Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Automatic Identification Tests
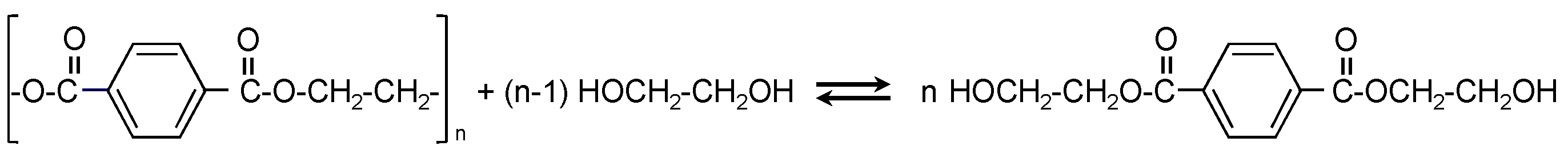
2.3. Glycolysis of PET
2.4. Analysis of Glycolysis Products/Characterisation of BHET
3. Results and Discussion
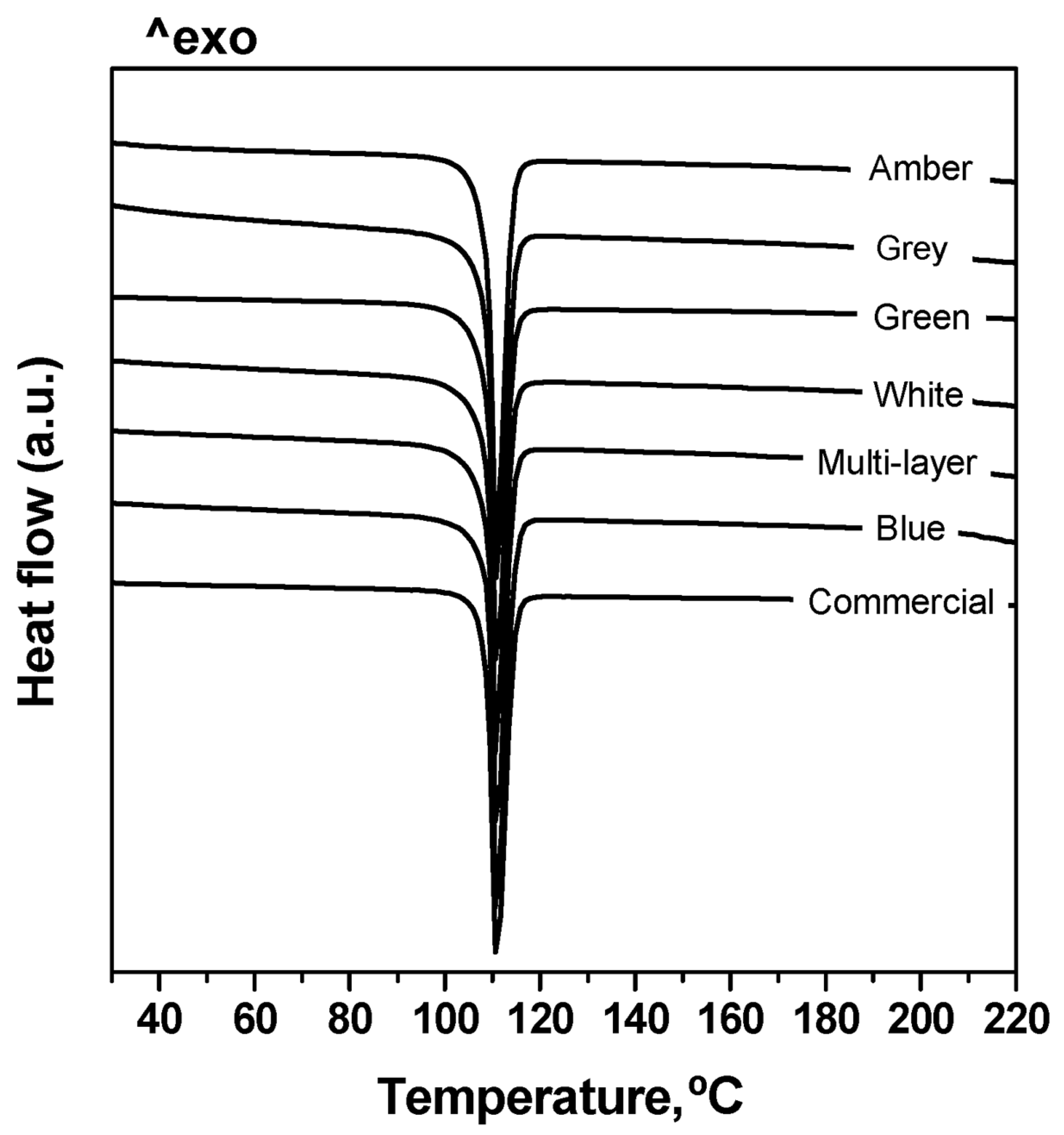
3.1. Automatic Identification Tests
