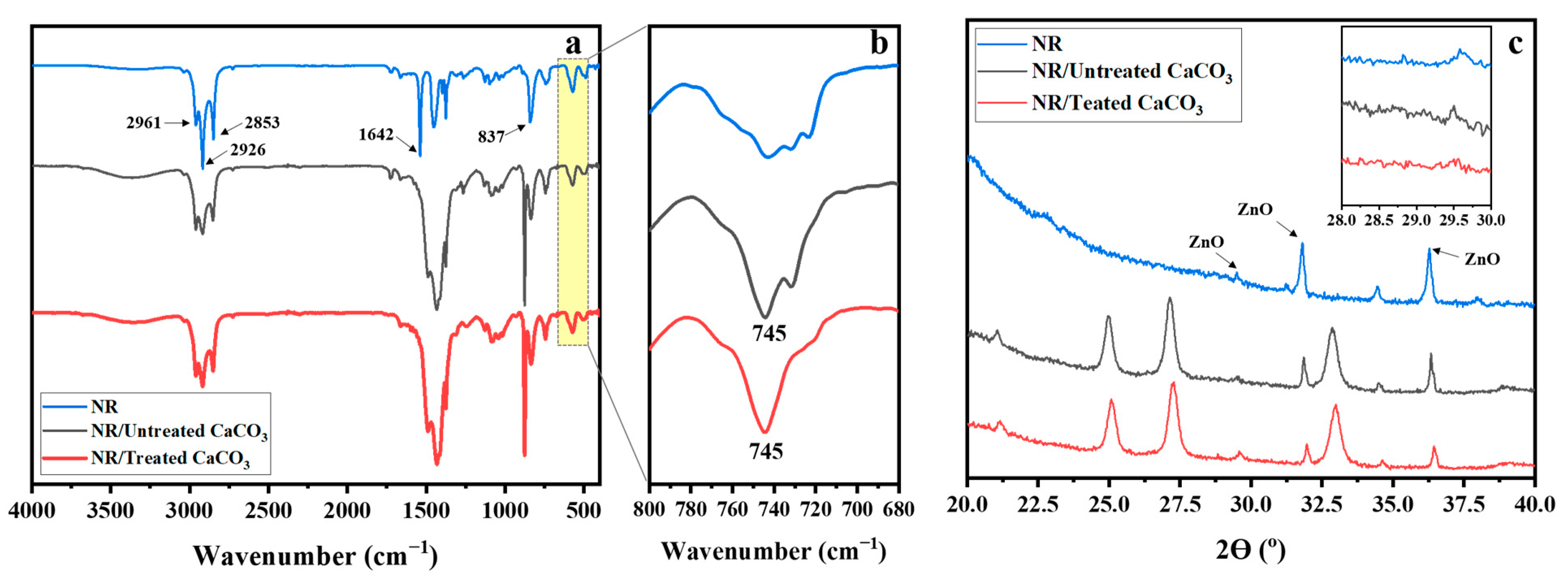
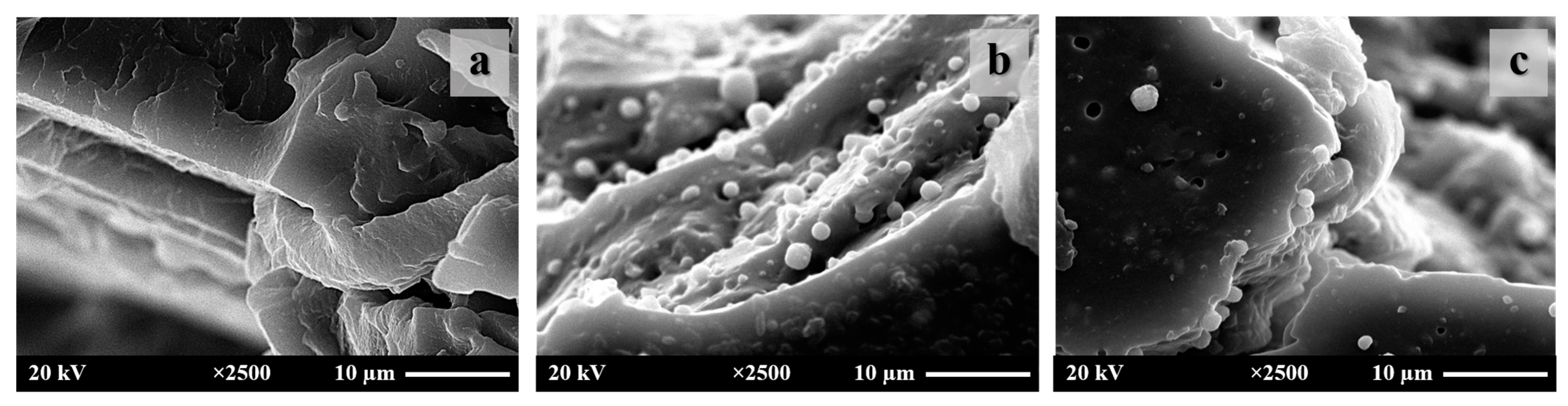
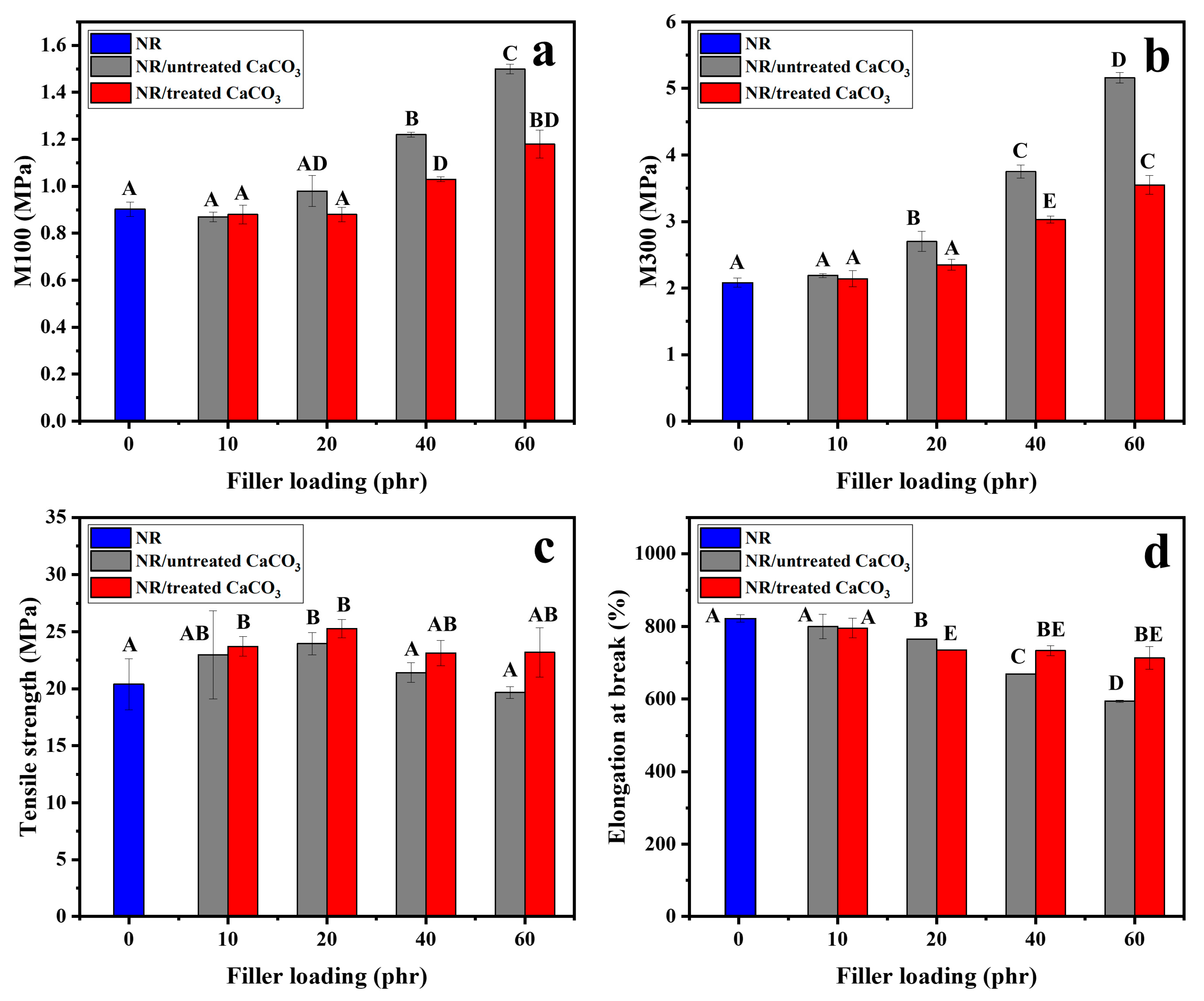
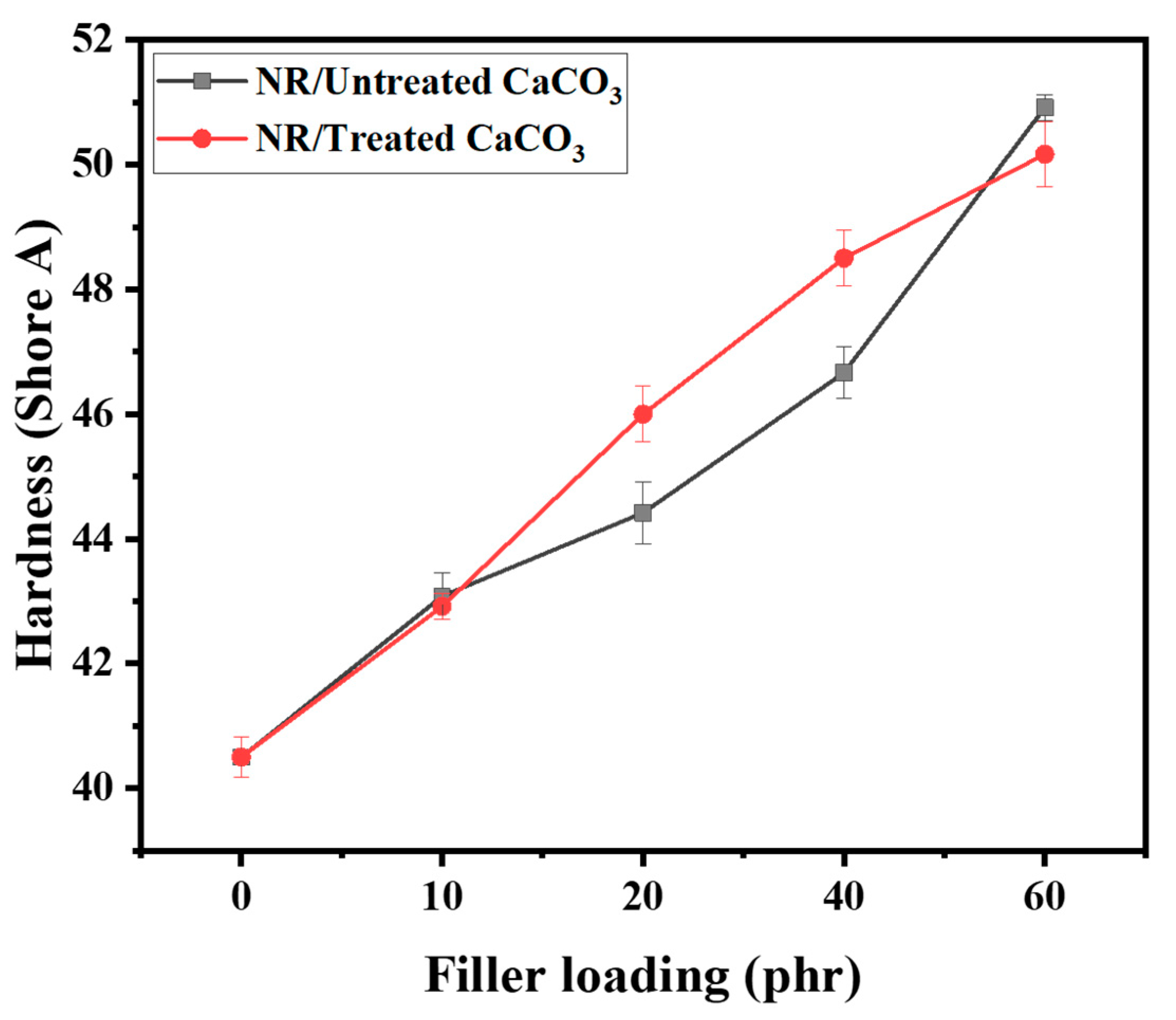
3.2. NR/Untreated CaCO3 and NR/Treated CaCO3 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phinyocheep, P.; Phetphaisit, C.W.; Derouet, D.; Campistron, I.; Brosse, J.C. Chemical degradation of epoxidized natural rubber using periodic acid: Preparation of epoxidized liquid natural rubber. J. Appl. Polym. Sci. 2005, 95, 6–15. [Google Scholar] [CrossRef]
- Phinyocheep, P. 3—Chemical modification of natural rubber (NR) for improved performance. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 68–118. [Google Scholar]
- Singh, M. The Colloidal Properties of Commercial Natural Rubber Latex Concentrates. J. Rubber Res. 2018, 21, 119–134. [Google Scholar] [CrossRef]
- Fisher, H.L. Vulcanization of rubber vulcanization of rubber. Ind. Eng. Chem. 1939, 31, 1381–1389. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber–filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Rothon, R. Particulate Fillers in Elastomers. In Fillers for Polymer Applications; Rothon, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 125–146. [Google Scholar]
- Turku, I.; Kärki, T. Research progress in wood-plastic nanocomposites: A review. J. Thermoplast. Compos. Mater. 2013, 27, 180–204. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.Y. A study on the material properties and fatigue life of natural rubber with different carbon blacks. Int. J. Fatigue 2005, 27, 263–272. [Google Scholar] [CrossRef]
