Abstract
Deep eutectic solvents (DESs) are promising for lignin dissolution and extraction. However, they usually possess high polarity and are difficult to recycle. To overcome this drawback, a variety of switchable ionic liquids (SILs) composed of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and alcohols was synthesized and screened. According to the thermodynamic modeling suggestions, the selected DBU–HexOH SIL was coupled with hydrogen-bond donors to form switchable-DES (SDES) systems with moderated viscosity, conductivity, and pH while maintaining switchability. The SDESs produced a well-improved lignin and lignin model compound solubility compared with those of SILs; charging CO2 into SDES (SDESCO2) caused a further increase in solubility. The solubility (25 °C) of syringic acid, ferulic acid, and milled wood lignin in SDESCO2 reached 230.57, 452.17, and 279.12 mg/g, respectively. Such SDES-dissolved lignin can be regenerated using acetone as an anti-solvent. The SDES-regenerated lignin exhibited a well-preserved structure with no noticeable chemical modifications. Furthermore, the SDESCO2 lignin possessed a higher molecular weight (Mw = 10,340 g/mol; Mn = 7672 g/mol), improved uniformity (polydispersity index = 1.35), and a higher guaiacyl lignin unit content compared with the original milled wood lignin. The SDES system proposed in the present work could benefit the fractionation of lignin compounds and facilitate downstream industrial processes.
1. Introduction
Abundant reserves of lignocellulosic biomass make it an important renewable resource [1]. As a main component within lignocellulose, lignin is a three-dimensional amorphous polymer composed of methoxylated phenylpropane units [2]. Its aromatic structure causes lignin to be recognized as an important natural source of phenolic chemicals [3]; it is estimated that around 20 billion tons/year of such chemicals are produced by plants [4]. However, the chemical and physical interactions between lignin and carbohydrate components within lignocellulosic biomass make the separation of lignin difficult to achieve [5,6]. Traditional lignin separation approaches, such as chemical treatment, organic solvent treatment, mechanical treatment, and ionic liquid (IL) treatment methods [7], are well developed but, nevertheless, reported to have several drawbacks. Chemical treatment methods, for example, acid or alkali treatment, can achieve lignin separation through the degradation and dissolution of lignin macromolecules [8]; however, their applications are limited by severe lignin deconstruction and unavoidable chemical modifications. In addition, other shortcomings, such as the corrosion of equipment, wastewater treatment, and chemical recycling, also prohibit further application [9]. Organic solvents, such as N-methylmorpholine-N-oxide [10], can be used to dissolve and separate lignin from lignocellulose. However, most of these solvents are toxic and volatile. Because of this, such methods are commonly used for laboratory research and small-scale production [11,12]. Lignin can also be separated through a ball mill treatment [13]; however, this approach is extremely energy-consuming. ILs can selectively remove lignin from the lignocellulose [14], effectively overcoming the lignocellulose obstinacy [12]. Although it is reported to produce lignin with an increased chemical activity [15], the IL method is limited by its high cost [16], poor reusability [17], and, in some cases, it even exhibits greater toxicity than organic solvents [1]. Therefore, it is in the interests of both academic and industrial fields to develop efficient and sustainable methods for lignin dissolution and separation.
A deep eutectic solvent (DES) is a eutectic solvent formulated by hydrogen-bond donors (HBDs) and hydrogen-bond acceptors (HBAs) [18]. ILs can sometimes act as typical HBAs in DES systems [19,20]. Indeed, the DESs share the advantages of ILs, which are low vapor pressure, easy synthesis, and adjustable physical and chemical properties [21]. With the addition of HBDs, a DES can be designed to be low cost [22], with reduced viscosity [23], good biodegradability [24], and low toxicity [25]. In recent years, DESs have been applied in many areas, for example, in organic reactions [26], biotransformations [27], sensor development [28], and pharmaceutical formulation [29]. Furthermore, DESs have been reported as promising solvents for lignocellulosic biomass fractionation due to their selective lignin extraction nature [30,31]. The extracted lignin is usually very pure and uniformly distributed. The selective cleavage of the ether bond in lignin macromolecules during DES treatment has been widely reported [32]. For example, choline chloride–lactic acid DES produced a mild acid-based catalytic environment, which triggered the cleavage of ether bonds between phenylpropane units, resulting in lignin depolymerization and dissolution [33]. The strong hydrogen-bond network within the DES system also facilitated the selective extraction of lignin from the lignocellulose [34,35]. It was reported that acidic DESs with strong HBDs caused the proton-catalyzed cleavage of the ether, ester, and glycosidic linkages present in the lignin–carbohydrate complex, leading to the extraction and depolymerization of lignin and xylan [36,37]. The DES treatment also led to the controllable cleavage of C–O–C and C–C bonds within the lignin macromolecules to produce a lignin stream with improved values [38]. Zhu et al. used a choline chloride–ethylene glycol DES to extract very pure lignin and well-preserved β-O-4 linkages [39]. Xu et al. used an ultrasound-assisted choline chloride–formic acid DES to extract lignin with a small molecular size, narrow distribution, and well-preserved β-O-4 bonds [1]. Although these DES treatments have been proven as efficient lignin dissolution and extraction methods, they still present defects of poor reusability, mainly caused by their high polarities [40,41]. It is, therefore, worth developing a “switchable” DES system, which could act as a polar solvent during the lignin dissolution and extraction processes and could also transfer into a non-polar state during recycling.
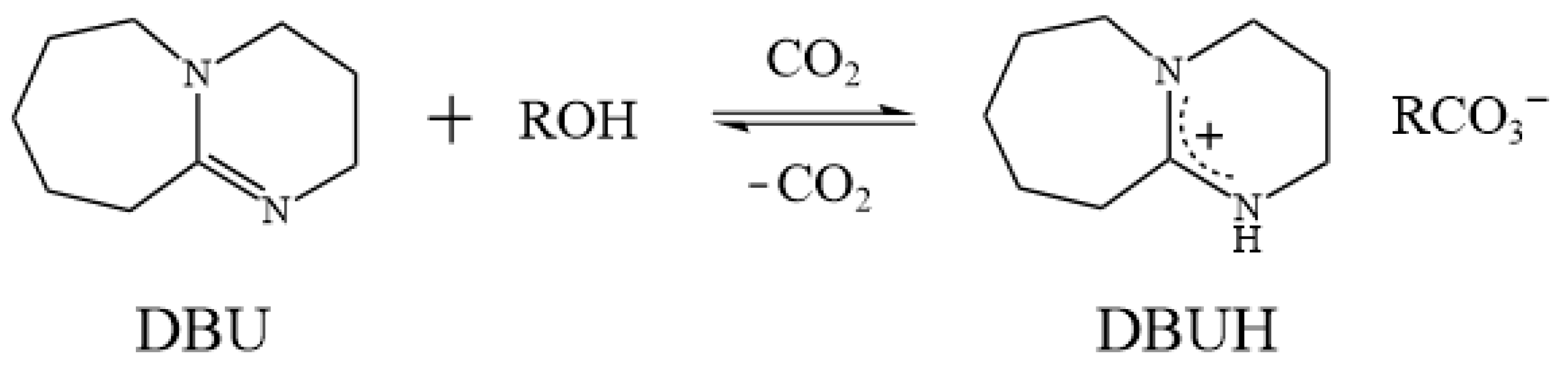
Mixing organic superbases and alcohols, followed by bubbling with CO2, generates a so-called switchable ionic liquid (SIL) [42,43], whose chemical and physical properties can be altered by the addition of acid gases, such as CO2 and SO2 [43]. These SILs can be recycled by reversibly transforming to their molecular precursors by heating or by bubbling N2 [44]. Lam Phan et al. found that the exposure of a 1:1 mixture of two neutral liquids, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and monohydric alcohols, to gaseous CO2 at 1 atm caused an exothermic conversion of the liquid phase to an ionic-form substance (Figure 1) [42,45]. Anugwom et al. tested the possibility of using these SILs for lignocellulosic biomass treatment [46]. However, like IL defects, SILs are also expensive and highly viscous. Recently, several switchable DESs (SDESs) with a reversible phase transition nature, triggered by CO2, pH, and heat, were also developed [47]. Therefore, it would be beneficial to use the concept of DESs, taking SIL as an HBA and coupling it with a selected HBD, to generate a brand-new switchable solvent system, thereby overcoming the defects mentioned previously. A detailed review of the possibility of using switchable solvents for lignin dissolution and extraction was presented in our previous work [48]. The proposed solvent system could provide a low-cost, high-efficiency, and sustainable method for lignin dissolution and extraction; this could further facilitate the development of plant fiber pretreatment technology.
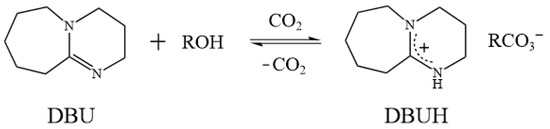
Figure 1.
The reaction scheme for the switchable ionic liquids (SILs) [42].
A variety of SILs composed of DBU and different alcohol compounds was synthesized and screened in this work. Optimized SIL was coupled with an HBD to form stable SDES systems; this was followed by using the systems to dissolve lignin and its model compounds. Lignin solubility was checked using ultraviolet spectroscopy, and characterizations such as Fourier-transform infrared (FTIR) spectrum, heteronuclear single-quantum coherence nuclear magnetic resonance (HSQC NMR), gel permeation chromatography (GPC), and thermogravimetric analysis (TGA) were carried out, to confirm the possibility of using the proposed SDES system for lignin dissolution and extraction processes.
2. Materials and Methods
2.1. Materials
Poplar wood chips were provided by a pulp mill in Shandong, China; 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, analytical reagent (AR), 99%), ethylene glycol (EG, AR, 98.0%), n-propanol (PrOH, AR, 99.5%), sec-butanol (sBuOH, AR, 99.5%), ethanolamine (ETA, AR, ≥99.0%), decane (AR, 98%), 1,4-dioxane (AR, 99%), tetrahydrofuran (THF, high-pressure liquid chromatography grade, ≥99.9%), syringic acid (AR, 98%), vanillic acid (AR, 98%), syringaldehyde (AR, 98%), ferulic acid (AR, 99%), alkaline lignin (Biopurity), ammonium sulfamate (AR, 99%), imidazole (AR, 99%), dimethyl sulfoxide-d6 (DMSO-d6, 99.9%), and potassium bromide (KBr, spectrum pure) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Methanol (MeOH, AR, ≥99.5%), glycerol (Gly, AR, ≥99.0%), isopropanol (iPrOH, AR, ≥99.7%), tert-butanol (tBuOH, AR, ≥99.0%), choline chloride (AR, 98.0~101.0%), acetic anhydride (AR, ≥98.5%), and urea (AR, ≥99.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ethanol (EtOH, AR, ≥99.7%), benzene (AR, ≥99.5%), n-hexane (AR, ≥97.0%), and butanol (BuOH, AR, ≥99.5%) were purchased from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China). Hexanol (HexOH, AR, ≥99.0%), octanol (OctOH, AR, ≥99.0%), and pyridine (AR, ≥99.5%) were purchased from the Tianjin Damao Chemical Reagent Factory (Tianjin, China). Acetone (AR, ≥99.5%) was purchased from the Yantai Far East Fine Chemical Co., Ltd. (Yantai, China). All chemicals were used as received, without further purification.