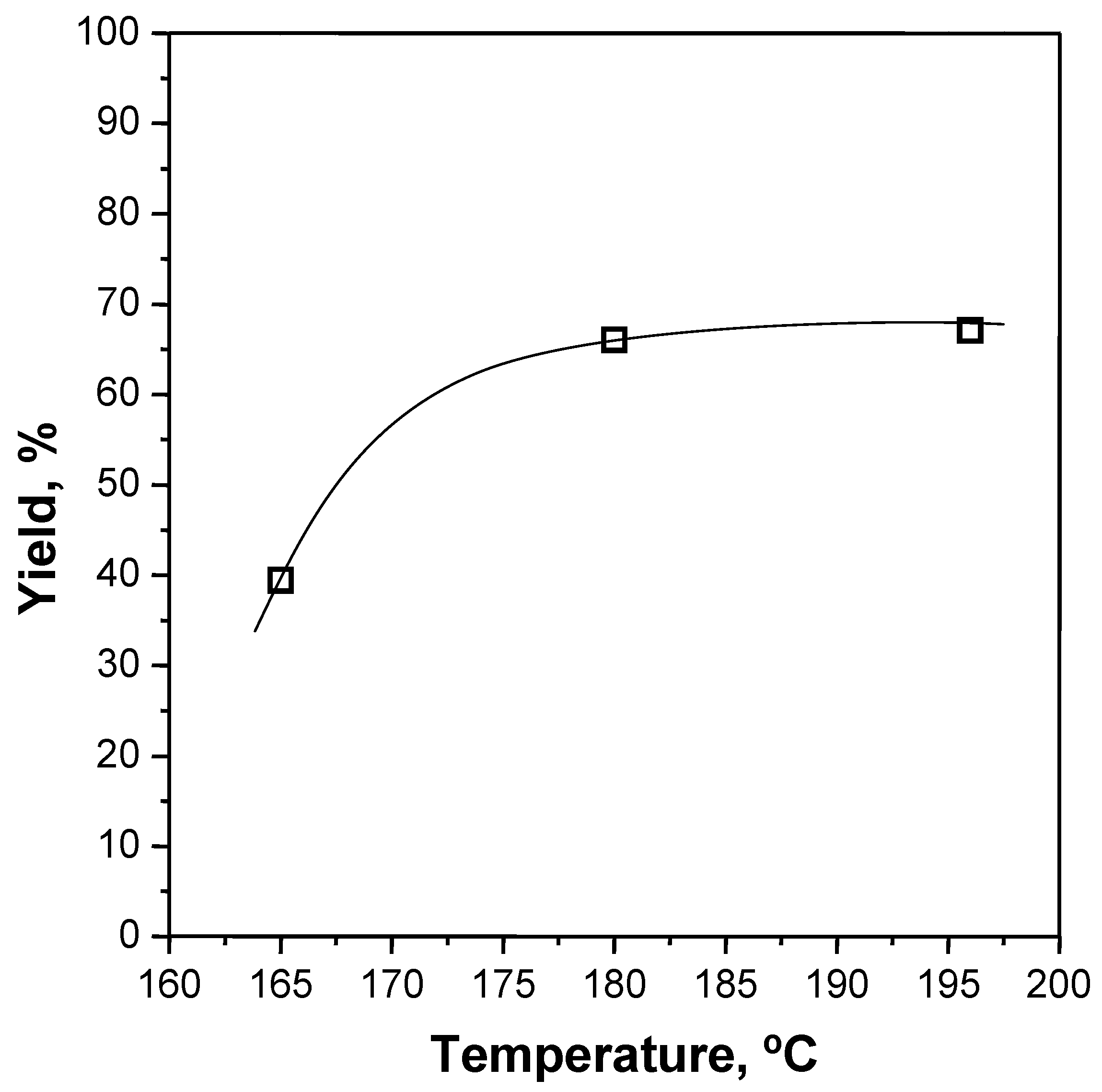
3.2. Glycolysis Reactions
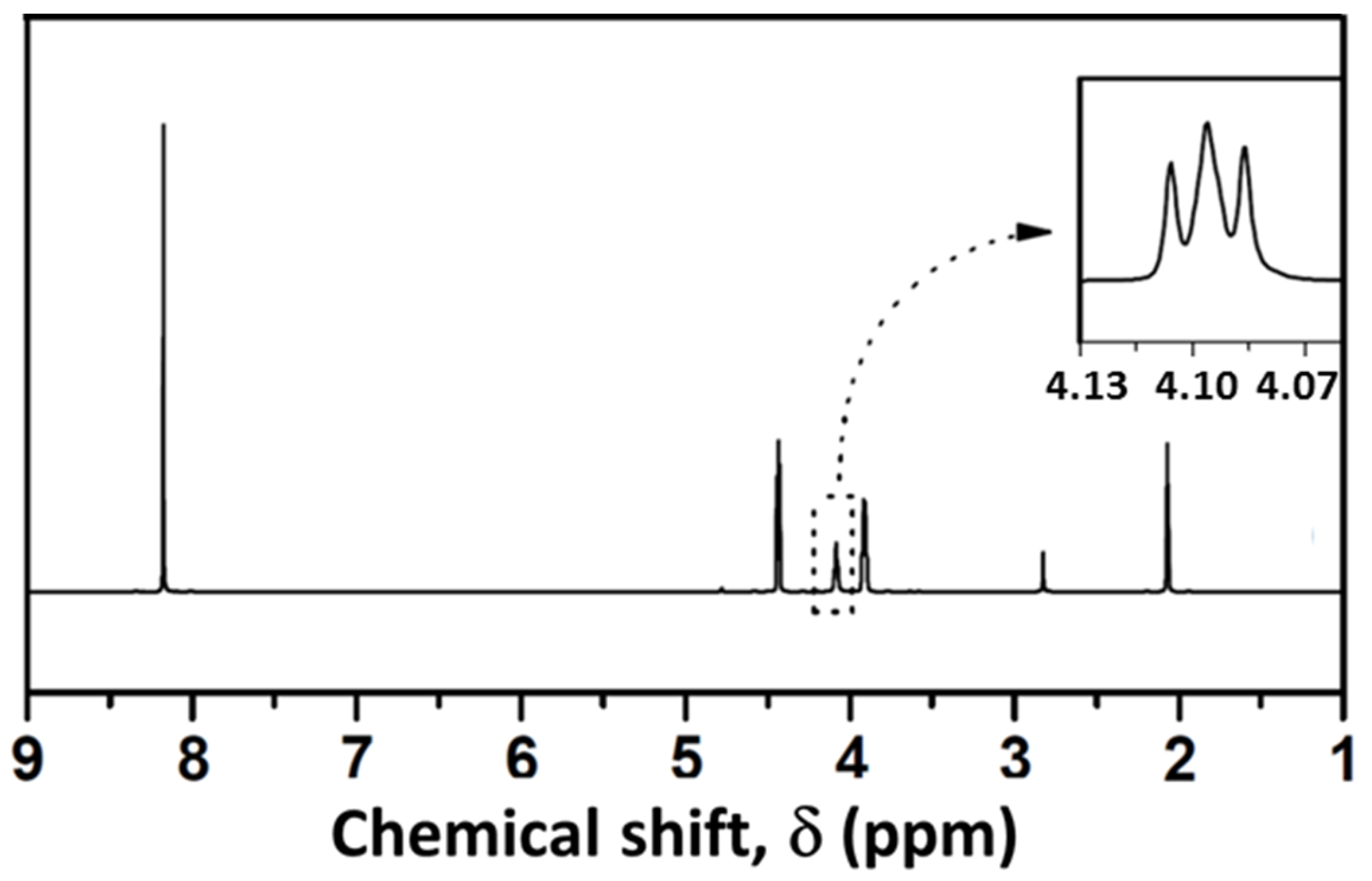
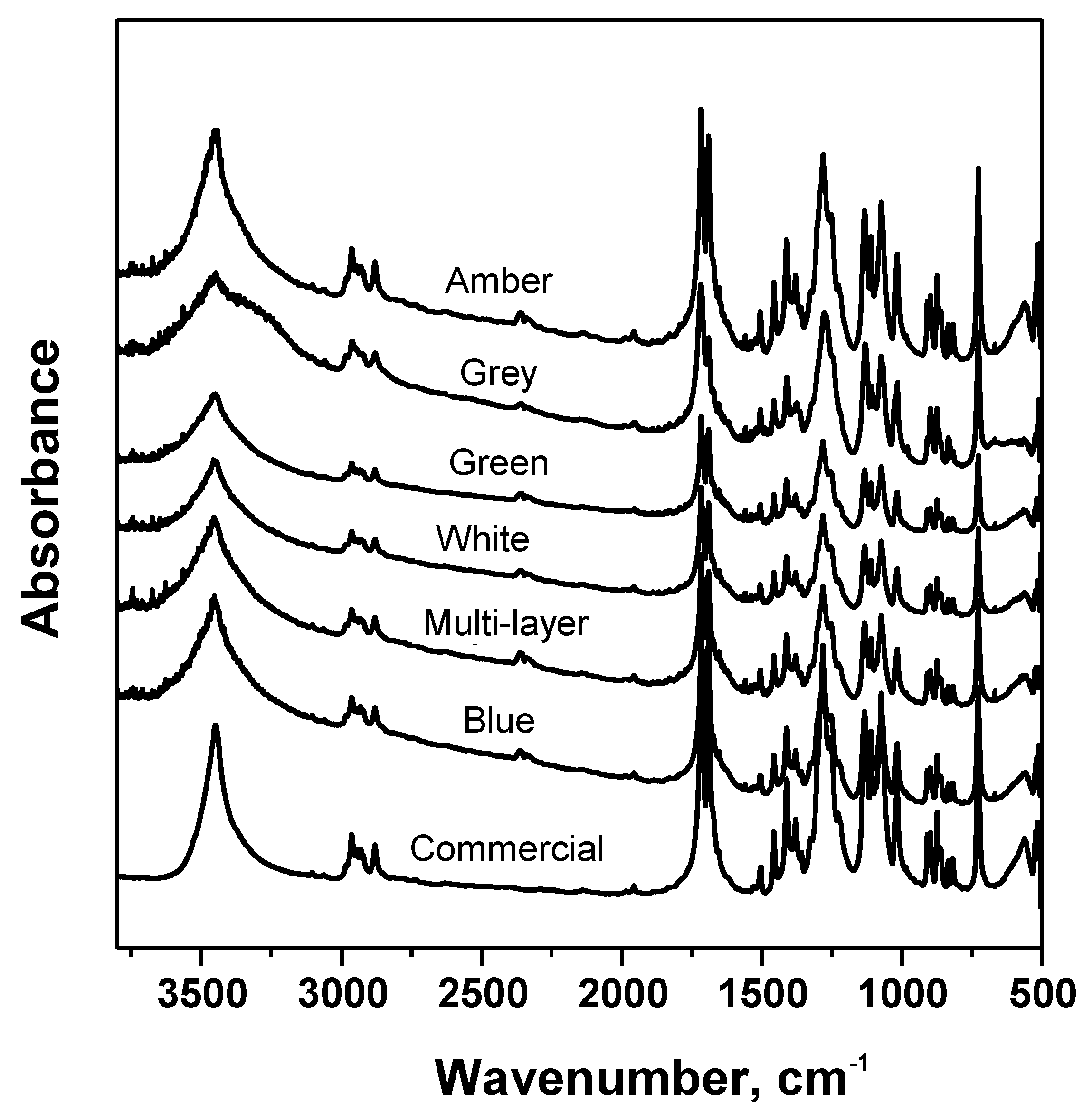
3.3. Analysis of Glycolysis Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palm, E.; Svensson Myrin, E. Mapping the Plastics System and Its Sustainability Challenges; Lund University: Lund, Sweden, 2018. [Google Scholar]
- Statista. Demand for Polyethylene Terephthalate Worldwide from 2010 to 2020, with a Forecast for 2021 to 2030. 2023. Available online: https://www.statista.com/statistics/1128658/polyethylene-terephthalate-demand-worldwide/ (accessed on 11 August 2023).
