Biorenewable Oxypropylated Pentane-1,2,5-triol as a Source for Incorporation in Rigid Polyurethane Foams
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Instruments and Methods
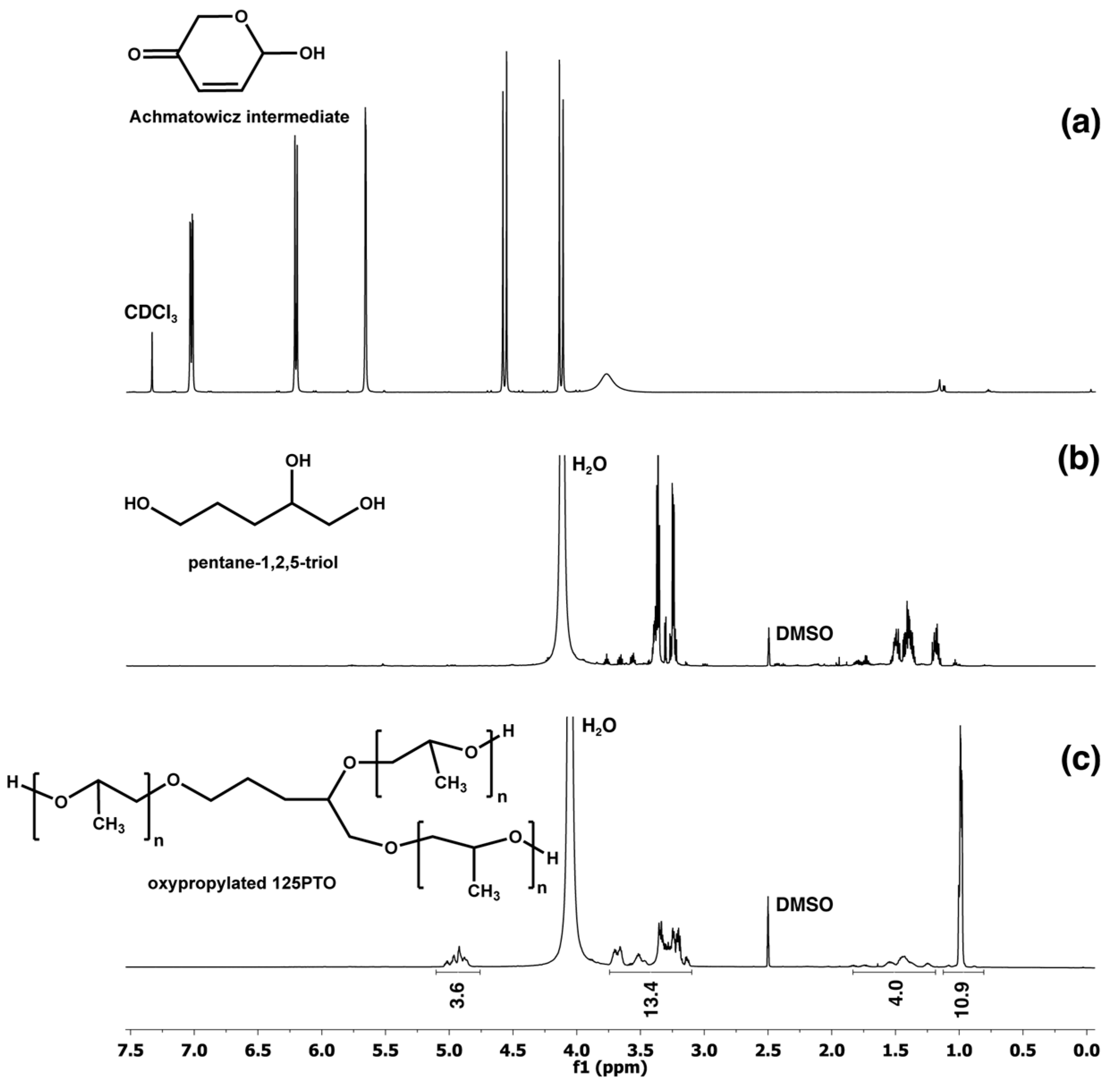
2.3. Synthesis of 6-Hydroxy-(2H)-pyran-3(6H)-one
2.4. Synthesis of Pentane-1,2,5-triol
2.5. Treatment of Pentane-1,2,5-triol by Propylene Oxide
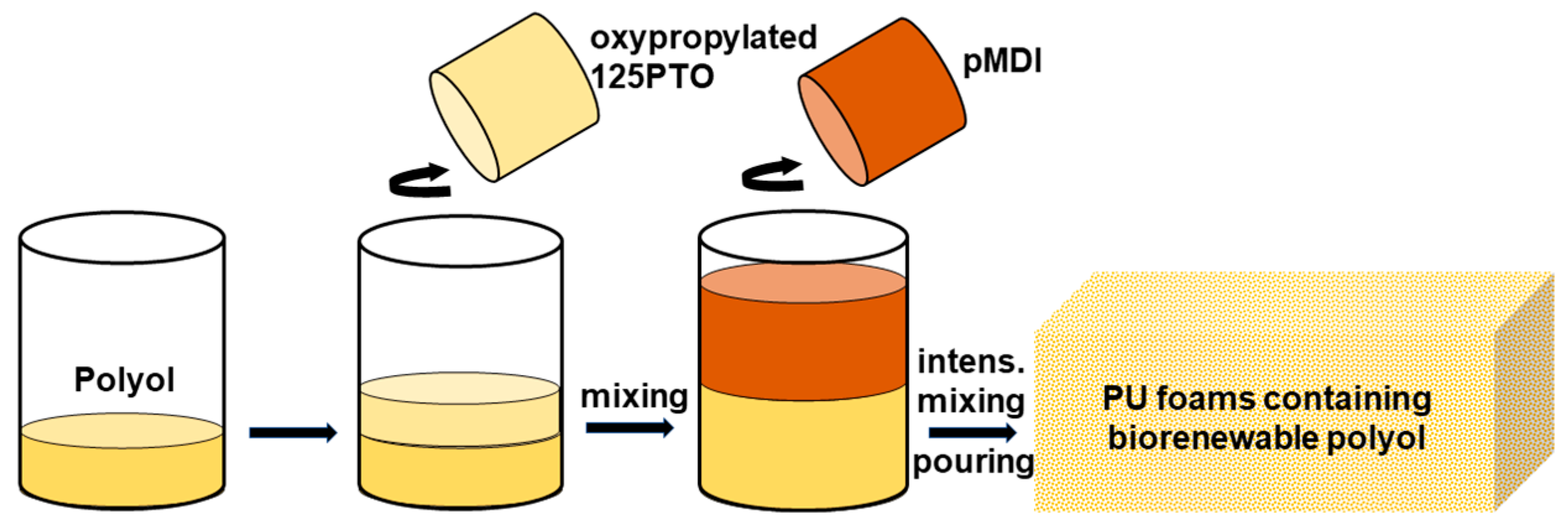
2.6. Preparation of Rigid Polyurethane Foams
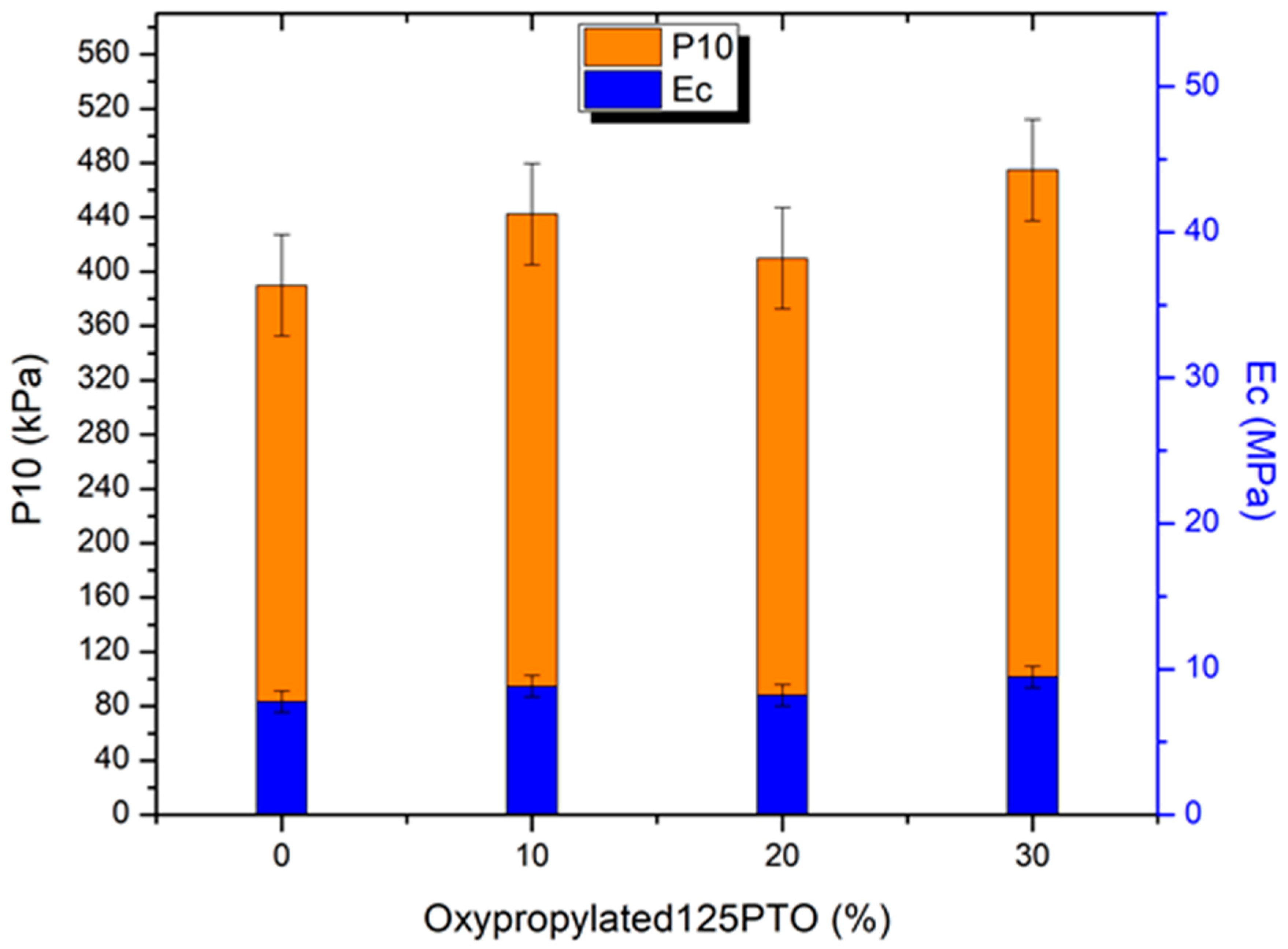
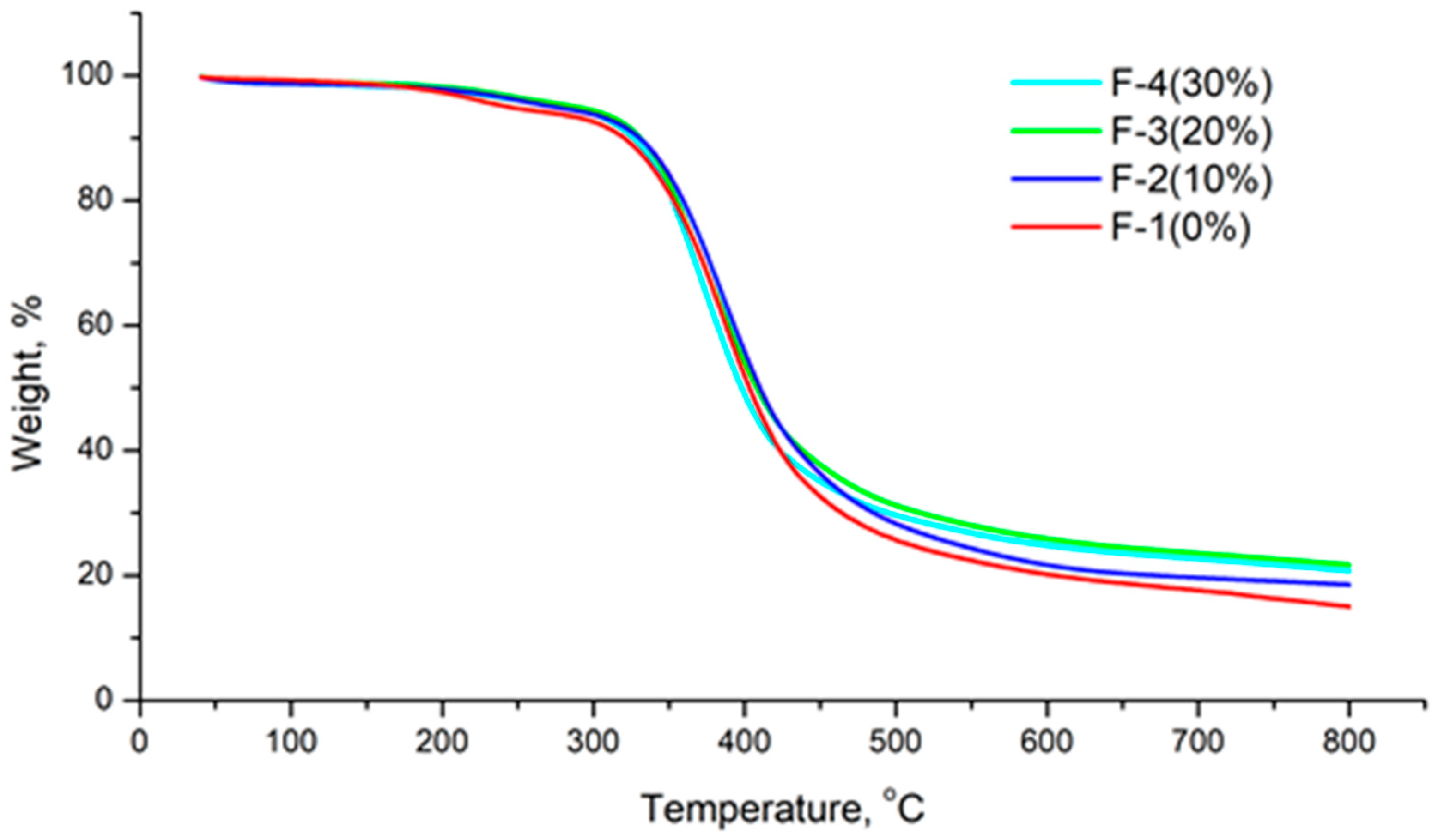
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Smithers Rapra Publishing: Shrewsbury, UK, 2005. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: New York, NY, USA, 2006. [Google Scholar]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Eaves, D. Handbook of Polymer Foams; Rapra Technology Ltd.: Shawbury, UK, 2004. [Google Scholar]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure–function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef]
- Ashida, K. Polyurethane and Related Foams: Chemistry and Technology; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Furtwengler, P.; Avérous, L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials. Green Mater. 2013, 1, 137–160. [Google Scholar] [CrossRef]
- Kazzaz, A.E.; Feizi, Z.H.; Fatehi, P. Grafting strategies for hydroxy groups of lignin for producing materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Ma, Y.; Xiao, Y.; Zhao, Y.; Bei, Y.; Hu, L.; Zhou, Y.; Jia, P. Biomass based polyols and biomass based polyurethane materials as a route towards sustainability. React. Funct. Polym. 2022, 175, 105285. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of bio-based polyurethanes: Perspective on applications and bio-degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Brenes-Granados, D.; Cubero-Sesin, J.M.; Gutiérrez, F.O.; Vega-Baudrit, J.; Gonzalez-Paz, R. Variation of physical properties of rigid polyurethane foams synthesized from renewable sources with different commercial catalysts. J. Renew. Mat. 2017, 5, 280–289. [Google Scholar] [CrossRef]