2.2. Preparation of Switchable Ionic Liquids
Switchable ionic liquids (SILs) were synthesized according to the method described in the literature [42,49], where DBU and alcohols (MeOH, EtOH, EG, PrOH, iPrOH, Gly, BuOH, sBuOH, tBuOH, HexOH, OctOH, and ETA) were mixed at a 1:1 molar ratio and stirred at 25 °C. Transparent and uniform SIL systems were obtained, namely, DBU–MeOH, DBU–EtOH, DBU–EG, DBU–PrOH, DBU–iPrOH, DBU–Gly, DBU–BuOH, DBU–sBuOH, DBU–tBuOH, DBU–HexOH, DBU–OctOH, and DBU–ETA. These SILs presented switchable physical and chemical properties upon the absorption or release of CO2. According to the method used by [50], polar SIL (SILCO2) can be obtained by bubbling CO2 into SIL under room temperature conditions for 30 min until the viscosity of the system increases significantly, whereas non-polar SIL (SIL′) can be restored by heating the SILCO2 at 80 °C for 10 h.
2.3. Preparation of Switchable Deep Eutectic Solvents
The switchable deep eutectic solvents (SDES) were synthesized according to the method employed by [51], by using SIL as the hydrogen-bond acceptor (HBA) and water (H2O) as the hydrogen-bond donor (HBD). The HBA and HBD were mixed at a 1:10 to 10:1 weight ratio and stirred at 25 °C. A transparent and uniform SDES system was obtained, namely, DBU–HexOH/H2O. DBU–HexOH/H2O demonstrated switchable physical and chemical properties upon the absorption or release of CO2. Polar SDES (SDESCO2) can be obtained by bubbling CO2 into SDES at room temperature for 30 min until the viscosity of the system increases significantly, whereas non-polar SDES (SDES′) can be restored by heating the SDESCO2 at 80 °C for 10 h.
2.4. Characterizations of Solvent Properties
The pH of the testing sample was detected using a pH meter (PHS-3G, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China) at 25 °C. The conductivity of the testing sample was detected using a conductivity meter (DDS-307A, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China). The viscosity of the DES was measured according to the method described in [49], by placing 1 g of sample solution on the fixture of a rotational rheometer (ARES-G2, TA Instruments Vorster, New Castle, DE, USA), operating at 30 rpm. The polarity of the sample to be measured is illustrated by their miscibility in a low polarity reagent (decane) [42].
2.5. Preparation of Milled Wood Lignin
The milled wood lignin (MWL) was prepared according to the method recorded in [52]. A specific amount of 80~40 mesh poplar powder was extracted by a solution with a volume ratio of benzene to ethanol of 2:1, and the dried raw material was placed in a planetary ball mill (Pulverisette 5, FRITSCH, Markt Einersheim, Germany). The milled sample was extracted with 1,4-dioxane and deionized water at a volume ratio of 96:4 (v/v). The extract was centrifuged, and the supernatant was evaporated, concentrated, and freeze-dried to obtain the MWL.
2.6. Solubility of the Lignin Model Compound
Lignin solubility was detected according to the method described in [53]. Lignin and its model compounds were added to the testing solvent under continuous stirring at room temperature—25 °C—to fully dissolve until saturation. The liquid phase was separated by filtration using a 0.45 μm organic filter, and the absorption of the dissolved lignin was determined using an ultraviolet-visible spectrophotometer (Agilent Cary 8454, Agilent Technologies, Santa Clara, CA, USA). The lignin concentration was then calculated using Lambert–Beer’s law. The characteristic absorption peaks for lignin and lignin model compounds were listed as follows: alkali lignin 280 nm, syringaldehyde 307 nm, vanillic acid 256 nm, syringic acid 265 nm, and ferulic acid 314 nm [54].
2.7. Dissolution and Regeneration of Lignin
Lignin dissolution and regeneration were carried out according to the method employed in [9,55]. MWL was added into the testing solvents (1:100, w/w) under continuous stirring at room temperature. The fully dissolved lignin solution was filtrated with a 0.45 μm organic filter. Acetone was added to the lignin solution (1:10, v/v) as an anti-solvent and then kept at 4 °C for 12 h to enable lignin regeneration. The precipitate was recovered using centrifugation (8000 rpm, 10 min) followed by filtration and then oven-dried at 105 °C until a constant weight was achieved.
2.8. Characterizations of Lignin
The number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity index (PDI) were measured by gel permeation chromatography (GPC, e2695, Agilent Technologies Inc., Palo Alto, CA, USA) equipped with an Agilent 1200 series high-performance liquid chromatograph (HPLC) and an ultraviolet detector (UV) [34,56]. Acetylated lignin samples (2 mg) were dissolved in THF (1 mL) and filtered through a 0.45 μm filter. The injection volume was 100 μL, and the wavelength of the UV detector was 280 nm. THF was used as the mobile phase under a flow rate of 100 mL/min. A calibration curve was prepared using polystyrene in the range of 1480~1,233,000 g/mol. The thermal oxidative degradation and stability of the lignin samples were subjected to thermogravimetric analysis (TGA SDT650, TA Instruments, Milford, MA, USA) [57]. The lignin samples were placed in aluminum crucibles and tested between room temperature and 800 °C at a heating rate of 10 °C/min under nitrogen conditions.
The structural characterization of the lignin samples was carried out using Fourier-transform infrared spectroscopy (FTIR, Bruker ALPHA, Ettlingen, Germany) [3]. A total of 1 mg lignin sample was mixed with 100 mg dried KBr, ground, and pressed into tablets. Samples were scanned 32 times over a range of 400~4000 cm–1 at a resolution of 4 cm−1. Detailed structural characterization was performed using nuclear magnetic resonance (NMR) spectroscopy (HSQC NMR, BRUKER AVANCE III HD 500 M, Karlsruhe, Germany); around 50 mg of lignin was dissolved in 0.5 mL of DMSO-d6 [58]. NMR spectra of lignin samples were obtained using a Bruker Avance III HD500 MHz spectrometer at a room temperature of 25 °C. The 1H-13C heteronuclear single-quantum coherence (HSQC) spectral standard pulse sequence was as follows: spectral width 1H of 11 ppm with 2048 sampling points; 13C spectral width of 190 ppm with 256 data points; 64 scans; and 1 s scan delay. Volume integration of the signals in the HSQC NMR spectra was performed in Bruker Top Spin 2.1 software.
2.9. Molecular Simulation
The molecular simulation was conducted using the conductor-like screening model for real solvents (COSMO-RS) model (BIOVIA COSMOtherm 2020, Version 20.0.0, Dassault Systèmes, Paris, France), in which quantum chemical calculations were combined with statistical mechanics to explore the thermodynamic behaviors of the chemicals used in this work.
The structure of DBUH and HexCO3 was drawn by Turbomole (BIOVIA TmoleX 2021, Version 21.0.0, Dassault Systèmes, Paris, France). The geometry optimizations were performed at the density functional theory (DFT) level and utilized the BP function with resolution of identity (RI) approximation; a triple-ξ valence polarized basis set (TZVP) was utilized [59,60]. All the other chemicals were obtained from the built-in database. For the COSMO-RS calculations, it was assumed that ILs were set as a mixture of an equimolar composition of cations and anions, and the DESs were set as a mixture of an equimolar composition of HBAs and HBDs [61].
3. Results and Discussion
3.1. Characterizations of Switchable Ionic Liquids
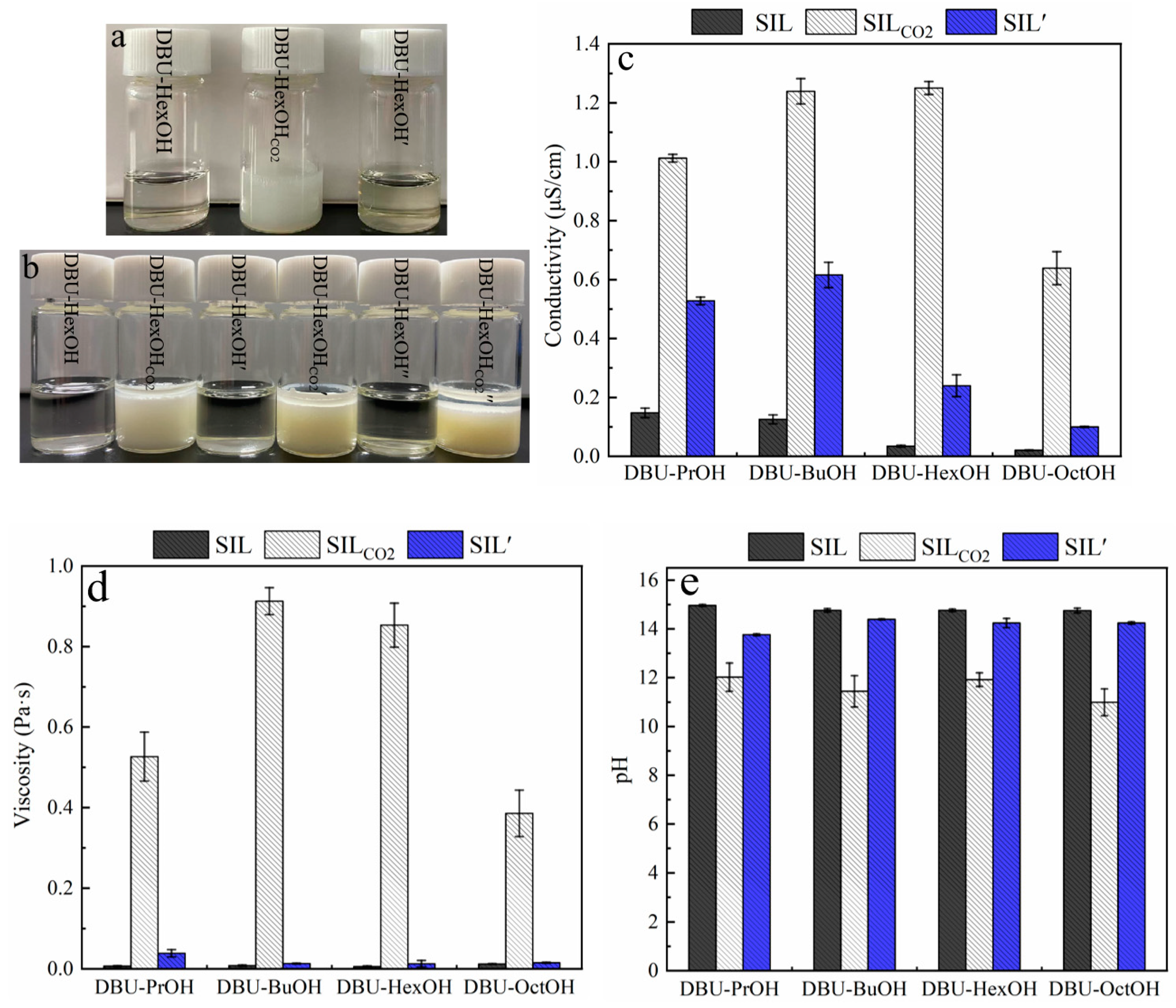
As shown in Figure 2a, a transparent and homogeneous SIL was obtained by simply mixing DBU and monohydric alcohol at room temperature. According to Figure 1, bubbling CO2 into SIL caused the formation of its ionic state (SILCO2), leading to a significant increase in viscosity, and sometimes even the formation of solids (Table A1). Among the testing SIL systems, most of the SILCO2 remained as a transparent and homogeneous liquid. For example, DBU–PrOHCO2, DBU–BuOHCO2, DBU–HexOHCO2, and DBU–OctOHCO2 were still viscous liquids in the presence of CO2. However, DBU–MeOHCO2 and DBU–ETACO2 formed rigid solids. The CO2 in SILCO2 was removed by heating at 80 °C, after which a transparent SIL’ was obtained. As the presented work focuses on the dissolution and extraction of lignin, the proposed solvent system should maintain the liquid state either with or without the presence of CO2. The solvent properties of the SIL system were then investigated; there were notable changes in SIL polarity, conductivity, pH, and viscosity triggered by introducing and removing CO2 (Figure 2).