- Statista. Distribution of Polyethylene Terephthalate (PET) Packaging Consumption Worldwide in 2019, by End-Use Sector. 2023. Available online: https://www.statista.com/statistics/858624/global-polyethylene-terephthalate-consumption-distribution-by-end-use/ (accessed on 11 August 2023).
- GlobeNewswire. The Worldwide Recycled PET Industry is Expected to Reach $11.7 Billion by 2026. 2021. Available online: https://www.globenewswire.com/fr/news-release/2021/11/18/2337529/28124/en/The-Worldwide-Recycled-PET-Industry-is-Expected-to-Reach-11-7-Billion-by-2026.html (accessed on 11 August 2023).
- PETCORE Europe. PET Market in Europe State of Play—2022. 2022. Available online: https://www.petcore-europe.org/news-events/409-pet-market-in-europe-state-of-play-2022.html (accessed on 11 August 2023).
- PETCORE Europe. PETCORE Europe. 2023. Available online: https://www.petcore-europe.org/about.html (accessed on 11 August 2023).
- PlasticsEurope. The Circular Economy for Plastics. A European Overview. 2019. Available online: https://plasticseurope.org/fr/knowledge-hub/the-circular-economy-for-plastics-a-european-overview/ (accessed on 11 August 2023).
- Zhu, S.; Chen, H.; Wang, M.; Guo, X.; Lei, Y.; Jin, G. Plastic solid waste identification system based on near infrared spectroscopy in combination with support vector machine. Adv. Ind. Eng. Polym. 2019, 2, 77–81. [Google Scholar] [CrossRef]
- Pakhomova, S.; Zhdanov, I.; van Bavel, B. Polymer Type Identification of Marine Plastic Litter Using a Miniature Near-Infrared Spectrometer (MicroNIR). Appl. Sci. 2020, 10, 8707. [Google Scholar] [CrossRef]
