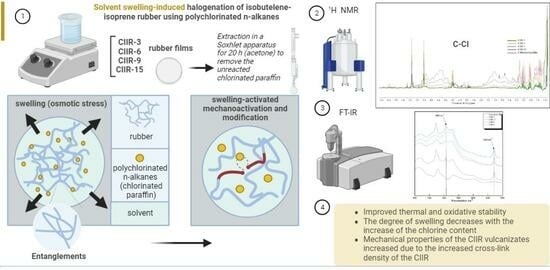
Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties
Abstract
:1. Introduction
- Chlorination or bromination of the IIR solution with gaseous halogen;
- Neutralization of the formed halogenated IIR;
- Washing of the halogenated IIR solution to remove salts;
- Introduction of a stabilizing antioxidant into the halogenated IIR;
- Degassing, isolating the halogenated IIR, and drying.
- Efficient mixing of the viscous elastomer mass requires high energy consumption;
- There is difficulty in obtaining a homogeneous product;
- The process is accompanied by the self-heating of the reaction mass; therefore, strict process control is required, and, if necessary, measures to cool the mixing equipment;
- This method is limited in terms of the halogen content in the modified rubbers.
2. Materials
3. Methods
3.1. Modification in Polychlorinated N-Alkane (CP) Solution
3.2. Extraction
3.3. Oxygen Flask Combustion Method
3.4. X-ray Photoelectron Spectroscopy
3.5. 1H NMR Spectroscopy
3.6. FT-IR Spectroscop
3.7. Preparation of CIIR (IIR) Vulcanizates
3.8. Oxidative Degradation
3.9. Mechanical Properties
3.10. Chemical Resistance
4. Results and Discussion