- Da Silva, V.R.; Mosiewicki, M.A.; Yoshida, M.I.; Da Silva, M.C.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash I: Synthesis and physical chemical characterization. Polym. Test. 2013, 32, 438–445. [Google Scholar] [CrossRef]
- Czlonka, S.; Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam/cellulose whisker nanocomposites: Preparation, characterization, and properties. J. Nanosci. Nanotechnol. 2011, 11, 6904–6911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ragauskas, A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Saffar, T.; Bouafif, H.; Braghiroli, F.L.; Magdouli, S.; Langlois, A.; Koubaa, A. Production of bio-based polyol from oxypropylated pyrolytic lignin for rigid polyurethane foam application. Waste Biomass Valorization 2020, 11, 6411–6427. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Ionescu, M.; Radojcic, D.; Wan, X.; Shrestha, M.L.; Petrovic, Z.S.; Upshaw, T.A. Highly functional polyols from castor oil for rigid polyurethanes. Eur. Polym. J. 2016, 84, 736–749. [Google Scholar] [CrossRef]
- Miao, S.; Sun, L.; Wang, P.; Liu, R.; Su, Z.; Zhang, S. Soybean oil-based polyurethane networks as candidat biomaterials: Synthesis and biocompatibility. Eur. J. Lipid Sci. Technol. 2012, 114, 1165–1174. [Google Scholar] [CrossRef]
- Adnan, S.; Tuan Noor, M.T.I.; ‘Ain, N.H.; Devi, K.P.P.; Mohd, N.S.; Kian, Y.S.; Idris, Z.B.; Campara, I.; Schiffman, C.M.; Pietrzyk, K.; et al. Impact of the hard-segment concentration on highly resilient polyurethane foams based on palm olein polyol. J. Appl. Polym. Sci. 2017, 134, 45440. [Google Scholar] [CrossRef]
- Zhou, X.; Sain, M.M.; Oksman, K. Semi-rigid biopolyurethane foams based on palm-oil polyol and reinforced with cellulose nanocrystals. Compos. Part A 2016, 83, 56–62. [Google Scholar] [CrossRef]