- Kaliyathan, A.V.; Rane, A.V.; Huskic, M.; Kunaver, M.; Kalarikkal, N.; Rouxel, D.; Thomas, S. Carbon black distribution in natural rubber/butadiene rubber blend composites: Distribution driven by morphology. Compos. Sci. Technol. 2020, 200, 108484. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Reinforcement of natural rubber by precipitated silica: The influence of processing temperature. Rubber Chem. Technol. 2014, 87, 103–119. [Google Scholar] [CrossRef][Green Version]
- Fang, Q.; Song, B.; Tee, T.-T.; Sin, L.T.; Hui, D.; Bee, S.-T. Investigation of dynamic characteristics of nano-size calcium carbonate added in natural rubber vulcanizate. Compos. Part. B Eng. 2014, 60, 561–567. [Google Scholar] [CrossRef]
- Lay, M.; Hamran, N.; Rashid, A.A. Ultrafine calcium carbonate-filled natural rubber latex film: Mechanical and post-processing properties. Iran. Polym. J. 2019, 28, 849–858. [Google Scholar] [CrossRef]
- Sadeghi Ghari, H.; Jalali-Arani, A. Nanocomposites based on natural rubber, organoclay and nano-calcium carbonate: Study on the structure, cure behavior, static and dynamic-mechanical properties. Appl. Clay Sci. 2016, 119, 348–357. [Google Scholar] [CrossRef]
- Akbari, A.; Jawaid, M.; Hassan, A.; Balakrishnan, H. Epoxidized natural rubber toughened polylactic acid/talc composites: Mechanical, thermal, and morphological properties. J. Compos. Mater. 2013, 48, 769–781. [Google Scholar] [CrossRef]
- Khan, I.; Bhat, A.H. Micro and Nano Calcium Carbonate Filled Natural Rubber Composites and Nanocomposites. In Natural Rubber Materials, Volume 2: Composites and Nanocomposites; Thomas, S., Maria, H.J., Joy, J., Chan, C.H., Pothen, L.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2013; Volume 2. [Google Scholar]