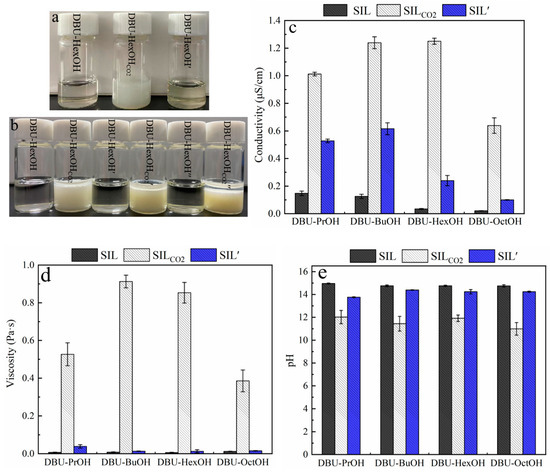
Figure 2.
Switchable solvent properties of SILs triggered by CO2: (a) effect of CO2 on the state of DBU–HexOH SIL system; (b) effect of CO2 on the miscibility of DBU–HexOH in decane; (c) effect of CO2 on the electric conductivity of DBU-based SILs; (d) effect of CO2 on viscosity of DBU-based SILs; and (e) effect of CO2 on pH of DBU-based SILs.
The polarity of the SIL system can be regulated reversibly by introducing and removing CO2. This polarity conversion was verified by mixing SILs with a low-polarity reagent (decane). Taking the DBU–HexOH SIL system as an example, the DBU–HexOH is miscible with decane, whereas its ionic form (DBU–HexOHCO2) is not. As shown in Figure 2b, the conversion in polarity remained after three CO2 charging–discharging cycles. Pumping CO2 through the homogenous SIL–decane solution caused the formation of emulsion, while simply removing CO2 by heating restored miscibility. DBU–PrOH, DBU–BuOH, and DBU–OctOH SIL systems (Table A1) demonstrated a similar polarity conversion performance to that of DUB–HexOH. Other SIL systems, however, encountered difficulties in restoring their non-polar state from SILCO2 during the heating process, possibly due to the strong interaction formed between the alcohol compound and CO2 [62]. Our study, therefore, focused on the analysis of DBU–PrOH, DBU–BuOH, DBU–HexOH, and DBU–OctOH SIL systems.
The formation of ionic compounds was confirmed by analyzing the conductivity and viscosity of the SIL systems. Generally, SIL and SIL′ demonstrated significantly lower conductivity than that of SILCO2. Alcohol and DBU (0.012 μS/cm) both possess low electroconductivities. As expected, the SILs generated by mixing DBU and alcohols produced low conductivities. The conductivity of SIL, shown in Figure 2c, decreased with the growth of carbon chains, and the conductivity of DBU–PrOH, DBU–BuOH, DBU–HexOH, and DBU–OctOH gradually decreased from 0.148 μS/cm to 0.021 μS/cm. Introducing CO2 allowed the SIL to convert into its ionic form, producing a dramatic increase in conductivity in the SILCO2. Among all the tested SIL systems, DBU–HexOHCO2 presented the highest conductivity of 1.250 μS/cm. Discharging CO2 allowed the electroconductivity to be restored. However, the conductivity of SIL′ was generally higher than that of the SIL system; this could be attributed to the incomplete removal of CO2 during the heating process. In terms of their viscosities, SILs presented slightly higher viscosities than those of DBU (0.0005 Pa·s) and alcohols (~10−4 Pa·s). As shown in Figure 2d, the viscosities of SILs were within the range of 0.007 Pa·s–0.0126 Pa·s. Similar to conductivity, charging with CO2 caused a surge in SIL viscosity, especially for the DBU–BuOHCO2 (0.9127 Pa·s) and DBU–HexOHCO2 (0.8531 Pa·s) samples. Similarly, viscosity was restored by removing CO2 from the system. The significant increase in viscosity relates to the formation of an ionic compound: This created more and stronger ionic interactions, enhancing the internal friction in SILCO2. As shown in Figure 2, the DBU–BuOH and DBU–HexOH SIL systems demonstrated the most notable switching ability for conductivity and viscosity, whereas DBU–BuOHCO2 (Figure 2c) presented some difficulties in terms of CO2 release when heated. Although there was only a minor difference in their SIL and SIL’ viscosities, the conductivity of DBU–BuOH’ (0.616 μS/cm) was much higher than that of DBU–BuOH (0.126 μS/cm), indicating the incomplete removal of CO2. Therefore, DBU–HexOH was deemed the preferred SIL system, as its solvent properties can easily be restored from 1.250 μS/cm to 0.240 μS/cm, demonstrating a preferred switching ability.
DBU is a strong organosuperbase with a pH of 15.01; SILs composed of DBU and alcohols, therefore, also present strong basicity. As shown in Figure 2e, no noticeable variations in the pH of DBU–PrOH, DBU–BuOH, DBU–HexOH, or DBU–OctOH, which ranges from 14.96 to 14.75, could be obtained. Charging with CO2 caused the formation of SILCO2 and decreased its pH to around 12, while removing CO2 restored the SIL pH to about 14, which is close to that of SIL. Therefore, the SIL system pH was also regulated by charging and discharging CO2. For example, DBU–HexOH had a pH of 14.76; charging CO2 decreased the pH to 11.92 (DBU–HexOHCO2), and removing CO2 by heating restored the pH to 14.24 in DBU–HexOH’. However, it is proposed that a strong basicity environment may affect the aromatic structure of lignin, destroy the C–O single bond in lignin, and lead to a significant chemical modification of lignin macromolecules [63]. It would be wise to introduce a cosolvent to moderate the alkaline operating condition, as this may ease the side reactions. In addition, the cosolvent would help reduce the high viscosity of SILCO2, which may prohibit lignin dissolution.
3.2. From Switchable Ionic Liquids to Switchable Deep Eutectic Solvents
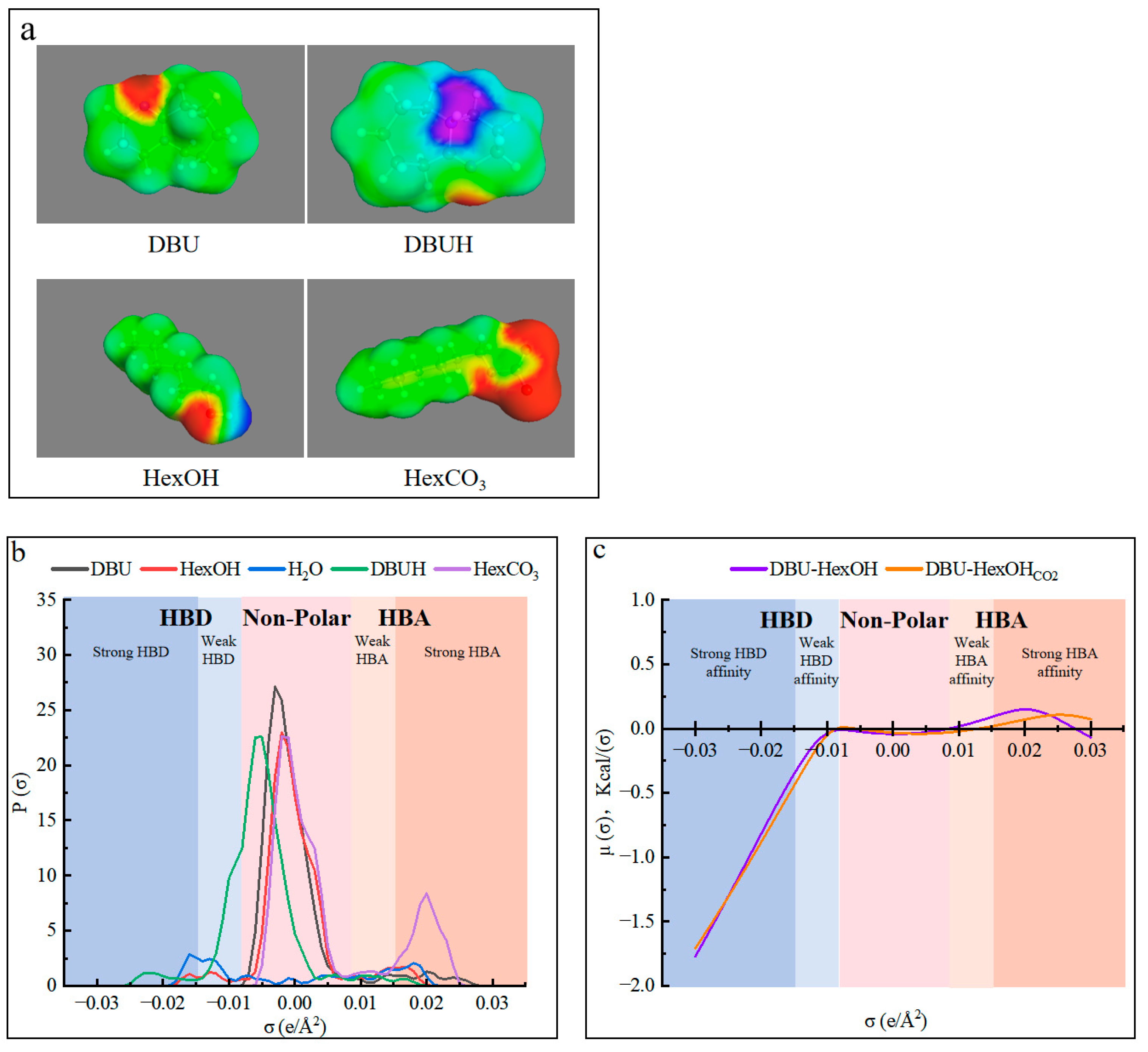
The use of cosolvent has been reported to enhance the solubility of lignin derivatives [54,64]. However, for this specific use, it should be carefully selected to stabilize the SIL system and promote the hydrogen-bond network within. The molecular simulation was, therefore, conducted with the conductor-like screening model for real solvents (COSMO-RS), in which quantum chemical calculations were combined with statistical mechanics to explore the thermodynamic behavior of the DBU and HexOH used in this work [61]. As was previously demonstrated, charging CO2 into SIL caused the formation of ionic SILCO2. The molecule structures and their charge distributions of the chemicals in the DBU–HexOH SIL system are shown in Figure 3. As can be seen in Figure 3a, charging CO2 caused the formation of a strong hydrogen-bond donor (HBD) center (blue-purple area) on the DBUH from DBU. In addition, a strong hydrogen-bond acceptor (HBA) zone (red area) was created on the HexCO3 from the HexOH. Therefore, in addition to the strong ionic interaction in SILCO2, possible hydrogen bonds could also exist in the DBU–HexOHCO2 mixtures. Figure 3b shows the charge distribution on the molecular surface, where the σ values lower than −0.0082 e/Å2 are the HBD region, σ values between −0.0082 e/Å2 and 0.0082 e/Å2 represent the non-polar region, and values higher than 0.00821 e/Å2 are the HBA region. As shown in Figure 3b, DBU and HexOH acted as mild polar molecules, as most of their peaks were located in the non-polar and weak HBD (−0.0082~−0.015 e/Å2)/weak HBA (0.0082~0.015 e/Å2) regions. According to the reaction scheme (Figure 1), charging with CO2 converted them into DBUH and HexCO3, where a strong HBD peak at −0.021 e/Å2 and an HBA peak at 0.020 e/Å2 are noticed. The sigma potential results in Figure 3c indicate that only a small change in the mixture was induced by CO2 charging, both DBU–HexOH and its ionic form (DBU–HexOHCO2) presenting a strong HBD affinity. Due to charging with CO2, the formation of ionic substances in SILCO2, therefore, mainly contributes to the internal ionic interactions and only produces a minor effect on the overall hydrogen-bonding ability of the mixture. In addition, based on the COSMO-RS modeling results, introducing a cosolvent such as an HBD is an effective method of enriching the hydrogen-bonding network inside the proposed SIL solvent system. Water normally acts as both an HBD and HBA, presenting characteristic peaks located at −0.016 and 0.017 e/Å2, respectively. The addition of water allowed it to perform as an ideal HBD in both the non-ionic (SIL) and ionic (SILCO2) states of the DBU–HexOH system. As shown in Figure 3b, water presented peaks both in the strong (−0.016 e/Å2) and weak (−0.013 e/Å2) HBD regions, both of which overwhelmed the HBD assignments within the DBU–HexOH and DBU–HexOHCO2 (DBUH and HexCO3) systems. Therefore, the addition of an HBD as a cosolvent enriched the hydrogen-bond network within the DBU–HexOH SIL system. In this case, the SIL/SILCO2 worked as the HBA and water acted as the HBD within the mixture system.