- Kairyte, A.; Vejelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crops Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Prociak, A.; Kuranska, M.; Cabulis, U.; Kirpluks, M. Rapeseed oil as main component in synthesis of bio-polyurethane-polyisocyanurate porous materials modified with carbon fibers. Polym. Test. 2017, 59, 478–486. [Google Scholar] [CrossRef]
- Da Silva, V.R.; Mosiewicki, M.A.; Yoshida, M.I.; Da Silva, M.C.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash II: Mechanical characterization. Polym. Test. 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czuprynski, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New bio-polyol based on white mustard seed oil for rigid PUR-PIR foams. Pol. J. Chem. Technol. 2018, 20, 24–31. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Karimi, M.B.; Khanbabaei, G.; Sadeghi, G.M.M. Unsaturated canola oil-based polyol as effective nucleating agent for polyurethane hard segments. J. Polym. Res. 2019, 26, 253. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Czlonka, S.; Strakowska, A.; Strzelec, K.; Kairyte, A.; Kremensas, A. Bio-based polyurethane composite foams with improved mechanical, thermal, and antibacterial properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pang, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and characterization of water-blown polyurethane foams from liquefied cornstalk polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tuohedi, N. Polyurethane foams and bio-polyols from liquefied cotton stalk agricultural waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Li, H.; Feng, S.; Yuan, Z.; Wei, Q.; Xu, C. Highly efficient liquefaction of wheat straw for the production of bio-polyols and bio-based polyurethane foams. Ind. Crops Prod. 2017, 109, 426–433. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Yuan, Z.; Wei, Q. Synthesis of bio-based polyurethane foams with liquefied wheat straw: Process optimization. Biomass Bioenergy 2018, 111, 134–140. [Google Scholar] [CrossRef]