- Wang, L.; Nelson, G.; Toland, J.; Holbrey, J.D. Glycolysis of PET using 1,3-dimethylimidazolium-2-carboxylate as an organocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 13362–13368. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent advances in chemical recycling of polyethylene terephthalate waste into value added products for sustainable coating solutions—Hope vs. hype. Mater. Adv. 2022, 3, 1974. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ohshima, M.-A.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Enthaler, S. Zinc-catalyzed depolymerization reactions. In Zinc Catalysis: Applications in Organic Synthesis; Enthaler, S., Wu, X.-F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 207–218. [Google Scholar]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Han, M. 5-Depolymerization of PET bottle via methanolysis and hydrolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, CT, USA, 2019; pp. 85–108. [Google Scholar]
- Aguado, A.; Martínez, L.; Becerra, L.; Arieta-araunabeña, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical depolymerisation of PET complex waste: Hydrolysis vs. glycolysis. J. Mater. Cycles Waste Manag. 2014, 16, 201–210. [Google Scholar] [CrossRef]
- Barredo, A.; Asueta, A.; Amundarain, I.; Leivar, J.; Miguel-Fernández, R.; Arnaiz, S.; Epelde, E.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I. Chemical recycling of monolayer PET tray waste by alkaline hydrolysis. J. Environ. Chem. Eng. 2023, 11, 109823. [Google Scholar] [CrossRef]
- Bertelli, C. Recycling PET: Bottle to Bottle Process Application. In Proceedings of the Polyester 2005 10th World Congress, Amsterdam, The Netherlands, 13–15 December 2005. [Google Scholar]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Synthesis of poly(ethylene terephthalate) based on glycolysis of waste PET fiber. J. Macromol. Sci. A 2020, 57, 430–438. [Google Scholar] [CrossRef]