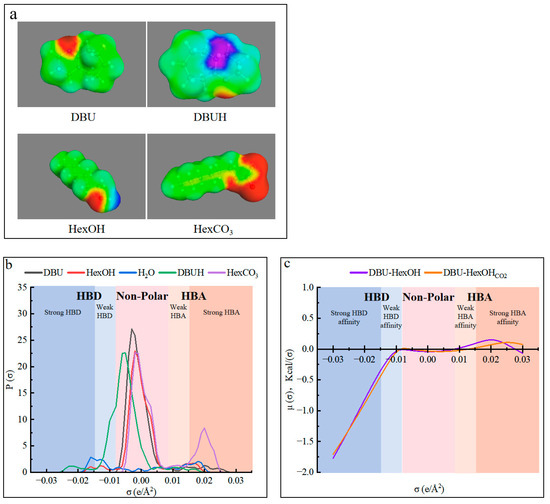
Figure 3.
Molecular modeling results from COSMO-RS: (a) surface charge distribution for DBU and HexOH before and after charging CO2; (b) sigma profiles of water and the chemicals in the SIL system; and (c) sigma potentials for the DBU–HexOH and DBU–HexOHCO2 mixtures.
Based on the COSMO-RS thermodynamic modeling results, a switchable deep eutectic solvent (SDES) system was proposed by adding HBDs as cosolvents into the SILs to improve their hydrogen-bonding networks. The addition of the HBD should be able to reduce the viscosity and cost of the solvent, expand the liquid range, and enhance the solubility [36,37]. In this work, SIL and various HBDs were mixed (Figure A1); water was selected as an ideal HBD for the formation of a stable homogeneous and transparent DBU–HexOH/H2O system with a 1:5 mass ratio, either with or without the presence of CO2 (Figure A2). It can be seen from Table A1 that charging CO2 into SDES caused the elevation of conductivity from 4.848 μS/cm (DBU–HexOH/H2O) to 23.030 μS/cm (DBU–HexOH/H2OCO2), and removing CO2 allowed the electroconductivity to be restored. However, the conductivity of DBU–HexOH/H2O′ (10.066 μS/cm) was higher than that of DBU–HexOH/H2O; this could be attributed to the incomplete removal of CO2 due to the presence of water. Charging with CO2 also increased the viscosity of the SDES system, from 0.0186 Pa·s (DBU–HexOH/H2O) to 0.0825 Pa·s (DBU–HexOH/H2OCO2). Moreover, releasing CO2 by heating allowed the viscosity of the DBU–HexOH/H2O to be restored to 0.0466 Pa·s. It should be noted that the addition of the HBD significantly reduced the viscosity in the ionic state to about 1/10 of that for the SIL system (0.8531 Pa·s for SILCO2 vs. 0.0825 for SDESCO2). In addition, the pH of the DBU–HexOH/H2O was 14.26: Charging with CO2 decreased the pH to 9.50 (DBU–HexOH/H2OCO2), which was much lower than that of SILCO2 (DBU–HexOHCO2, pH 11.92). Therefore, the proposed SDES, composed of a mixture of DBU–HexOH and water, could moderate the viscosity and pH of the SIL system, while allowing it to maintain its switchable nature, making the SDES more suitable for the lignin dissolution process.
3.3. Lignin Dissolution in Switchable Solvents
The solubility of lignin model compounds in the SIL and SDES systems is shown in Table 1. The DBU–HexOH SIL system demonstrated limited lignin solubility, dissolving 12.12 mg/g syringic acid, 2.01 mg/g vanillic acid, 2.24 mg/g syringaldehyde, 22.87 mg/g ferulic acid, and 10.88 mg/g alkaline lignin at room temperature. However, its ionic form (DBU–HexOHCO2) did not demonstrate any lignin solubility. This phenomenon is very similar to that of DBU, which is a major component in SIL. DBU dissolved 22.79 mg/g syringic acid, 4.54 mg/g vanillic acid, 1.17 mg/g syringaldehyde, 2.26 mg/g ferulic acid, and 1.91 mg/g alkaline lignin at room temperature but did not demonstrate lignin solubility after being charged with CO2. As the other component in SIL, monohydric alcohol, however, demonstrated a different lignin solubility. Both HexOH and HexOHCO2 presented a very small but similar solubility to vanillic acid, syringaldehyde, and ferulic acid. However, charging with CO2 increased their solubility for syringic acid, and alkaline lignin increased from 34.9 mg/g and 9.72 mg/g to 40.24 and 16.30, respectively. Although water presented limited lignin solubility and no notable change after charging with CO2, the proposed DBU–HexOH/H2O SDES system, mixed with SIL and water, demonstrated a notable increase in lignin solubility. The solubility of syringic acid, vanillic acid, syringaldehyde, and ferulic acid in DBU–HexOH/H2O achieved 207.58 mg/g, 21.95 mg/g, 7.98 mg/g, and 58.12 mg/g, respectively. These could be further increased by charging CO2 into the system. The solubility of syringic acid, vanillic acid, syringaldehyde, and ferulic acid in the DBU–HexOH/H2OCO2 system was 230.57 mg/g, 78.43 mg/g, 11.64 mg/g, and 452.17 mg/g, respectively. Therefore, the lignin solubility in the SDES system could also be regulated by the addition of CO2, and this would vary depending on the type of lignin model compounds. Particularly, for vanillic acid and ferulic acid, after charging CO2 into SDES, their solubility increased by 357% and 778%, respectively. However, the solubility of alkaline lignin in SDES decreased from 5.67 mg/g to 4.44 mg/g after forming its ionic state. Therefore, the proposed SDES mixed with DBU–HexOH and H2O demonstrated improved lignin solubility, and this was also regulated by charging and discharging CO2.

Table 1.
Solubility of switchable solvent system to lignin model compounds (25 °C, mg/g).
The proposed SDES system was further tested for the dissolution of milled wood lignin (MWL). The DBU–HexOH SIL dissolved 9.05 mg/g MWL, while the DBU–HexOH/H2O SDES dissolved up to 213.35 mg/g MWL. Charging CO2 into the SDES further increased MWL solubility to 279.12 mg/g. The SDES-dissolved lignin was easily regenerated using acetone as an anti-solvent, with a yield of 84.31% (SDESCO2–MWL). The variations in weight average molecular weight (Mw), number average molecular weight (Mn), and the polydispersity index (PDI) before and after SDES treatment were tested using gel permeation chromatography (GPC); the results are given in Table 2. In comparison with the MWL lignin, both regenerated lignin samples demonstrated a higher molecular weight. The native lignin sample (MWL) was 7701 g/mol Mw and 2959 g/mol Mn, with a PDI of 2.60. The SDES-treated MWL sample had a similar PDI to that of its raw material (MWL), but the regenerated lignin demonstrated an elevated molecular weight of 9823 g/mol Mw and 3397 g/mol Mn. SDESCO2 treatment resulted in a lignin stream with a much higher molecular weight (Mw = 10,340 g/mol, Mn = 7672 g/mol) and with an improved uniformity (PDI = 1.35). Compared with the traditional DES–lignin (Table 2), the lignin stream produced in this work had a well-preserved long-chain structure and a much higher molecular weight. Therefore, the GPC results indicate that the SDES treatment is a promising method for lignin dissolution and extraction, as it not only regulated the lignin solubility with CO2 but also produced a lignin stream with a high molecular weight and improved uniformity, both of which may benefit the downstream process.

Table 2.
Molecular weights and polydispersity indices of lignin.
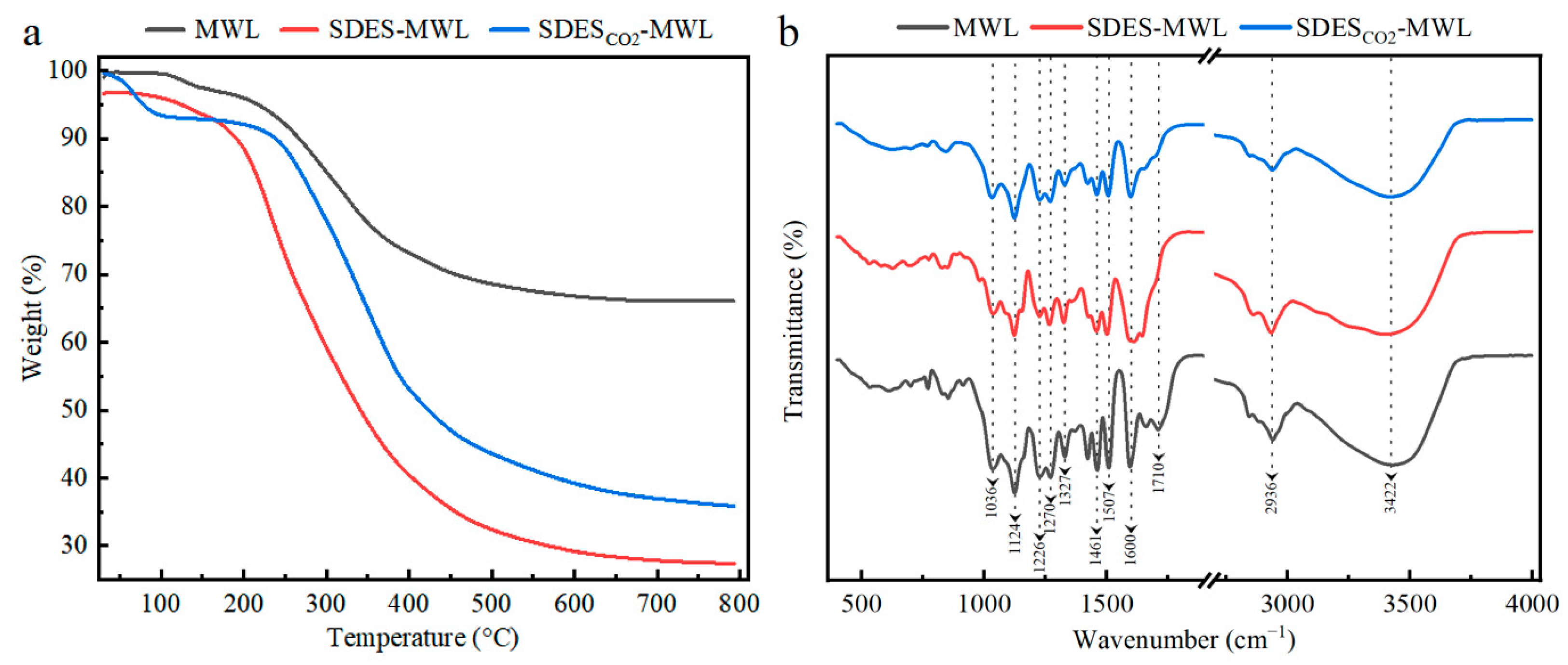
The thermal oxidative degradation and stability of the MWL before and after SDES treatments were investigated using the thermogravimetric analysis (TGA) [65]. As shown in Figure 4a, all the testing samples exhibited a similar thermal performance, divided into three stages. The first stage was the initial degradation stage (80~200 °C), most likely caused by the removal of moisture and volatile components [66]. The second stage was the main degradation stage of lignin (200~400 °C), where lignin degradation occurred. Carboxylation breakdown of aliphatic hydroxyl groups and ether bonds present in the structure of lignin occurred, while degradation can be attributed to the side chain dehydrogenation reaction [67]. Both SDES–MWL and SDESCO2–MWL samples demonstrated a more significant mass loss, which is possibly due to the partial breakage of connections between lignin macromolecules after regeneration. The third stage was the carbonization stage (400~600 °C), where methoxy groups and C–C bonds of lignin were disrupted, with the release of volatiles and production of bio-oils [68]. The decomposition of the lignin sample in Figure 4a occurred slowly at this stage, resulting in char residue [69]. This could be attributed to the production of highly branched and extremely condensed aromatic structures. The MWL sample produced a high char residue rate of 66.7%; this could be attributed to the fact that the sample had a highly complex and condensed lignin structure. MWL is easily converted to char residue owing to its structural resemblance [68]. Once the MWL had reached a temperature of 600 °C, the quality of the coke was stable and no further reaction occurred. However, both SDES- and SDESCO2-treated MWL produced lower char residue rates: 28.3% and 1.3%, respectively. This indicates the lower thermal stability of SDES–MWL and SDESCO2–MWL compared with that of the MWL sample.