- Serrano, L.; Rincon, E.; García, A.; Rodríguez, J.; Briones, R. Bio-degradable polyurethane foams produced by liquefied polyol from wheat straw biomass. Polymers 2020, 12, 2646. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Conversion of lignin into bio-based chemicals and materials, Ch. 8. Lignin-based polyurethane (PU) resins and foams. In Green Chemistry and Sustainable Technology; Springer Nature: Berlin, Germany, 2017; pp. 133–156. [Google Scholar]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef]
- Borges da Silva, E.A.; Zabkova, M.; Araujo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Hse, C.Y.; Qi, J.; Hu, T. Characterization of Biobased Polyurethane Foams Employing Lignin Fractionated from Microwave Liquefied Switchgrass. Int. J. Polym. Sci. 2017, 2017, 4207367. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Muller, L.C.; Marx, S.; Vosloo, H.C.M.J. Polyol preparation by liquefaction of technical lignins in crude glycerol. Renew. Mater. 2017, 5, 67–80. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzese, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of lignins and preparation of rigid polyurethane foams from the ensuing polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Ottati, C.; Lopretti, M.; Rodrigues, A.E.; Belgacem, M.N. Lignin-based rigid polyurethane foams with improved biodegradation. J. Cell. Plast. 2014, 50, 81–95. [Google Scholar] [CrossRef]
- Berrima, B.; Mortha, G.; Boufi, S.; El Aloui, E.; Belgacem, M.N. Oxypropylation of soda lignin: Characterization and application in polyurethane foams production. Cellul. Chem. Technol. 2016, 50, 941–950. [Google Scholar]
- Li, H.; Dun, C.; Jariwala, H.; Wang, R.; Cui, P.; Zhang, H.; Dai, Q.; Yang, S.; Zhang, H. Improvement of bio-based polyurethane and its optimal application in controlled release fertilizer. J. Control. Release 2022, 350, 748–760. [Google Scholar]