- Konopacka-łyskawa, D.; Lackowski, M. Influence of ethylene glycol on CaCO3 particles formation via carbonation in the gas–slurry system. J. Cryst. Growth 2011, 321, 136–141. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Takabait, F.; Mahtout, L.; Carrasco-Hurtado, B.; Eliche-Quesada, D.; Sánchez-Soto, P.J. Synthesis of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium. Ceram. Int. 2018, 44, 5291–5296. [Google Scholar] [CrossRef]
- Gibaud, A.; Younas, D.; Matthews, L.; Narayanan, T.; Longkaew, K.; Hageberg, I.U.; Chushkin, Y.; Breiby, D.W.; Chattopadhyay, B. Insights into the precipitation kinetics of CaCO3 particles in the presence of polystyrene sulfonate using in situ small-angle X-ray scattering. J. Appl. Crystallogr. 2023, 56, 1114–1124. [Google Scholar] [CrossRef]
- Longkaew, K.; Tessanan, W.; Daniel, P.; Phinyocheep, P.; Gibaud, A. Using sucrose to prepare submicrometric CaCO3 vaterite particles stable in natural rubber. Adv. Powder Technol. 2023, 34, 103924. [Google Scholar] [CrossRef]
- Baqiya, M.A.; Lailiyah, Q.; Riyanto, A.; Arifin, Z.; Triwikantoro; Zainuri, M.; Pratapa, S.; Darminto. Precipitation Process of CaCO3 from Natural Limestone for Functional Materials. J. AOAC Int. 2020, 103, 373–381. [Google Scholar] [CrossRef]