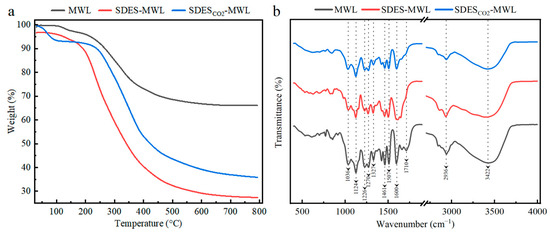
Figure 4.
Thermogravimetric analysis (a) and Fourier-transform infrared spectroscopy (b) of lignin samples.
Structural characterization of lignin before and after SDES treatment was performed using Fourier-transform infrared (FTIR) spectroscopy; the assignments of the characterizing peaks are shown in Table A2. As shown in Figure 4b, the stretching vibration for hydroxyl is observed at 3422 cm−1 [70]. The stretching vibrations for the lignin aromatic ring skeleton at 1600 cm−1 and 1507 cm−1 can be seen in all testing samples; this indicates that no significant change in the aromatic ring structure of MWL occurred during the SDES treatments [34,71]. The peak at 2936 cm−1 was attributed to the C–H vibration in CH3-/CH2 = groups. The characteristic peaks at the wave number of 1461 cm−1 were assigned to the C–H asymmetric vibration of CH2 = groups and C–H transformation in the aromatic rings [3]. All the characteristic peaks mentioned above were found in all the testing samples. Stretching vibrations for C=O bonds are typically found between 1740 and 1700 cm–1; this is where signals attributable to C=O bonds in unconjugated ketones, carbonyls, and ester groups are normally observed. Lower absorption energies (around 1700 cm–1) were reported for conjugated aldehydes and carboxyl acids [72]. MWL produced a characteristic peak at 1710 cm–1, while SDES–MWL and SDESCO2–MWL demonstrated lower intensities within this range, indicating lower carbonyl and carboxyl group content. The peak at 1327 cm−1 was attributed to the stretching vibration of the C–O in the syringyl (S) lignin unit, whereas the peaks at 1270 cm−1 represent the stretching vibration of the C–O in the guaiacyl (G) lignin unit [73]. The peak at 1124 cm−1 demonstrates the presence of syringyl moieties in lignin because it represents the C–H deformation of the lignin S unit. Therefore, a feature of GS-type lignin was demonstrated in the FTIR spectra. Additionally, the signal at 1036 cm−1, corresponding to the C–O deformation of primary –Ohs, was detected in all lignin fragments [74]. The FTIR spectra did not show any noticeable chemical modifications occurring in MWLs during the SDES treatments.
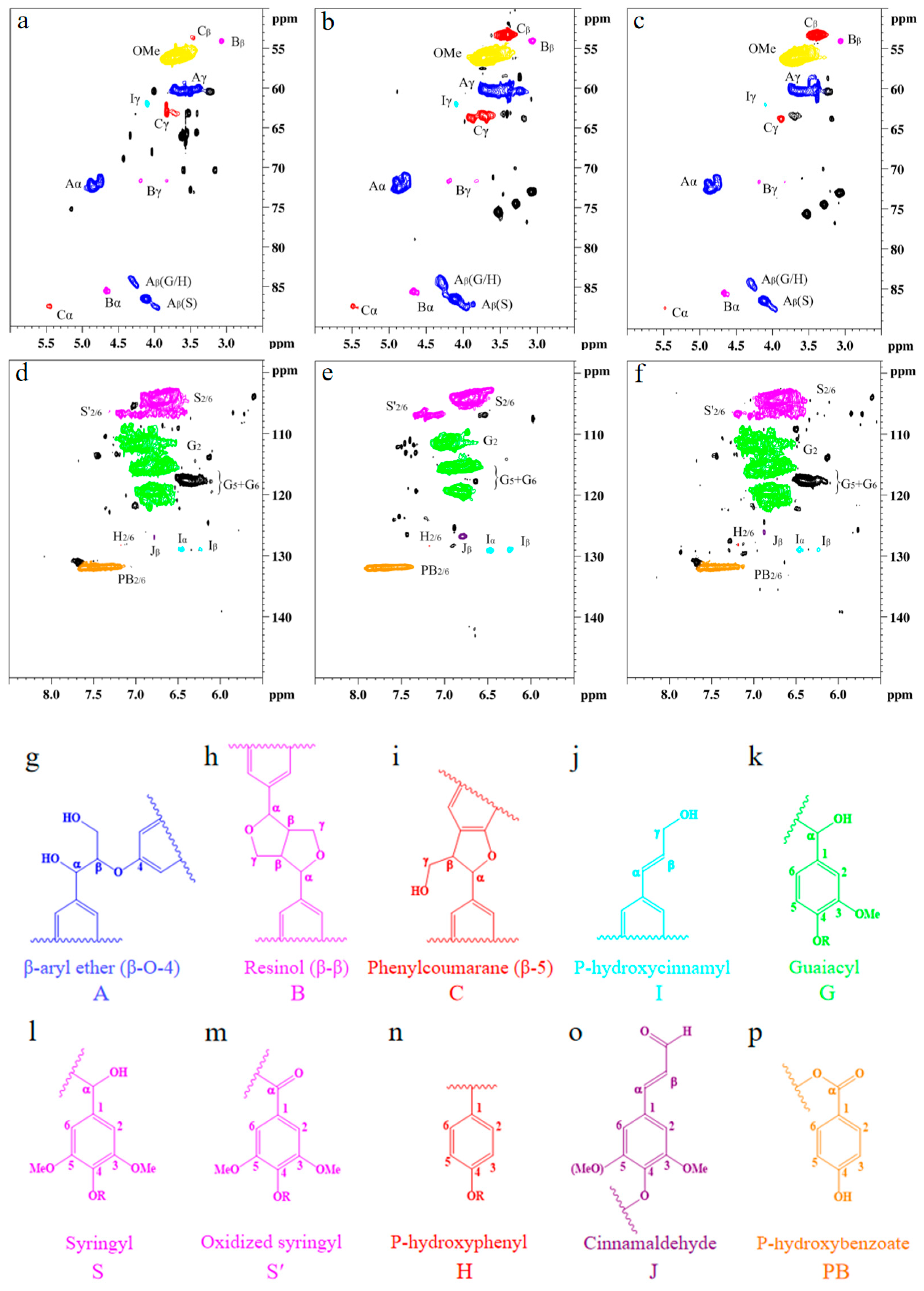
Detailed structural analysis of MWL and SDES–MWL and SDESCO2–MWL was characterized using 2D HSQC NMR spectroscopy [75,76]; the side chain (δC/δH 50–90/2.5–6.0) and the aromatic (δC/δH 100–135/5.5–8.5) regions of the lignin samples are shown in Figure 5, and the main lignin cross-signal assignments are listed in Table A3. The related signals for β-aryl ether ethers (A), resin alcohols (B), and phenylcoumaranes (C) in the lignin samples were found in all the testing samples, indicating a well-preserved lignin structure after treatment. The peaks corresponding to β-aryl ether connections are shown for Aα (δC/δH 71.8/4.86 ppm), Aγ (δC/δH 59.5–59.7/3.40–3.63 ppm), A′γ (δC/δH 63.2/4.33–4.49 ppm), Aβ(G/H) (δC/δH 83.9/4.29 ppm), and Aβ(S) (δC/δH 85.9/4.12 ppm). β-β connections are shown for Bα (δC/δH 84.8/4.65 ppm), Bβ (δC/δH 53.5/3.06 ppm), and Bγ (δC/δH 71.0/3.82 and 4.18 ppm). β-5 connections are shown for Cα (δC/δH 86.8/5.46 ppm), Cβ (δC/δH 53.3/3.46 ppm), and Cγ (δC/δH 62.5/3.73 ppm). Moreover, methoxyl connections were found at δC/δH 55.6/3.73 ppm. The signals for the Cα–Hα, Cβ–Hβ, and Cγ–Hγ correlations of substructures (I) were seen clearly at δC/δH 128.4/6.44, 128.2/6.25, and 61.4/4.10, respectively. It was found that all the lignin samples exhibited similar spectral patterns. Three main types of linkages, namely, aromatic ether bond β-O-4, β-β in resin, and β-5 in phenylcoumarane [73], were observed in all testing lignin samples, with only minor changes in their distributions. The MWL produced β-O-4 linkages of 40.71/100Ar, β-β linkages of 2.71/100Ar, and β-5 linkages of 3.57/100Ar cross-peaks; the SDES–MWL produced β-O-4 linkages of 38.29/100Ar, β-β linkages of 3.76/100Ar, and β-5 linkages of 4.03/100Ar; finally, the SDESCO2–MWL produced β-O-4 linkages of 38.27/100Ar, β-β linkages of 3.37/100Ar, and β-5 linkages of 3.63/100Ar. The main linkages within lignin macromolecules, therefore, remained unchanged after the SDES and SDESCO2 treatments, which agrees with the FTIR results. In addition, all the lignin samples exhibited strong signals for methoxy moieties, indicating that the –OCH3 groups also remained unchanged after the SDES and SDESCO2 treatments.