- Zhang, Q.; Lin, X.; Chen, W.; Zhang, H.; Han, D. Modification of rigid polyurethane foams with the addition of nano-SiO2 or lignocellulosic biomass. Polymers 2020, 12, 107. [Google Scholar] [CrossRef]
- Baysal, G.; Aydın, H.; Hosgoren, H.; Uzan, S.; Karaer, H. Improvement of synthesis and dielectric properties of polyurethane/Mt-QASs+ (novel synthesis). J. Polym. Environ. 2016, 24, 139–147. [Google Scholar] [CrossRef]
- Moghaddam, S.T.; Naimi-Jamal, M.R. Reinforced magnetic polyurethane rigid (PUR) foam nanocomposites and investigation of thermal, mechanical, and sound absorption properties. J. Thermoplast. Compos. Mater. 2019, 32, 1224–1241. [Google Scholar] [CrossRef]
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 2016, 7, 115–129. [Google Scholar] [CrossRef]
- Nanni, G.; Heredia-Guerrero, J.A.; Paul, U.C.; Dante, S.; Caputo, G.; Canale, C.; Athanassiou, A.; Fragouli, D.; Bayer, I.S. Poly(furfuryl alcohol)-polycaprolactone Blends. Polymers 2019, 11, 1069. [Google Scholar] [CrossRef]
- Sun, D.; Sato, S.; Ueda, W.; Primo, A.; Garcia, H.; Corma, A. Production of C4 and C5 alcohols from biomass-derived materials. Green Chem. 2016, 18, 2579–2597. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic conversions of furfural to pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Simeonov, S.P.; Ravutsov, M.A.; Mihovilovic, M.D. Biorefinery via Achmatowicz rearrangement: Synthesis of pentane-1,2,5-triol from furfuryl alcohol. ChemSusChem 2019, 12, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.; Lazarova, H.; Marinova, M.; Popova, M. Achmatowicz rearrangement enables hydrogenolysis-free gas-phase synthesis of pentane-1,2,5-triol from furfuryl alcohol. Green Chem. 2019, 21, 5657–5664. [Google Scholar] [CrossRef]
- ISO 844:2021; Rigid Cellular Plastics—Determination of Compression Properties. International Organization for Standardization: Geneva, Switzerland, 2021.