- Kárpáti, L.; Fogarassy, F.; Kovácsik, D.; Vargha, V. One-pot depolymerization and polycondensation of PET based random oligo- and polyesters. J. Polym. Environ. 2019, 27, 2167–2181. [Google Scholar] [CrossRef]
- Kathalewar, M.; Dhopatkar, N.; Pacharane, B.; Sabnis, A.; Raut, P.; Bhave, V. Chemical recycling of PET using neopentyl glycol: Reaction kinetics and preparation of polyurethane coatings. Prog. Org. Coat. 2013, 76, 147–156. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, D.; Son, C.; Lee, Y.; Kim, E.; Moon, I. Synthesis and applications of unsaturated polyester resins based on PET waste. Korean J. Chem. Eng. 2007, 24, 1076–1083. [Google Scholar] [CrossRef]
- Saravari, O.; Pathomwattanasak, K.; Pimpan, V. Synthesis of urethane oils from palm oil and waste PET bottles. J. Appl. Polym. Sci. 2007, 105, 1802–1807. [Google Scholar] [CrossRef]
- Hollstein, F.; Wohllebe, M.; Arnaiz, S.; Manjón, D.; O’Brien, N.; Kulcke, A. Challenges in Automatic Sorting of Bio-Plastics within the Recycling Chain by Means of NIR-Hyperspectral-Imaging. In Proceedings of the ICNIRS 2013-16th International Conference on Near Infrared Spectroscopy, La Grande-Motte, France, 2–7 June 2013; pp. 450–452. [Google Scholar]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Arnaiz, S.; Gutiérrez-Ortiz, J.I. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym. Degrad. Stab. 2010, 95, 1022–1028. [Google Scholar] [CrossRef]
- Baliga, S.; Wong, W.T. Depolymerization of poly(ethylene terephthalate) recycled from post-consumer soft-drinks bottles. J. Polym. Sci. A Polym. Chem. 1989, 27, 2071–2082. [Google Scholar] [CrossRef]