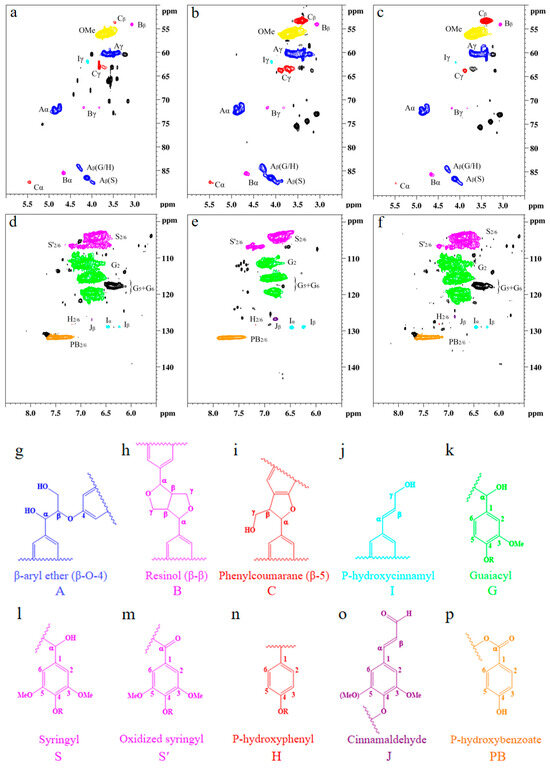
Figure 5.
Nuclear magnetic resonance analysis of lignin with or without treatment: (a) side-chain regions of the milled wood lignin; (b) side-chain regions of SDES-treated lignin; (c) side-chain regions of the SDESCO2-treated lignin; (d) aromatic regions of the milled wood lignin; (e) aromatic regions of the SDES-treated lignin; (f) aromatic regions of the SDESCO2-treated lignin; (g) β-O-4 aryl ethers; (h) resinol; (i) phenylcoumarane; (j) p-hydroxycinnamyl alcohol end group; (k) guaiacyl unit; (l) syringyl unit; (m) oxidized (Cα = O) syringyl unit; (n) p-hydroxyphenyl unit; (o) cinnamaldehyde end group; and (p) p-hydroxybenzoate. The abbreviations A, B, C, I, G, S, S’, H, J, and PB listed in (g–p) are used to indicate the different types of lignin structures shown in (a–f).
In the aromatic region, the C2,6–H2,6 correlations from S-type units and the sum of C2–H2, C5–H5, and C6–H6 correlations from G-type units were used to estimate the S/G ratio [74]. A strong signal at δC/δH 103.8/6.71 belonged to the S unit at C2,6–H2,6, whereas the G units showing the cross-peak signals for C2–H2 (δC/δH 110.9/6.98), C5–H5 (δC/δH 114.9/6.77), and C6–H6 (δC/δH 119.0/6.80) can be found in all the lignin samples. It can, therefore, be confirmed that the MWL used in this work was predominantly comprised of S and G units. The S/G ratios of MWL, SDES–MWL, and SDESCO2–MWL were 0.88, 0.73, and 0.76, respectively, indicating that the SDES and SDESCO2 treatments caused slightly more S-type lignin loss than G-type lignin loss. Other signals observed in the aromatic regions could be assigned to p-hydroxycinnamyl alcohol end groups (I), cinnamaldehyde end groups (J), and p-hydroxybenzoate substructures (PB). The C–H correlated signals for I and J were found at δC/δH 128.2/6.44 and 126.1/6.76 ppm, respectively. The C2,6–H2,6 correlations for PB were observed as a strong signal at δC/δH 131.2/7.67. These characterization results clearly demonstrate, again, that the lignin macromolecule framework remained intact following both the SDES and SDESCO2 treatments.
4. Conclusions
In this work, switchable ionic liquids (SILs) composed of DBU and different alcohols were screened, based on their solvent properties. DBU–HexOH was selected as the optimized SIL for its stable liquid state and notable switchable nature in conductivity, viscosity, and pH, created by the addition and removal of CO2. According to the thermodynamic molecular modeling results, the selected SIL was coupled with a hydrogen-bond donor (HBD) to form a switchable deep eutectic solvent (SDES) system, providing moderate viscosity and pH while maintaining its switchable nature. The DBU–HexOH SIL system resulted in a switchable lignin dissolution performance, which was regulated by charging and discharging CO2. The addition of water as an HBD improved lignin solubility in the SDES, while preserving its switchable nature. The DBU–HexOH SIL dissolved 9.05 mg/g MWL, while the DBU–HexOH/H2O SDES dissolved up to 213.35 mg/g MWL. Charging CO2 into the SDES further increased MWL solubility to 279.12 mg/g. The lignin dissolved in SDES systems can be regenerated using acetone as an anti-solvent. Both FTIR and HSQC results indicate that the SDES- and SDESCO2-treated lignin did not produce any notable chemical modifications compared to the MWL sample. Interestingly, the regenerated lignin treated by SDESCO2 produced a high molecular weight (Mw = 10,340 g/mol; Mn = 7672 g/mol) and improved uniformity (PDI = 1.35). On the other hand, the original MWL was only 7701 g/mol Mw and 2959 g/mol Mn, with a PDI of 2.60. Therefore, the presented work proposed a notable SDES system, whose solvent properties can be regulated by the charging and discharging of CO2. Moreover, it can be used for lignin dissolution and extraction to produce a lignin stream with a well-preserved structure, high molecular weight, and improved uniformity, all of which may facilitate the downstream process.
Author Contributions
Conceptualization, L.Q. and D.L.; methodology, L.Q. and J.C.; software, D.L. and Y.G.; modeling, Y.X. and L.Q.; validation, D.L. and M.Y.; formal analysis, D.L., Y.G. and M.Y.; resources, L.Q., J.C. and G.Y.; data curation, D.L. and Y.X.; writing—original draft preparation, D.L.; writing—review and editing, L.Q., G.Y. and M.H.; visualization, D.L. and L.Q.; supervision, L.Q. and J.C.; project administration, L.Q.; funding acquisition, L.Q., G.Y. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Provincial Key Research and Development Program of Shandong (Grant No. 2021CXGC010601), the National Natural Science Foundation of China (Grant No. 22108134), the Pilot Project for Integrating Science, Education and Industry (Grant No. 2022JBZ01-05), and the Taishan Scholar Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The work was financially supported by the Provincial Key Research and Development Program of Shandong (Grant No. 2021CXGC010601), the National Natural Science Foundation of China (Grant No. 22108134), the Pilot Project for Integrating Science, Education and Industry (Grant No. 2022JBZ01-05), and the Taishan Scholar Project.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Solvent properties of the proposed switchable ionic liquids and switchable deep eutectics.
Table A1.
Solvent properties of the proposed switchable ionic liquids and switchable deep eutectics.
| SIL/SDES | pH | Conductivity (μS/cm) | Viscosity (Pa·s) | Polarity |
|---|---|---|---|---|
| DBU–MeOH | 15.14 ± 0.16 | 0.307 ± 0.089 | 0.1095 ± 0.0972 | Non-polar |
| DBU–MeOHCO2 | - | - | - | Polar |
| DBU–MeOH′ | 14.48 ± 0.21 | 0.863 ± 0.161 | 0.0205 ± 0.1590 | Polar |
| DBU–EtOH | 15.08 ± 0.20 | 0.224 ± 0.037 | 0.1337 ± 0.1894 | Non-polar |
| DBU–EtOHCO2 | 12.48 ± 0.56 | 1.075 ± 0.365 | 0.6755 ± 0.3039 | Polar |
| DBU–EtOH′ | 14.58 ± 0.12 | 0.681 ± 0.003 | 0.0190 ± 0.0022 | Polar |
| DBU–EG | 14.32 ± 0.02 | 0.112 ± 0.010 | 0.0474 ± 0.0007 | Polar |
| DBU–EGCO2 | 12.83 ± 0.17 | 0.278 ± 0.024 | 2.0262 ± 0.0322 | Polar |
| DBU–EG′ | 13.25 ± 0.37 | 0.690 ± 0.245 | 0.2110 ± 0.0007 | Polar |
| DBU–PrOH | 14.96 ± 0.04 | 0.148 ± 0.016 | 0.0070 ± 0.0013 | Non-polar |
| DBU–PrOHCO2 | 12.02 ± 0.58 | 1.012 ± 0.013 | 0.5267 ± 0.0607 | Polar |
| DBU–PrOH′ | 13.76 ± 0.04 | 0.528 ± 0.013 | 0.0389 ± 0.0090 | Non-polar |
| DBU–PrOH | 15.01 ± 0.11 | 0.195 ± 0.011 | 0.1734 ± 0.1444 | Non-polar |
| DBU–iPrOHCO2 | 12.62 ± 0.93 | 1.066 ± 0.012 | 0.4049 ± 0.0453 | Polar |
| DBU–iPrOH′ | 14.33 ± 0.09 | 0.402 ± 0.006 | 0.0296 ± 0.2647 | Polar |
| DBU–Gly | 13.95 ± 0.35 | 0.063 ± 0.041 | 0.5086 ± 0.0017 | Polar |
| DBU–GlyCO2 | 13.27 ± 1.09 | 0.158 ± 0.061 | 1.2097 ± 0.0047 | Polar |
| DBU–Gly′ | 13.68 ± 0.23 | 0.163 ± 0.067 | 1.0338 ± 0.0145 | Polar |
| DBU–BuOH | 14.76 ±0.07 | 0.126 ± 0.015 | 0.0085 ± 0.0015 | Non-polar |
| DBU–BuOHCO2 | 11.44 ± 0.64 | 1.239 ± 0.043 | 0.9127 ± 0.034 | Polar |
| DBU–BuOH′ | 14.39 ± 0.02 | 0.616 ± 0.043 | 0.0138 ± 0.0009 | Non-polar |
| DBU–sBuOH | 14.94 ± 0.05 | 0.047 ± 0.010 | 0.0057 ± 0.0019 | Non-polar |
| DBU–sBuOHCO2 | 13.01 ± 0.14 | 0.799 ± 0.039 | 0.3782 ± 0.0769 | Polar |
| DBU–sBuOH′ | 14.38 ± 0.10 | 0.328 ± 0.259 | 0.0173 ± 0.0008 | Non-polar |
| DBU–tBuOH | 14.75 ± 0.30 | 0.018 ± 0.014 | 0.0005 ± 0.0003 | Non-polar |
| DBU–tBuOHCO2 | 13.49 ± 0.26 | 0.252 ± 0.047 | 0.3252 ± 0.1020 | Polar |
| DBU–tBuOH′ | 14.36 ± 0.46 | 0.202 ± 0.098 | 0.0458 ± 0.0012 | Non-polar |
| DBU–HexOH | 14.76 ± 0.05 | 0.035 ± 0.003 | 0.0060 ± 0.0019 | Non-polar |
| DBU–HexOHCO2 | 11.92 ± 0.28 | 1.250 ± 0.022 | 0.8531 ± 0.055 | Polar |
| DBU–HexOH′ | 14.24 ± 0.19 | 0.240 ± 0.037 | 0.0131 ± 0.0080 | Non-polar |
| DBU–OctOH | 14.75 ± 0.10 | 0.021 ± 0.001 | 0.0126 ± 0.0007 | Non-polar |
| DBU–OctOHCO2 | 10.99 ± 0.55 | 0.639 ± 0.056 | 0.3857 ± 0.0576 | Polar |
| DBU–OctOH′ | 14.24 ± 0.05 | 0.100 ± 0.002 | 0.0156 ± 0.0011 | Non-polar |
| DBU–ETA | 14.91 ± 0.05 | 0.100 ± 0.017 | 0.0116 ± 0.0007 | Polar |
| DBU–ETACO2 | - | - | - | - |
| DBU–ETA′ | - | - | - | - |
| DBU–HexOH/H2O | 14.26 ± 0.28 | 4.848 ± 0.422 | 0.0186 ± 0.0011 | Polar * |
| DBU–HexOH/H2OCO2 | 9.50 ± 0.60 | 23.030 ± 3.010 | 0.0825 ± 0.0205 | Polar * |
| DBU–HexOH/H2O′ | 11.19 ± 0.50 | 10.066 ± 2.854 | 0.0466 ± 0.0046 | Polar * |
Note: - Data that cannot be determined as the sample is solid; * Data test with the presence of water.