- ASTM D7487-13; Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test. ASTM International: West Conshohocken, PA, USA, 2016.
- ISO 845:2006; Cellular Plastics and Rubbers—Determination of Apparent Density. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 4590:2016; Rigid Cellular Plastics—Determination of the Volume Percentage of Open Cells and of Closed Cells. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 2896:2001; Rigid Cellular Plastics—Determination of Water Absorption. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 2796:1986; Cellular Plastics, Rigid—Test for Dimensional Stability. International Organization for Standardization: Geneva, Switzerland, 1986.
- ISO 16620-4:2016; Plastics—Biobased Content—Part 4: Determination of Biobased Mass Content. International Organization for Standardization: Geneva, Switzerland, 2016.
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P. K A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Gosz, K.; Kowalkowska-Zedler, D.; Haponiuk, J.; Piszczyk, L. Liquefaction of alder wood as the source of renewable and sustainable polyols for preparation of polyurethane resins. Wood Sci. Technol. 2020, 54, 103–121. [Google Scholar] [CrossRef]

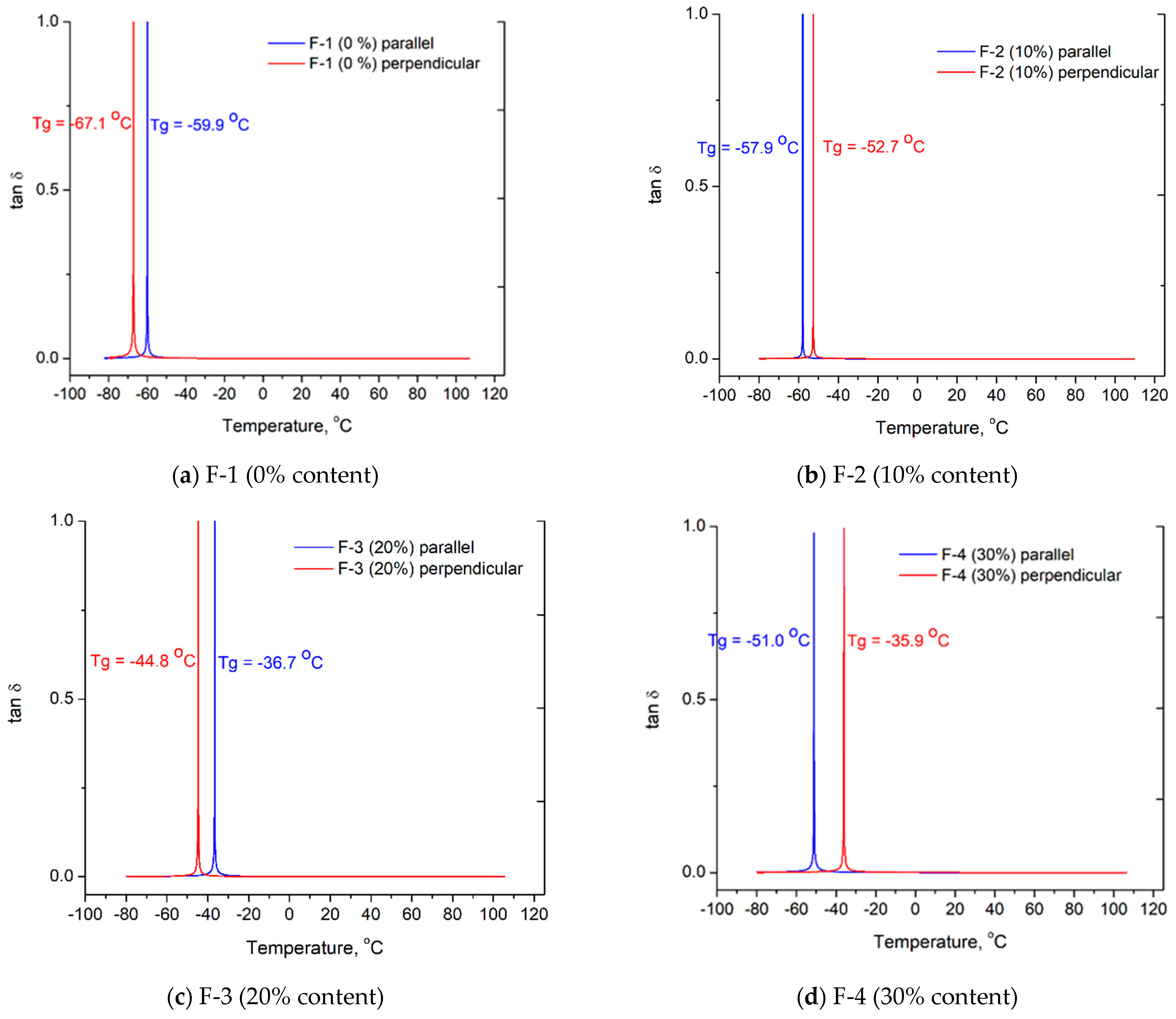
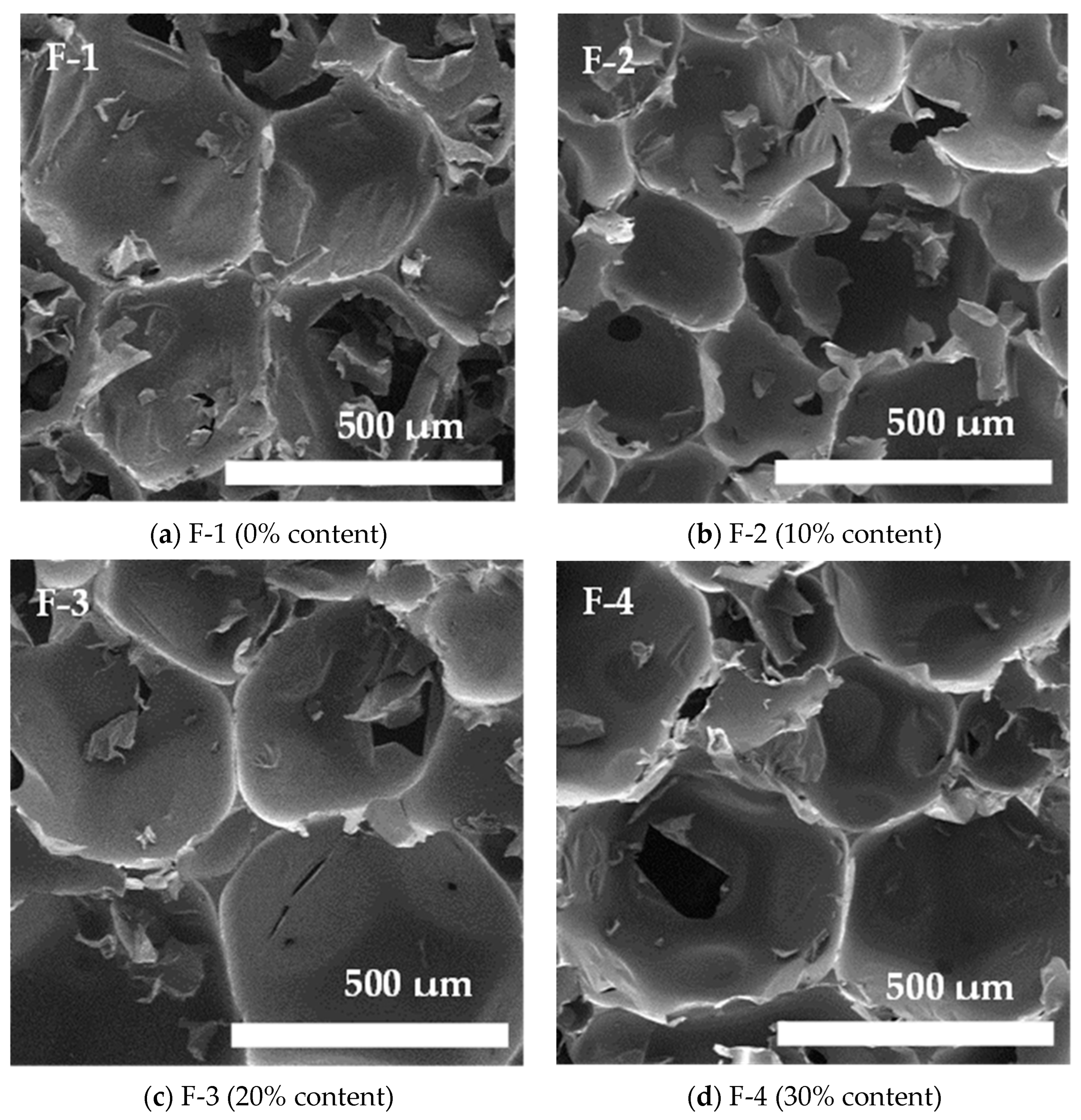
| Property | Pentane-1,2,5-triol | Oxypropylated 125PTO | Polyol Component |