- Ghaemy, M.; Mossaddegh, K. Depolymerisation of poly(ethylene terephthalate) fibre wastes using ethylene glycol. Polym. Degrad. Stab. 2005, 90, 570–576. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. Influences of glycolysis conditions. J. Appl. Polym. Sci. 2001, 80, 943–948. [Google Scholar] [CrossRef]
- Güçlü, G.; Kas¸göz, A.; Özbudak, S.; Özgümüs, S.; Orbay, M. Glycolysis of poly(ethylene terephthalate) wastes in xylene. J. Appl. Polym. Sci. 1998, 69, 2311–2319. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ou, C.F.; Hu, Y.C.; Lin, C.C. Depolymerization of poly(ethylene terephthalate) resin under pressure. J. Appl. Polym. Sci. 1991, 42, 1501–1507. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.P.; Zhang, S.; Zhang, Y. Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids. Europ. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
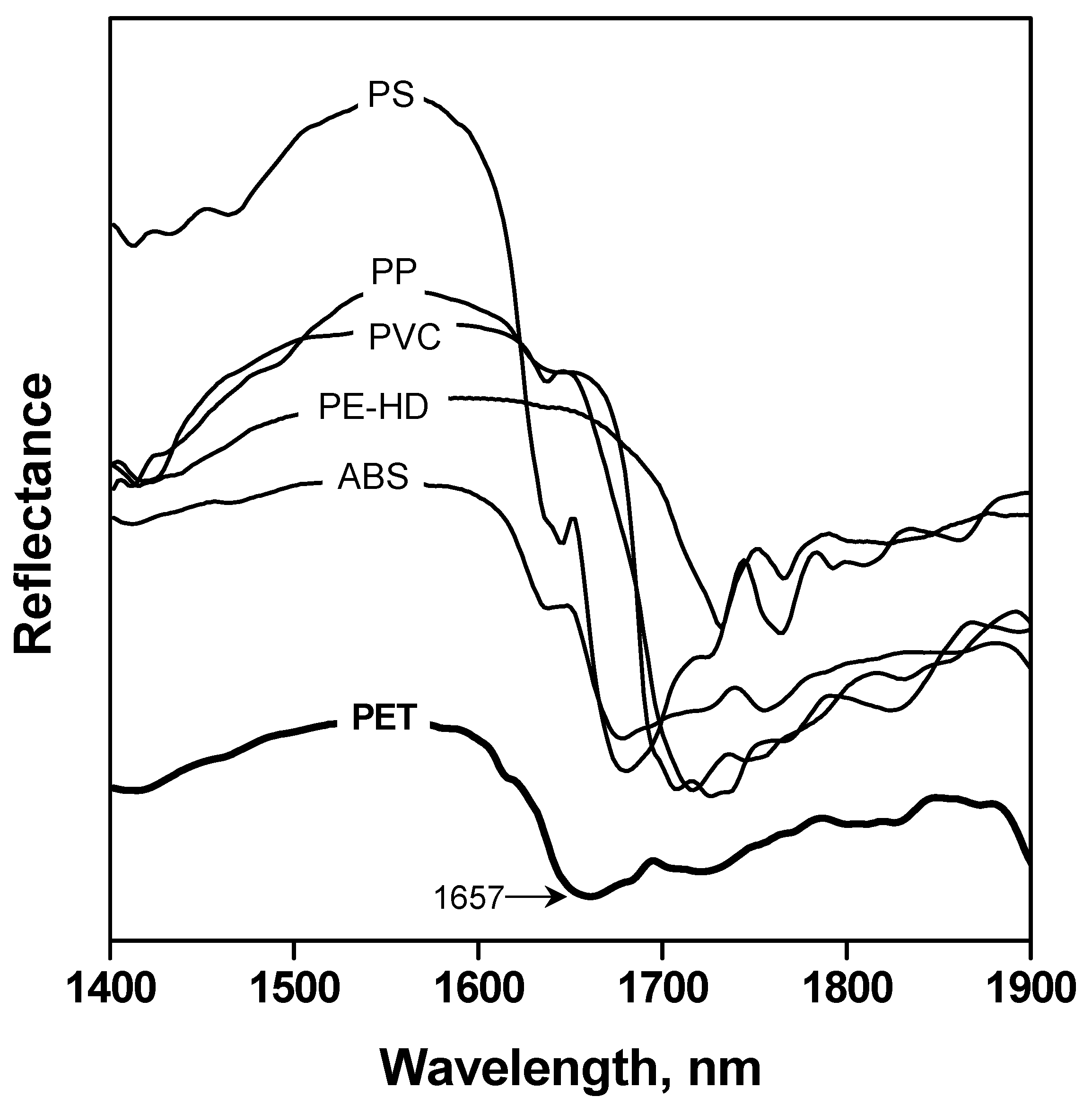
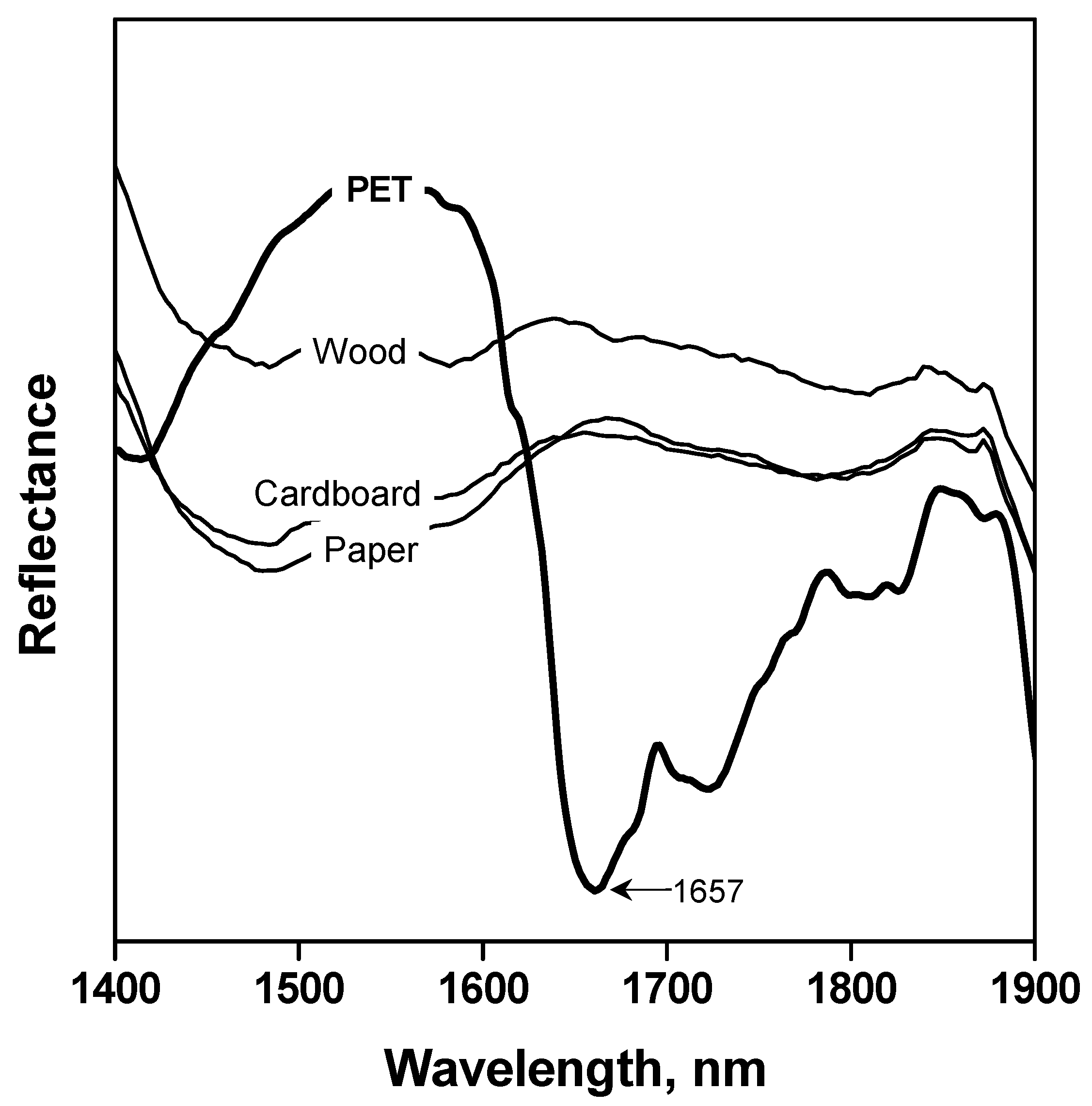


| PET Material | Type | Colour | Origin | Product | Content |
|---|---|---|---|---|---|
| PET R-CL | Reference | Clear | Mechanical recycling | Bottles | Water |
| PET C-BL | Highly coloured | Blue | Post-consumer waste | Bottles | Water |
| PET C-GY | Highly coloured | Grey | Post-consumer waste | Bottles | Soft drink |
| PET C-GN | Highly coloured | Green | Post-consumer waste | Bottles | Soft drink |
| PET C-WH | Highly coloured | White | Post-consumer waste | Bottles | Various |
| PET C-AM | Highly coloured | Amber | Post-consumer waste | Packages | Miscellaneous |
| PET M-AM | Multi-layered | Amber | Post-consumer waste | Bottles | Beer |
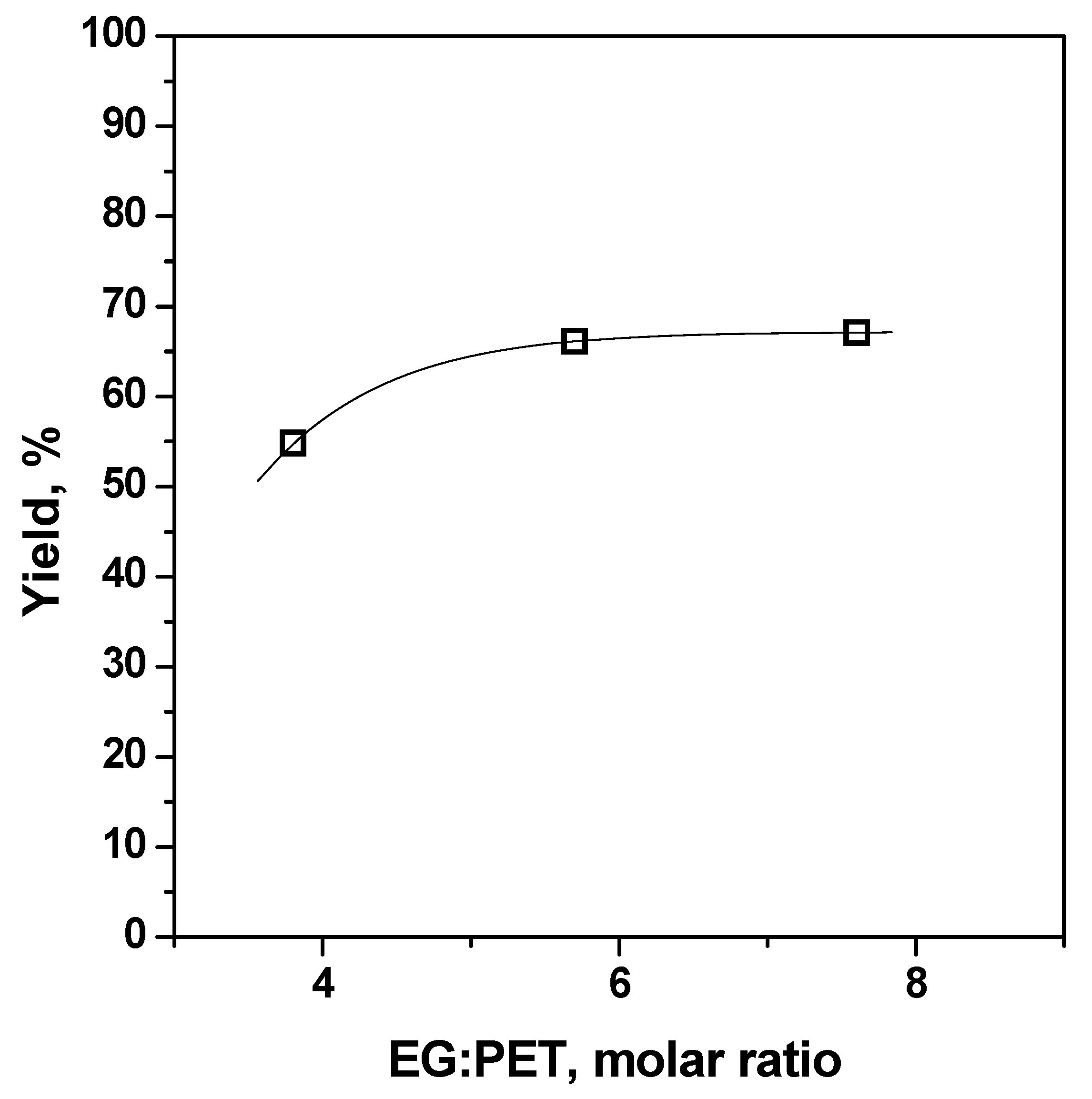
| Run | t (h) | T (°C) | PET Material | PET (g) | EG/PET (mol/mol) | Zn(OAc)2/PET (w/w) | BHET Yield (%) |
|---|---|---|---|---|---|---|---|
| #1 | 8 | 196 | PET R-CL | 30 | 7.6/1 | 1% | 67.1 |