Table A2.
Assignment of Fourier-transform infrared spectrum analysis of lignin samples.
Table A2.
Assignment of Fourier-transform infrared spectrum analysis of lignin samples.
| Wavenumbers (cm−1) | Assignment (Bond) | MWL | SDES-MWL | SDESCO2-MWL |
|---|---|---|---|---|
| 3422 | O–H stretching vibration | √ | √ | √ |
| 2936 | C–H stretching vibration in methyl | √ | √ | √ |
| 1710 | C=O stretching vibration | √ | × | × |
| 1600, 1507 | Aromatic ring skeleton vibration | √ | √ | √ |
| 1461 | C–H deformation vibration in –CH2– | √ | √ | √ |
| 1373 | C–H bending vibration of aliphatic compounds in carbohydrate | √ | × | × |
| 1126, 1327 | C–O stretching vibration of syringyl units | √ | √ | √ |
| 1270 | C–O stretching vibration of guaiacyl units | √ | √ | √ |
| 1124 | C–H stretching vibration of syringyl units | √ | √ | √ |
| 1036 | C–H bending vibration of guaiacyl units | √ | √ | √ |

Table A3.
Assignments of 13C-1H correlation signals in the HSQC NMR spectra of MWL and SDES-MWL and SDESCO2-MWL.
Table A3.
Assignments of 13C-1H correlation signals in the HSQC NMR spectra of MWL and SDES-MWL and SDESCO2-MWL.
| Labels | δC/δH (ppm) | Assignment |
|---|---|---|
| Cβ | 53.3/3.46 | Cβ−Hβ in phenylcoumarane substructures (C) |
| Bβ | 53.5/3.06 | Cβ−Hβ in resinol substructures (B) |
| –OCH3 | 55.6/3.73 | C−H in methoxyls |
| Aγ | 59.5-59.7/3.40-3.63 | Cγ−Hγ in β-O-4′ substructures (A) |
| Iγ | 61.4/4.10 | Cγ−Hγ in p-hydroxycinnamyl alcohol end groups (I) |
| A′γ | 63.2/4.33-4.49 | Cγ−Hγ in γ-acylated β-O-4′ substructures (A′) |
| Cγ | 62.5/3.73 | Cγ−Hγ in phenylcoumarane substructures (C) |
| Bγ | 71.0/3.82 and 4.18 | Cγ−Hγ in resinol substructures (B) |
| Aα | 71.8/4.86 | Cα−Hα in β-O-4′ substructures (A) |
| Aβ(G/H) | 83.9/4.29 | Cβ−Hβ in β-O-4′ substructures linked to G/H units (A) |
| Bα | 84.8/4.65 | Cα−Hα in resinol substructures (B) |
| Aβ(S) | 85.9/4.12 | Cβ−Hβ in β-O-4′ substructures linked to S units (A) |
| Cα | 86.8/5.46 | Cα−Hα in phenylcoumarane substructures (C) |
| S2,6 | 103.8/6.71 | C2,6−H2,6 in etherified syringyl units (S) |
| S′2,6 | 106.2/7.23 and 7.07 | C2,6−H2,6 in oxidized (Cα = O) syringyl units (S′) |
| G2 | 110.9/6.98 | C2−H2 in guaiacyl units (G) |
| G5 | 114.9/6.77 | C5−H5 in guaiacyl units (G) |
| G6 | 119.0/6.80 | C6−H6 in guaiacyl units (G) |
| Iβ | 128.2/6.25 | Cβ−Hβ in p-hydroxycinnamyl alcohol end groups (I) |
| Iα | 128.4/6.44 | Cα−Hα in p-hydroxycinnamyl alcohol end groups (I) |
| PB2,6 | 131.2/7.67 | C2,6−H2,6 in p-hydroxybenzoate substructures (PB) |
| Jβ | 126.1/6.76 | Cβ−Hβ in cinnamaldehyde end groups (J) |
| H2,6 | 127.9/7.19 | C2,6−H2,6 in p-hydroxyphenyl units (H) |
Appendix B

Figure A1.
Effect of CO2 on the switchable deep eutectics composed of DBU–HexOH and various hydrogen-bond donors: (a) DBU–HexOH mixed with various hydrogen-bond donors and (b) DBU–HexOHCO2 mixed with various hydrogen-bond donors.
Figure A1.
Effect of CO2 on the switchable deep eutectics composed of DBU–HexOH and various hydrogen-bond donors: (a) DBU–HexOH mixed with various hydrogen-bond donors and (b) DBU–HexOHCO2 mixed with various hydrogen-bond donors.


Figure A2.
Switchable deep eutectics composed of DBU–HexOH and H2O at various proportions (DBU–HexOH/H2O (w/w)): (a) DBU–HexOH mixed with H2O at various proportions and (b) DBU–HexOHCO2 mixed with H2O at various proportions.
Figure A2.
Switchable deep eutectics composed of DBU–HexOH and H2O at various proportions (DBU–HexOH/H2O (w/w)): (a) DBU–HexOH mixed with H2O at various proportions and (b) DBU–HexOHCO2 mixed with H2O at various proportions.

References
- Xu, Y.Y.; Ren, T.T.; Wu, J.N.; Meng, G.H.; Yang, S.C.; Cui, L.; Liu, Z.Y.; Guo, X.H. Ultrasound-assisted formic acid–choline chloride deep eutectic solvent pretreatment of cotton straw to extracted lignin. J. Appl. Polym. Sci. 2023, 140, 54082–54094. [Google Scholar] [CrossRef]
- Dodge, L.A.; Kalinoski, R.M.; Das, L.; Bursavich, J.; Muley, P.; Boldor, D.; Shi, J. Sequential Extraction and Characterization of Lignin-Derived Compounds from Thermochemically Processed Biorefinery Lignins. Energy Fuels 2019, 33, 4322–4330. [Google Scholar] [CrossRef]
- Bagh, F.S.G.; Ray, S.; Peng, T. Optimizing conditions for using deep eutectic solvents to extract lignin from black liquor. Wood Sci. Technol. 2022, 56, 759–792. [Google Scholar] [CrossRef]
- Balk, M.; Sofia, P.; Neffe, A.T.; Tirelli, N. Lignin, the Lignification Process, and Advanced, Lignin-Based Materials. Int. J. Mol. Sci. 2023, 24, 11668. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.T.; Chua, A.S.M.; Ngoh, G.C. Strategizing Assistive Heating Techniques on Delignification of Empty Fruit Bunch with Incorporation of Deep Eutectic Solvent. Waste Biomass Valorization 2023, 14, 2801–2814. [Google Scholar] [CrossRef]
- Weng, S.X.; Zhang, G.X.; Hu, Y.; Bo, C.Y.; Song, F.; Feng, G.D.; Hu, L.H.; Zhou, Y.H.; Jia, P.Y. Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization. Int. J. Mol. Sci. 2023, 24, 1479. [Google Scholar] [CrossRef]
- Achinivu, E.C. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef]
- Shi, F.X.; Wang, Y.J.; Davaritouchaee, M.; Yao, Y.Q.; Kang, K. Directional Structure Modification of Poplar Biomass-Inspired High Efficacy of Enzymatic Hydrolysis by Sequential Dilute Acid-Alkali Treatment. ACS Omega 2020, 538, 24780–24789. [Google Scholar] [CrossRef]
- Li, X.-Y.; Guo, T.-S.; Li, M.-F.; Peng, F. Comparison of structure, thermal stability, and pyrolysis products of lignin extracted with ChCl-formic acid/lactic acid systems. J. Mater. Res. Technol. 2021, 14, 841–850. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lu, H.L.; Yu, J.; Wang, Z.G.; Fan, Y.M.; Zhou, X.F. Dissolution of Lignocelluloses with a High Lignin Content in a N-Methylmorpholine-N-oxide Monohydrate Solvent System via Simple Glycerol-Swelling and Mechanical Pretreatments. J. Agric. Food Chem. 2017, 65, 9587–9594. [Google Scholar] [CrossRef]
- Li, P.H.; Zhang, Z.H.; Zhang, X.X.; Li, K.Y.; Jin, Y.C.; Wu, W.J. DES: Their effect on lignin and recycling performance. RSC Adv. 2023, 13, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Li, R.J.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237–126260. [Google Scholar] [CrossRef]
- Takada, M.; Okazaki, Y.; Kawamoto, H.; Sagawa, T. Solubilization of sulfuric acid lignin by ball mill treatment with excess amounts of organic compounds. RSC Adv. 2023, 13, 1059–1065. [Google Scholar] [CrossRef]
- Almeida, R.O.; Moreira, A.; Moreira, D.; Pina, M.E.; Carvalho, M.G.V.S.; Rasteiro, M.G.; Gamelas, J.A.F. High-performance delignification of invasive tree species wood with ionic liquid and deep eutectic solvent for the production of cellulose-based polyelectrolytes. RSC Adv. 2022, 12, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Szalaty, T.J.; Kubiak, A.; Skrzypczak, A.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. The controlled oxidation of kraft lignin in mild conditions using ionic liquid as a crucial point in fabrication of antibacterial hybrid materials. J. Mol. Liq. 2018, 274, 370–378. [Google Scholar] [CrossRef]
- Yu, H.T.; Xue, Z.M.; Shi, R.F.; Zhou, F.Y.; Mu, T.C. Lignin dissolution and lignocellulose pretreatment by carboxylic acid based deep eutectic solvents. Ind. Crops Prod. 2022, 184, 115049–115057. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986–112009. [Google Scholar] [CrossRef]
- Lou, R.; Ma, R.S.; Lin, K.-t.; Ahamed, A.; Zhang, X. Facile Extraction of Wheat Straw by Deep Eutectic Solvent (DES) to Produce Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar] [CrossRef]
- Wang, J.; Baker, S.N. Pyrrolidinium salt based binary and ternary deep eutectic solvents: Green preparations and physiochemical property characterizations. Green. Process. Synth. 2018, 7, 353–359. [Google Scholar] [CrossRef]
- Zhang, H.; Vicent-Luna, J.M.; Tao, S.X.; Calero, S.; Jiménez Riobóo, R.J.; Ferrer, M.L.; del Monte, F.; Gutiérrez, M.C. Transitioning from Ionic Liquids to Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2022, 10, 1232–1245. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C.X. Lignin extraction and upgrading using deep eutectic solvents. Ind. Crops Prod. 2020, 147, 112241–112248. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.K.; Xue, Z.M.; Mu, T.C. Deep eutectic solvents as a green toolbox for synthesis. Cell Rep. Phys. Sci. 2022, 3, 100809–100831. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green. Chem. 2022, 25, 59–105. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 141–163. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.-W.; Zong, M.-H.; Li, N.; Lou, W.-Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 37, 1814–1823. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Mezzetta, A.; Grecchi, S.; Longhi, M.; Emanuele, E.; Rizzo, S.; Arduini, F.; Micheli, L.; Guazzelli, L.; Mussini, P.R. Natural-based chiral task-specific deep eutectic solvents: A novel, effective tool for enantiodiscrimination in electroanalysis. Electrochim. Acta 2021, 380, 138189–138197. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Li, T.T.; Lyu, G.J.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.H.; Chen, J.C.; Saeed, H.A.M. Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.S.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Hou, X.T.; Li, Z.X.; Yao, Z.L.; Zhao, L.X.; Luo, J.; Shen, R.X. Research advances on deep eutectic solvent pretreatment of lignocellulosic biomass. Chin. Sci. Bull. 2022, 67, 2736–2748. [Google Scholar] [CrossRef]