|---|---|---|---|
| Viscosity (mPa·s) | 60 | 180 | 430 |
| OH no. (mg KOH/g) | 1380 * | 410 | 300 |
| Characteristic Data | F-1 (0%) | F-2 (10%) | F-3 (20%) | F-4 (30%) |
|---|---|---|---|---|
| Polyol (g) | 10.0 | 9.0 | 8.0 | 7.0 |
| pMDI (g) | 12.0 | 12.0 | 12.0 | 12.0 |
| Oxypropylated 125PTO (g) | 0 | 1.0 | 2.0 | 3.0 |
| wt (%) (based on polyol component) | 0 | 10 | 20 | 30 |
| wt (%) bio-based content (based on PU foam) | 0 | 1.95 | 3.90 | 5.85 |
| Reaction time at 25 °C cup test | ||||
| - Cream time (s) | 20.2 ± 0.4 | 19.7 ± 0.4 | 19.5 ± 0.3 | 19.7 ± 0.3 |
| - Gel time (s) | 133.8 ± 2.5 | 119.1 ± 2.3 | 112.8 ± 2.2 | 116.5 ± 2.1 |
| - Tack-free time (s) | 185.6 ± 2.6 | 203.5 ± 2.8 | 198.7 ± 2.7 | 186.8 ± 2.5 |
| Apparent density (kg/m3) | 52.8 ± 1.5 | 56.0 ± 1.7 | 59.1 ± 1.8 | 64.3 ± 2.0 |
| Closed cell content (%) | 97.4 ± 1.3 | 97.2 ± 1.4 | 93.0 ± 1.4 | 90.4 ± 1.5 |
| Dimensional Stability (%) | F1 (0%) | F2 (10%) | F3 (20%) | F4 (30%) |
|---|---|---|---|---|
| 48 h, −25 °C | ||||
| - Length (%) | 2.1 ± 0.4 | 1.8 ± 0.3 | 1.1 ± 0.3 | 0.0 ± 0.0 |
| - Width (%) | 2.2 ± 0.5 | 0.0 ± 0.0 | 0.9 ± 0.2 | 0.0 ± 0.0 |
| - Thickness (%) | 2.9 ± 0.8 | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.0 ± 0.0 |
| 48 h, 100 °C | ||||
| - Length (%) | −2.1 ± 0.3 | −0.9 ± 0.2 | 0.0 ± 0.0 | −0.4 ± 0.1 |
| - Width (%) | 2.6 ± 0.5 | 1.7 ± 0.4 | 1.8 ± 0.4 | 0.6 ± 0.2 |