- Owuamanam, S.; Cree, D. Progress of Bio-Calcium Carbonate Waste Eggshell and Seashell Fillers in Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Hussin, H.B.; Temitope, A.E. Synthesis of precipitated calcium carbonate: A review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Nehrke, G.; Van Cappellen, P. Framboidal vaterite aggregates and their transformation into calcite: A morphological study. J. Cryst. Growth 2006, 287, 528–530. [Google Scholar] [CrossRef]
- Bragg, W.L. The structure of aragonite. Proc. R. Soc. London Ser. A Contain. Pap. A Math. Phys. Character 1997, 105, 16–39. [Google Scholar] [CrossRef]
- Kogo, M.; Suzuki, K.; Umegaki, T.; Kojima, Y. Control of aragonite formation and its crystal shape in CaCl2-Na2CO3-H2O reaction system. J. Cryst. Growth 2021, 559, 125964. [Google Scholar] [CrossRef]
- Tas, A.C. Monodisperse Calcium Carbonate Microtablets Forming at 70 °C in Prerefrigerated CaCl2–Gelatin–Urea Solutions. Int. J. Appl. Ceram. Technol. 2009, 6, 53–59. [Google Scholar] [CrossRef]
- Lu, J.; Ruan, S.; Liu, Y.; Wang, T.; Zeng, Q.; Yan, D. Morphological characteristics of calcium carbonate crystallization in CO2 pre-cured aerated concrete. RSC Adv. 2022, 12, 14610–14620. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Perera, R.; León, N.; Santana, M.H.; Martínez, N. Reinforcement of natural rubber using a novel combination of conventional and in situ generated fillers. Compos. Part C Open Access 2021, 5, 100133. [Google Scholar] [CrossRef]
- Zhao, R.; Yin, Z.; Zou, W.; Yang, H.; Yan, J.; Zheng, W.; Li, H. Preparation of High-Strength and Excellent Compatibility Fluorine/Silicone Rubber Composites under the Synergistic Effect of Fillers. ACS Omega 2023, 8, 3905–3916. [Google Scholar] [CrossRef]