- Liu, J.K.; Qi, L.T.; Yang, G.H.; Xue, Y.; He, M.; Lucia, L.A.; Chen, J.C. Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content. Polymers 2020, 12, 1599. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Wang, A.L.; Yan, C.C.; Liu, S.W.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.X.; Nie, G.K.; Nie, S.X.; et al. Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability 2023, 15, 7118. [Google Scholar] [CrossRef]
- Liang, X.Q.; Zhu, Y.; Qi, B.K.; Li, S.Q.; Luo, J.Q.; Wan, Y. H Structure-property-performance relationships of lactic acid-based deep eutectic solvents with different hydrogen bond acceptors for corn stover pretreatment. Bioresour. Technol. 2021, 336, 125312–125320. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Shen, X.-J.; Chen, T.; Wang, H.-M.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural and Morphological Transformations of Lignin Macromolecules during Bio-Based Deep Eutectic Solvent (DES) Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 2130–2137. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.G.; Lusing, A.; Zhang, H.W.; Wan, C.X. Insights into Structural Changes of Lignin toward Tailored Properties during Deep Eutectic Solvent Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.Y.L.; Liu, Y.Y.; Wu, K.J.; Zhu, Y.M.; Lu, H.F.; Liang, B. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef]
- Salehi, H.S.; Ramdin, M.; Moultos, O.A.; Vlugt, T.J.H. Computing solubility parameters of deep eutectic solvents from Molecular Dynamics simulations. Fluid Phase Equilib. 2019, 497, 10–18. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.W.; Eckert, C.A.; Liotta, C.L. Green chemistry: Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef] [PubMed]
- Anugwom, I.; Rujana, L.; Wärnå, J.; Hedenström, M.; Mikkola, J.P. In quest for the optimal delignification of lignocellulosic biomass using hydrated, SO2 switched DBU MEASIL switchable ionic liquid. Chem. Eng. J. 2016, 297, 256–264. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Le-That, T.; Mikkola, J.P. Carbohydrate Free Lignin: A Dissolution-Recovery Cycle of Sodium Lignosulfonate in a Switchable Ionic Liquid System. ACS Sustain. Chem. Eng. 2016, 4, 7032–7040. [Google Scholar] [CrossRef]
- Phan, L.; Chiu, D.; Heldebrant, D.J.; Huttenhower, H.; John, E.; Li, X.; Pollet, P.; Wang, R.; Eckert, C.A.; Liotta, C.L.; et al. Switchable Solvents Consisting of Amidine/Alcohol or Guanidine/Alcohol Mixtures. Ind. Eng. Chem. Res. 2008, 47, 539–545. [Google Scholar] [CrossRef]
- Anugwom, I.; Eta, V.; Virtanen, P.; Mäki-Arvela, P.; Hedenström, M.; Ma, Y.B.; Hummel, M.; Sixta, H.; Mikkola, J.-P. Towards optimal selective fractionation for Nordic woody biomass using novel amine–organic superbase derived switchable ionic liquids (SILs). Biomass Bioenergy 2014, 70, 373–381. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Yao, L.; Yi, Y.; Shen, L.; Li, Z.; Qiu, H. Responsive switchable deep eutectic solvents: A review. Chin. Chem. Lett. 2022, 34, 107750–107762. [Google Scholar] [CrossRef]
- Qi, L.T.; Li, D.B.; Yang, G.H.; Xue, Y.; Chen, J.C. Construction and prospects of a switching DES pretreatment system for selective separation of plant fibre lignin. Trans. China Pulp Pap. 2022, 37, 111–120. [Google Scholar] [CrossRef]
- Anugwom, I.; Maki-Arvela, P.; Virtanen, P.; Willfor, S.; Sjoholm, R.; Mikkola, J.P. Selective extraction of hemicelluloses from spruce using switchable ionic liquids. Carbohydr. Polym. 2012, 87, 2005–2011. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Shimoyama, Y. Controlled polarity of CO2 switchable solution with DBU and alcohols. Fluid Phase Equilib. 2019, 494, 115–124. [Google Scholar] [CrossRef]
- Shu, F.; Guo, Y.J.; Huang, L.; Zhou, M.G.; Zhang, G.Y.; Yu, H.; Zhang, J.H.; Yang, F.X. Production of lignin-containing nanocellulose from poplar using ternary deep eutectic solvents pretreatment. Ind. Crops Prod. 2021, 177, 114404–114413. [Google Scholar] [CrossRef]
- Xiong, B.L.; Ma, S.; Chen, B.L.; Feng, Y.C.; Peng, Z.Q.; Tang, X.; Yang, S.L.; Sun, Y.; Lin, L.; Zeng, X.H.; et al. Formic acid-facilitated hydrothermal pretreatment of raw biomass for co-producing xylo-oligosaccharides, glucose, and lignin. Ind. Crops Prod. 2023, 193, 116195–116202. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Abranches, D.O.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Kraft Lignin Solubility and Its Chemical Modification in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.P.; Amaral, J.L.; Silvestre, A.J.D.; Freire, C.S.R.; Coutinho, J.A.P. Enhanced Solubility of Lignin Monomeric Model Compounds and Technical Lignins in Aqueous Solutions of Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Huang, C.; Zhan, Y.N.; Han, S.M.; Wang, J.; Meng, X.Z.; Yoo, C.G.; Fang, G.G.; Ragauskas, A.J. Effective biomass fractionation and lignin stabilization using a diol DES system. Chem. Eng. J. 2022, 443, 136395–136404. [Google Scholar] [CrossRef]
- Park, C.-W.; Han, S.-Y.; Bandi, R.; Dadigala, R.; Lee, E.-A.; Kim, J.-K.; Cindradewi, A.W.; Kwon, G.-J.; Lee, S.-H. Esterification of Lignin Isolated by Deep Eutectic Solvent Using Fatty Acid Chloride, and Its Composite Film with Poly(lactic acid). Polymers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Pe, J.A.; Mun, J.S.; Mun, S.P. Thermal Characterization of Kraft Lignin Prepared from Mixed Hardwoods. Bioresources 2023, 18, 926–936. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Characteristics of Lignin Macromolecules from Different Eucalyptus Species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Rashid, Z.; Khamis, M.I.; Nancarrow, P.; Jabbar, N.A. COSMO-RS based screening of ionic liquids for extraction of phenolic compounds from aqueous media. J. Mol. Liq. 2021, 328, 115387–115413. [Google Scholar] [CrossRef]
- Muhammad, N.; Gonfa, G.; Rahim, A.; Ahmad, P.; Iqbal, F.; Sharif, F.; Khan, A.S.; Khan, F.U.; Khan, Z.U.L.H.; Rehman, F.; et al. Investigation of ionic liquids as a pretreatment solvent for extraction of collagen biopolymer from waste fish scales using COSMO-RS and experiment. J. Mol. Liq. 2017, 232, 258–264. [Google Scholar] [CrossRef]
- Xue, Y.; Li, W.D.; Yang, G.H.; Lin, Z.Y.; Qi, L.T.; Zhu, P.H.; Yu, J.H.; Chen, J.C. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef]
- Meesattham, S.; Kim-Lohsoontorn, P. Low-temperature alcohol-assisted methanol synthesis from CO2 and H2: The effect of alcohol type. Int. J. Hydrogen Energy 2022, 47, 22691–22703. [Google Scholar] [CrossRef]
- Li, Y.-X.; Hou, S.-X.; Wei, Q.-Y.; Ma, X.-S.; Qu, Y.-S. Effect of Alkali and 1,4-Butanediol Contents on the Extraction of Lignin and Lignin-Based Activated Carbon. ACS Omega 2021, 6, 34386–34394. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.; Silvestre, A.J.D.; Pinto, P.C.R.; Freire, C.S.R.; Coutinho, J.A.P. Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 12485–12493. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Si, H.; Hu, S.X.; Chen, L.; Liao, Y.; Wang, L.; Zhang, Y.F.; Jiang, J.G. Comparative investigation of the structural characteristics of tobacco stalk lignin during the DES and alkaline deconstruction toward sustainable materials. Front. Bioeng. Biotechnol. 2022, 10, 994760–994771. [Google Scholar] [CrossRef]
- Olgun, Ç.; Ateş, S. Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests 2023, 14, 882. [Google Scholar] [CrossRef]
- Xiong, S.-J.; Zhou, S.-J.; Wang, H.-H.; Wang, H.-M.; Yu, S.; Zheng, L.; Yuan, T.-Q. Fractionation of technical lignin and its application on the lignin/poly-(butylene adipate-co-terephthalate) bio-composites. Int. J. Biol. Macromol. 2022, 209, 1065–1074. [Google Scholar] [CrossRef]
- Gairola, S.; Sinha, S.; Singh, I. Thermal stability of extracted lignin from novel millet husk crop residue. Int. J. Biol. Macromol. 2023, 242, 124725–124735. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2017, 92, 27–37. [Google Scholar] [CrossRef]
- Li, C.C.; Huang, C.X.; Zhao, Y.; Zheng, C.J.; Su, H.X.; Zhang, L.Y.; Luo, W.R.; Zhao, H.; Wang, S.F.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Choi, M.-H.; Yang, S.-H.; Park, W.-K.; Shin, H.-J. Bamboo Lignin Fractions with In Vitro Tyrosinase Inhibition Activity Downregulate Melanogenesis in B16F10 Cells via PKA/CREB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 7462. [Google Scholar] [CrossRef] [PubMed]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of Industrial Softwood Kraft Lignin: Solvent Selection as a Tool for Tailored Material Properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, R.B.; Tang, S.Y.; Wu, K.J.; Wang, B.S.; Liu, Y.Y.; Zhu, Y.M.; Lu, H.F.; Liang, B. The structure and properties of lignin isolated from various lignocellulosic biomass by different treatment processes. Int. J. Biol. Macromol. 2023, 243, 125219–125228. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Ji, H.R.; Shi, X.Y.; Yang, X.; Zhang, X. Successive organic solvent fractionation and structural characterization of lignin extracted from hybrid poplar by deep eutectic solvent for improving the homogeneity and isolating narrow fractions. Renew. Energ. 2020, 157, 1025–1034. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.Y.; Xiao, J.X.; He, X.D.; Zhang, K.X.; Yuan, S.X.; Peng, Z.T.; Chen, Z.; Lin, X.Q. Enhanced Enzymatic Hydrolysis and Lignin Extraction of Wheat Straw by Triethylbenzyl Ammonium Chloride/Lactic Acid-Based Deep Eutectic Solvent Pretreatment. ACS Omega 2019, 4, 19829–19839. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-Q.; Sun, S.-N.; Xu, F.; Sun, R.-C. Characterization of Lignin Structures and Lignin–Carbohydrate Complex (LCC) Linkages by Quantitative 13C and 2D HSQC NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).