| #2 | 8 | 196 | PET R-CL | 30 | 7.6/1 | --- | 56.8 |
| #3 | 1 | 165 | PET R-CL | 30 | 7.6/1 | 1% | 39.4 |
| #4 | 1 | 180 | PET R-CL | 30 | 7.6/1 | 1% | 66.1 |
| #5 | 1 | 196 | PET R-CL | 30 | 3.8/1 | 1% | 54.9 |
| #6 | 1 | 196 | PET R-CL | 30 | 5.7/1 | 1% | 66.1 |
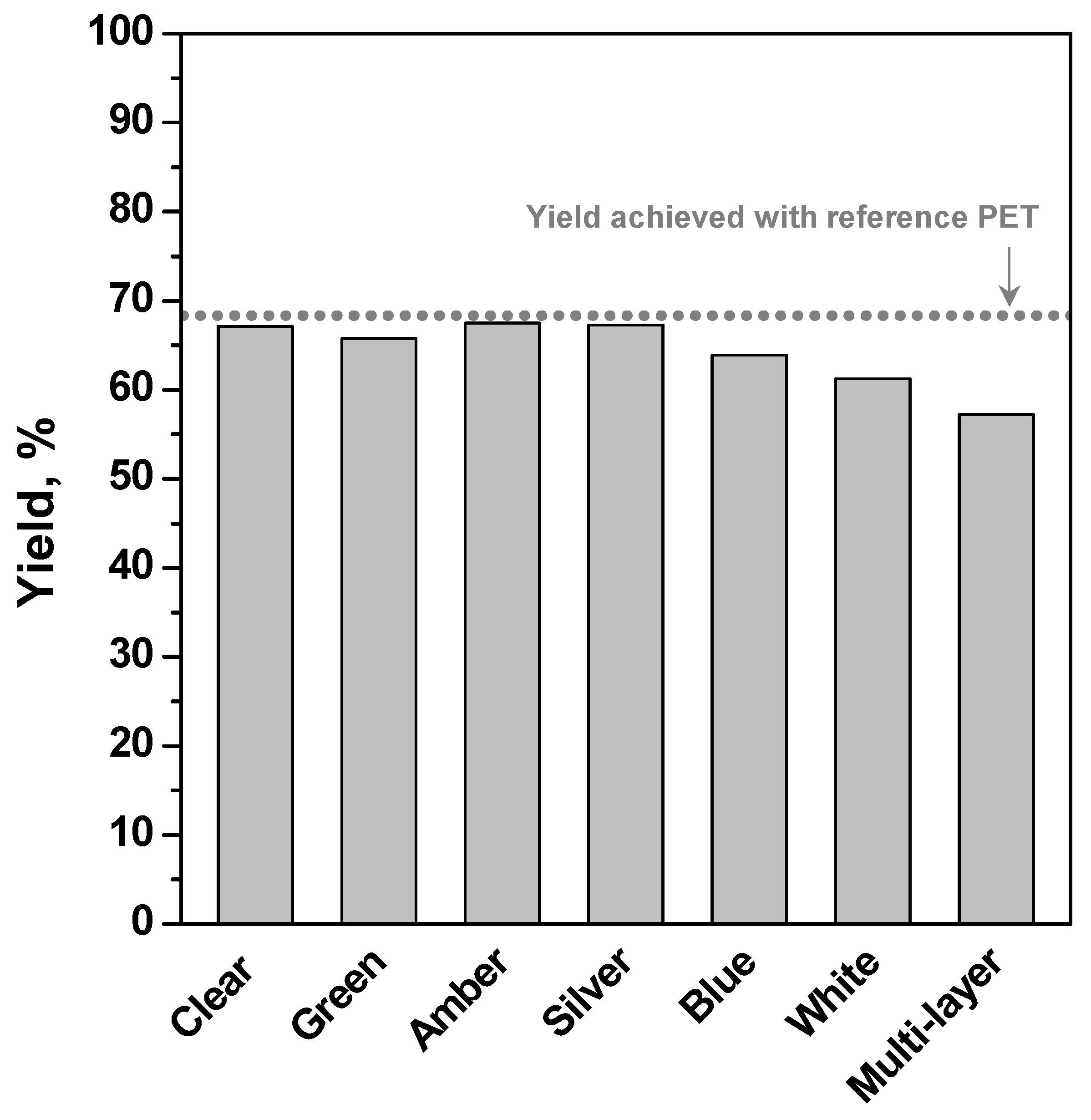
| #1 | 2 | 196 | PET R-CL | 30 | 7.6/1 | 1% | 67.1 |
| #7 | 2 | 196 | PET C-BL | 30 | 7.6/1 | 1% | 63.9 |
| #8 | 2 | 196 | PET C-GY | 30 | 7.6/1 | 1% | 67.3 |
| #9 | 2 | 196 | PET C-GN | 30 | 7.6/1 | 1% | 65.8 |
| #10 | 2 | 196 | PET C-WH | 30 | 7.6/1 | 1% | 61.2 |
| #11 | 2 | 196 | PET C-AM | 30 | 7.6/1 | 1% | 67.5 |
| #12 | 2 | 196 | PET M-AM | 30 | 7.6/1 | 1% | 57.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asueta, A.; Arnaiz, S.; Miguel-Fernández, R.; Leivar, J.; Amundarain, I.; Aramburu, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes. Polymers 2023, 15, 4196. https://doi.org/10.3390/polym15204196
Asueta A, Arnaiz S, Miguel-Fernández R, Leivar J, Amundarain I, Aramburu B, Gutiérrez-Ortiz JI, López-Fonseca R. Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes. Polymers. 2023; 15(20):4196. https://doi.org/10.3390/polym15204196
Chicago/Turabian StyleAsueta, Asier, Sixto Arnaiz, Rafael Miguel-Fernández, Jon Leivar, Izotz Amundarain, Borja Aramburu, Jose Ignacio Gutiérrez-Ortiz, and Rubén López-Fonseca. 2023. "Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes" Polymers 15, no. 20: 4196. https://doi.org/10.3390/polym15204196
APA StyleAsueta, A., Arnaiz, S., Miguel-Fernández, R., Leivar, J., Amundarain, I., Aramburu, B., Gutiérrez-Ortiz, J. I., & López-Fonseca, R. (2023). Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes. Polymers, 15(20), 4196. https://doi.org/10.3390/polym15204196