- Surya, I.; Sinaga, R. Tensile and rheometric properties of calcium carbonate-filled natural rubber compounds without/with lauryl alcohol. IOP Conf. Ser. Mater. Sci. Eng. 2019, 505, 012146. [Google Scholar] [CrossRef]
- Shi, X.; Rosa, R.; Lazzeri, A. On the Coating of Precipitated Calcium Carbonate with Stearic Acid in Aqueous Medium. Langmuir 2010, 26, 8474–8482. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Abidi, L.; Amiard, F.; Delorme, N.; Ouhenia, S.; Gibaud, A. Using saponified olive oil to make cost effective calcium carbonate particles superhydrophobic. Adv. Powder Technol. 2022, 33, 103399. [Google Scholar] [CrossRef]
- Subagjo; Wulandari, W.; Adinata, P.M.; Fajrin, A. Thermal decomposition of dolomite under CO2-air atmosphere. AIP Conf. Proc. 2017, 1805, 040006. [Google Scholar] [CrossRef]
- Erickson, K.L. Application of Low-Heating Rate TGA Results to Hazard Analyses Involving High-Heating Rates. In Proceedings of the SAMPE’08 Conference, Albuquerque, NM, USA, 18–22 May 2008. [Google Scholar]
- Koeipudsa, N.; Chanthateyanonth, R.; Daniel, P.; Phinyocheep, P. Development of natural rubber nanocomposites reinforced with cellulose nanocrystal isolated from oil palm biomass. J. Polym. Res. 2022, 29, 403. [Google Scholar] [CrossRef]
- ASTM D412-98; Standard Test Methods for Vulcanized Rubber and Thermoplastic Rubbers and Thermoplastic Elastomers-Tension. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2002.
- ASTM D2240-97; Standard Test Method for Rubber Property—Durometer Hardness. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2015.