| - Thickness (%) | 0.3 ± 0.1 | 0.7 ± 0.2 | −0.7 ± 0.2 | 0.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grancharov, G.; Atanasova, M.-D.; Kalinova, R.; Tuleshkov, P.; Petrov, P.D.; Marinova, M.K.; Ravutsov, M.A.; Simeonov, S.P. Biorenewable Oxypropylated Pentane-1,2,5-triol as a Source for Incorporation in Rigid Polyurethane Foams. Polymers 2023, 15, 4148. https://doi.org/10.3390/polym15204148
Grancharov G, Atanasova M-D, Kalinova R, Tuleshkov P, Petrov PD, Marinova MK, Ravutsov MA, Simeonov SP. Biorenewable Oxypropylated Pentane-1,2,5-triol as a Source for Incorporation in Rigid Polyurethane Foams. Polymers. 2023; 15(20):4148. https://doi.org/10.3390/polym15204148
Chicago/Turabian StyleGrancharov, Georgy, Mariya-Desislava Atanasova, Radostina Kalinova, Pencho Tuleshkov, Petar D. Petrov, Maya K. Marinova, Martin A. Ravutsov, and Svilen P. Simeonov. 2023. "Biorenewable Oxypropylated Pentane-1,2,5-triol as a Source for Incorporation in Rigid Polyurethane Foams" Polymers 15, no. 20: 4148. https://doi.org/10.3390/polym15204148
APA StyleGrancharov, G., Atanasova, M.-D., Kalinova, R., Tuleshkov, P., Petrov, P. D., Marinova, M. K., Ravutsov, M. A., & Simeonov, S. P. (2023). Biorenewable Oxypropylated Pentane-1,2,5-triol as a Source for Incorporation in Rigid Polyurethane Foams. Polymers, 15(20), 4148. https://doi.org/10.3390/polym15204148