- Rodrigues, N.; Casal, S.; Pinho, T.; Cruz, R.; Peres, A.M.; Baptista, P.; Pereira, J.A. Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year. Foods 2021, 10, 496. [Google Scholar] [CrossRef]
- Reig, F.B.; Adelantado, J.V.; Moya Moreno, M.C. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Dupont, L.; Portemer, F.; late Michel Figlarz, T. Synthesis and study of a well crystallized CaCO3 vaterite showing a new habitus. J. Mater. Chem. 1997, 7, 797–800. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 644–658. [Google Scholar] [CrossRef]
- Yang, L.-F.; Chu, D.-Q.; Sun, H.-L.; Ge, G. Room temperature synthesis of flower-like CaCO3 architectures. New J. Chem. 2016, 40, 571–577. [Google Scholar] [CrossRef]
- Thriveni, T.; Nam, S.Y.; Ahn, J.-W.; Um, N. Enhancement of arsenic removal efficiency from mining waste water by accelerated carbonation. In Proceedings of the IMPC 2014—27th International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Konopacka-Łyskawa, D.; Czaplicka, N.; Kościelska, B.; Łapiński, M.; Gębicki, J. Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method. Crystals 2019, 9, 117. [Google Scholar] [CrossRef]
- Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Rahman, M.Z.A.; Azizi, S.; Fatehi, A. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508–4522. [Google Scholar] [CrossRef] [PubMed]
- Buasri, A.; Chaiyut, N.; Borvornchettanuwat, K.; Chantanachai, N.; Thonglor, K. Thermal and Mechanical Properties of Modified CaCO3/PP Nanocomposites. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2012, 6, 689–692. [Google Scholar]
- Yu, Y.; Zhang, J.; Wang, H.; Xin, Z. Silanized Silica-Encapsulated Calcium Carbonate@Natural Rubber Composites Prepared by One-Pot Reaction. Polymers 2020, 12, 2668. [Google Scholar] [CrossRef]
- Umunakwe, R.; Oyetunji, A.; Adewuyi, B.; Eze, W.; Nwigwe, S.; Umunakwe, I. Mechanical properties and microstructure of hybrid vulcanized natural rubber filled with carbon black and Nano-CaCO3 from achatina achatina shells. J. Met. Mater. Miner. 2019, 29, 80–89. [Google Scholar] [CrossRef]
| Chemicals | Content (phr *) |
|---|---|
| NR | 100 |
| CaCO3 | 0, 10, 20, 40, 60 |
| ZnO | 1.8 |
| Stearic acid | 2.0 |
| CBS | 1.0 |
| Sulfur | 1.5 |
| Types of CaCO3 | Particle Size (µm) * | BET Value (m2/g) | Contact Angle (°) | Polymorphic Phase (%) | |
|---|---|---|---|---|---|
| Vaterite | Calcite | ||||
| Untreated | 0.42 ± 0.14 | 39.32 | 16.5 | 99.1 | 0.9 |
| Treated | 0.52 ± 0.16 | 34.45 | 145.4 | 99.8 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longkaew, K.; Gibaud, A.; Tessanan, W.; Daniel, P.; Phinyocheep, P. Spherical CaCO3: Synthesis, Characterization, Surface Modification and Efficacy as a Reinforcing Filler in Natural Rubber Composites. Polymers 2023, 15, 4287. https://doi.org/10.3390/polym15214287
Longkaew K, Gibaud A, Tessanan W, Daniel P, Phinyocheep P. Spherical CaCO3: Synthesis, Characterization, Surface Modification and Efficacy as a Reinforcing Filler in Natural Rubber Composites. Polymers. 2023; 15(21):4287. https://doi.org/10.3390/polym15214287
Chicago/Turabian StyleLongkaew, Khansinee, Alain Gibaud, Wasan Tessanan, Philippe Daniel, and Pranee Phinyocheep. 2023. "Spherical CaCO3: Synthesis, Characterization, Surface Modification and Efficacy as a Reinforcing Filler in Natural Rubber Composites" Polymers 15, no. 21: 4287. https://doi.org/10.3390/polym15214287
APA StyleLongkaew, K., Gibaud, A., Tessanan, W., Daniel, P., & Phinyocheep, P. (2023). Spherical CaCO3: Synthesis, Characterization, Surface Modification and Efficacy as a Reinforcing Filler in Natural Rubber Composites. Polymers, 15(21), 4287. https://doi.org/10.3390/polym15214287