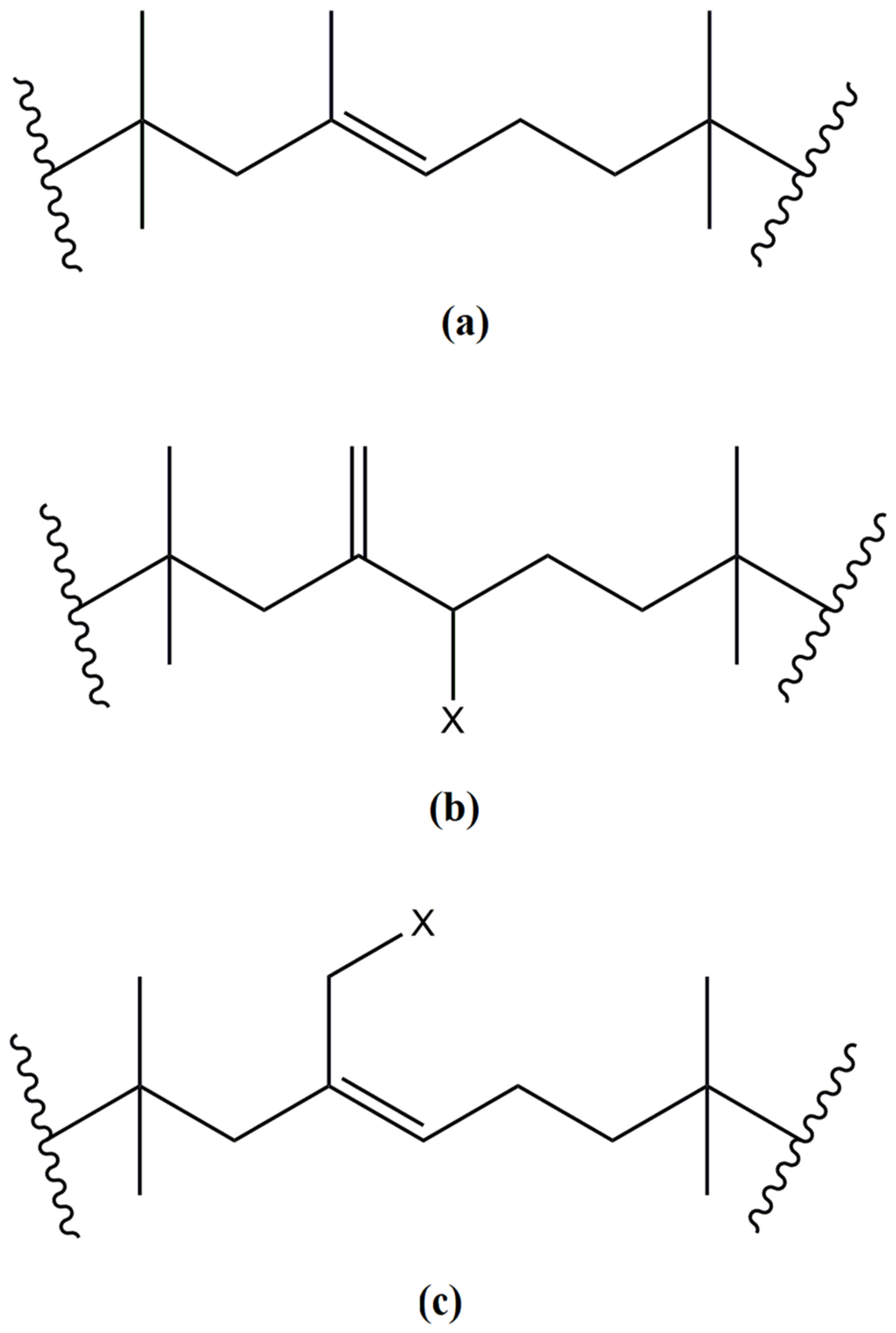
4.1. Microstructural Analysis of the IIR with Different Amounts of CP
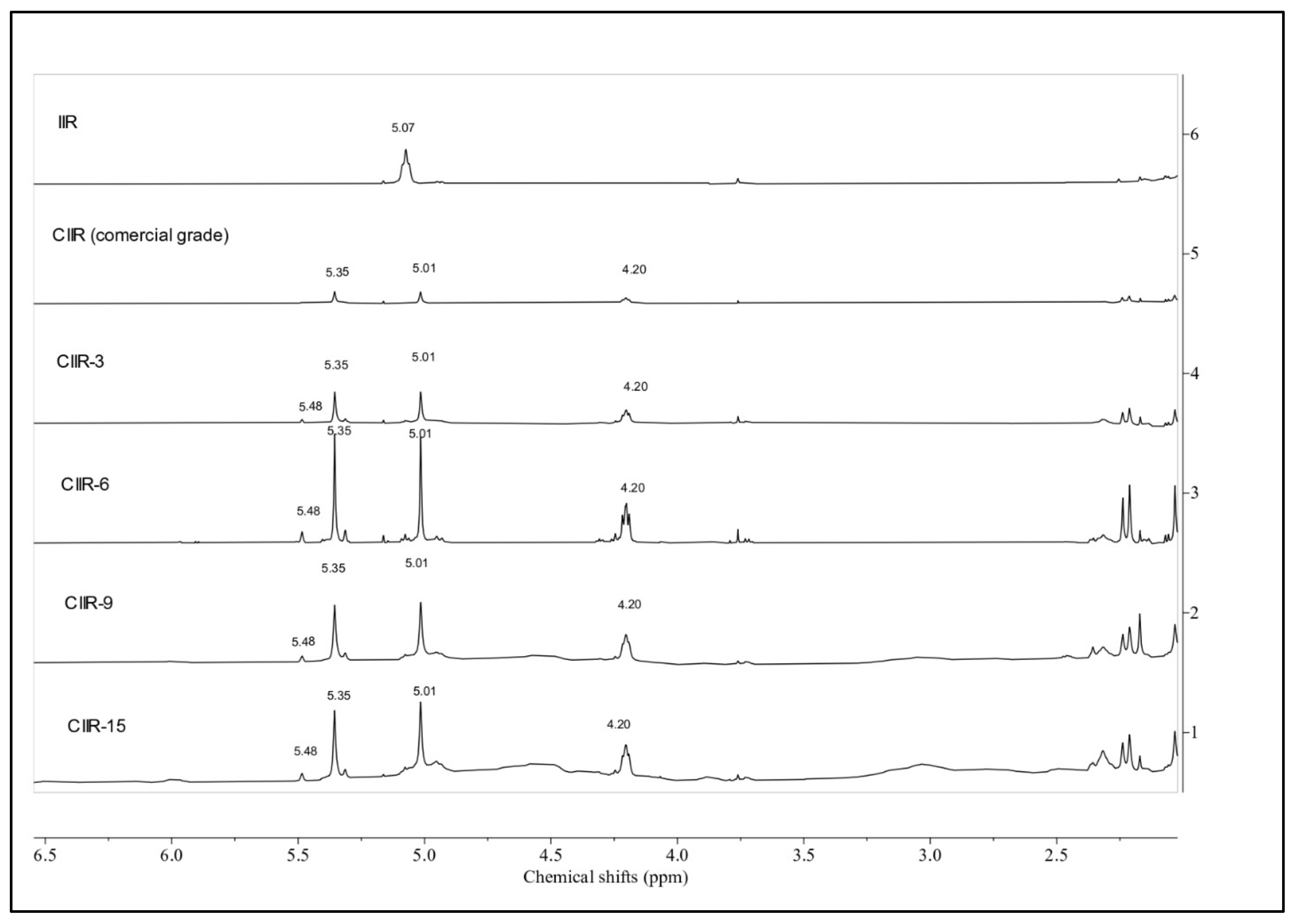
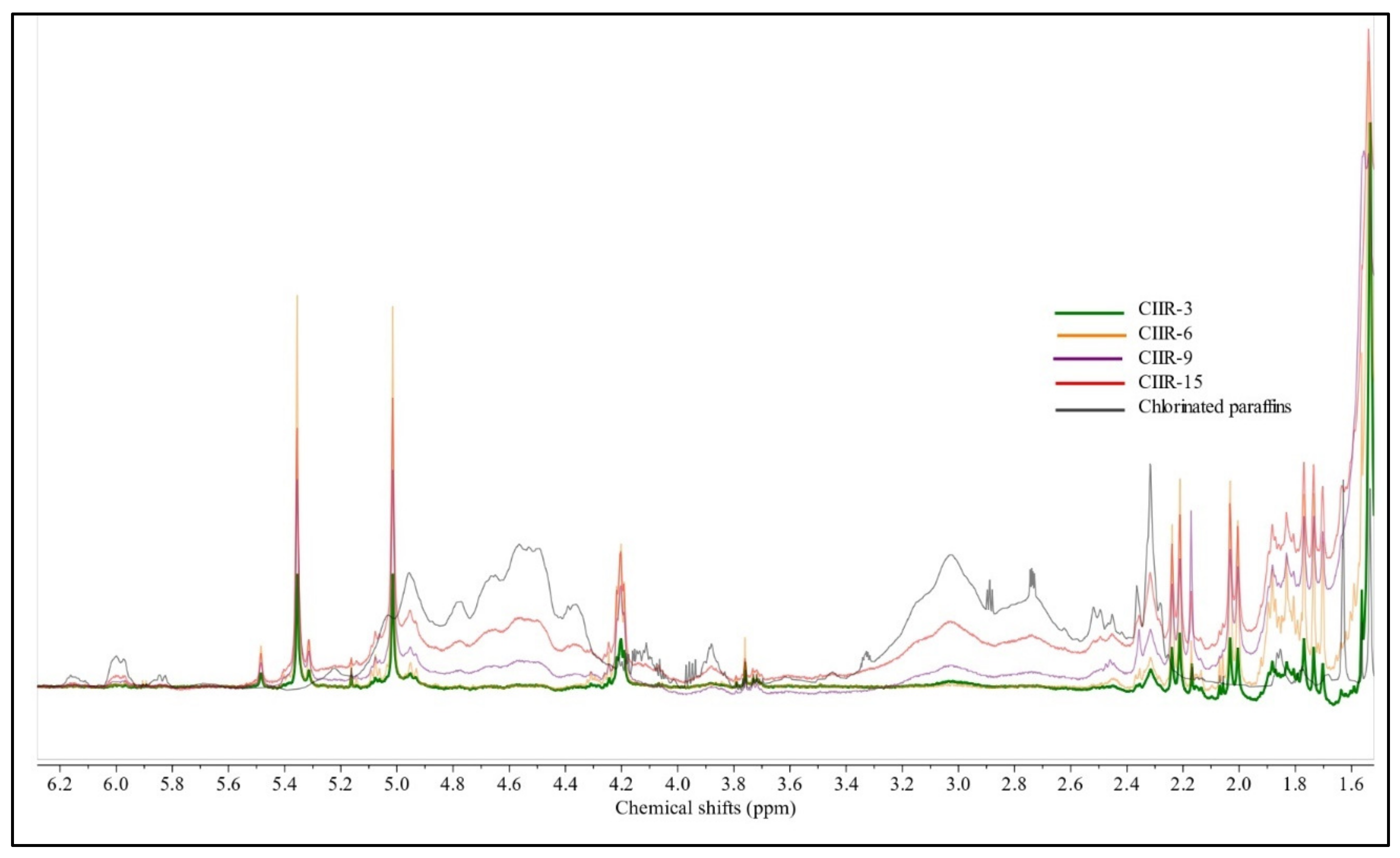
4.2. 1H NMR Spectroscopy
4.3. FT-IR Spectroscopy
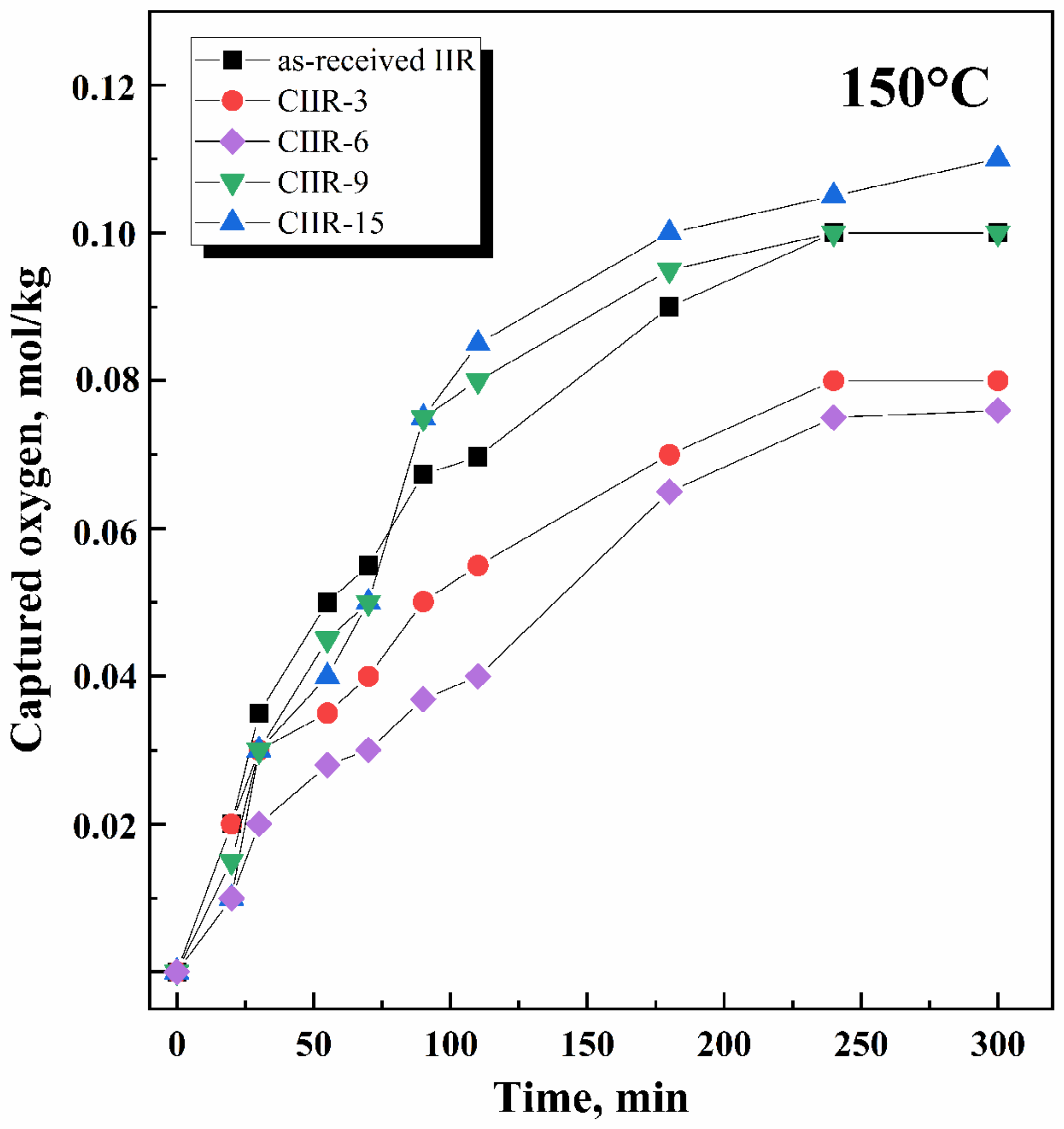
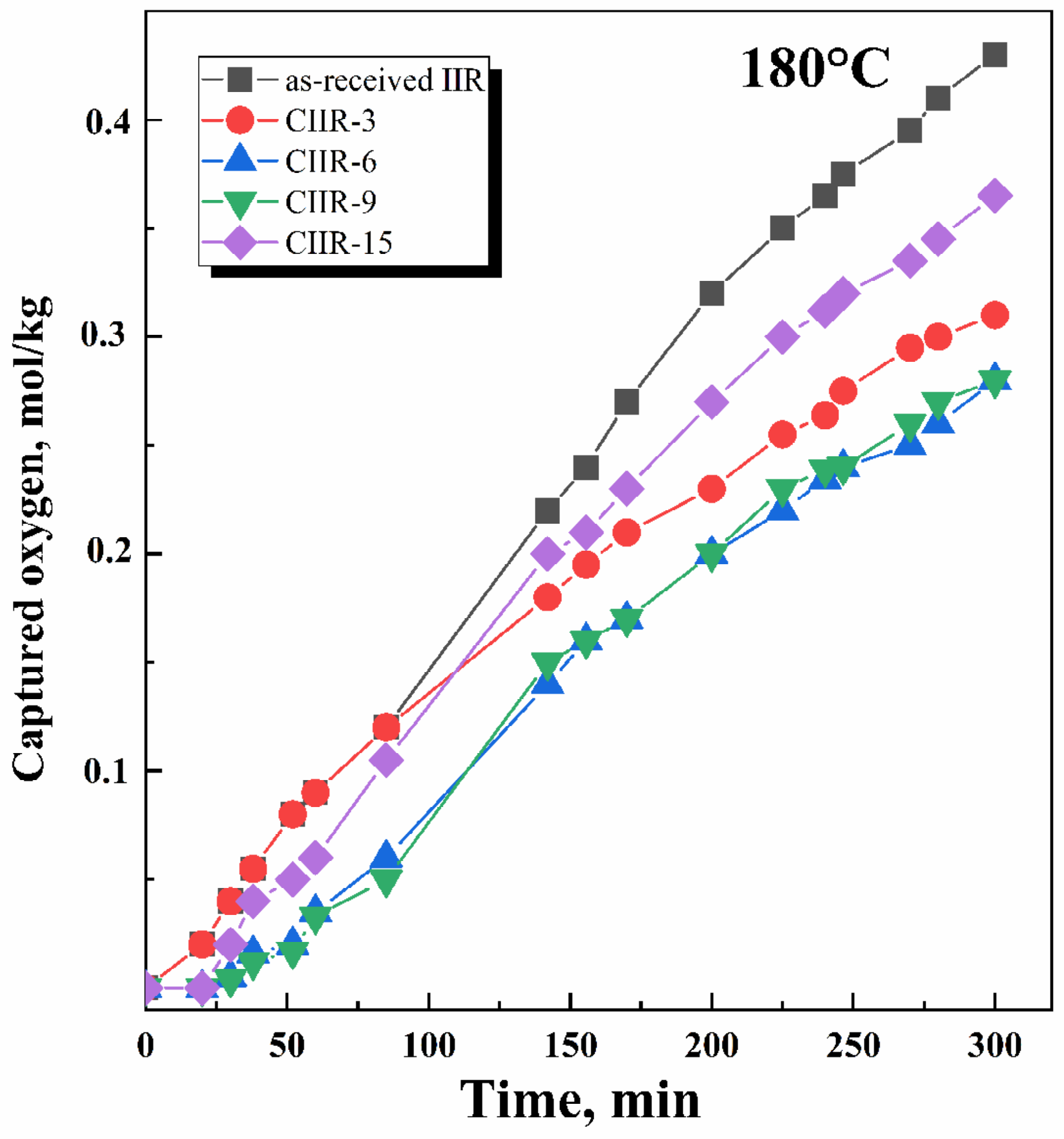
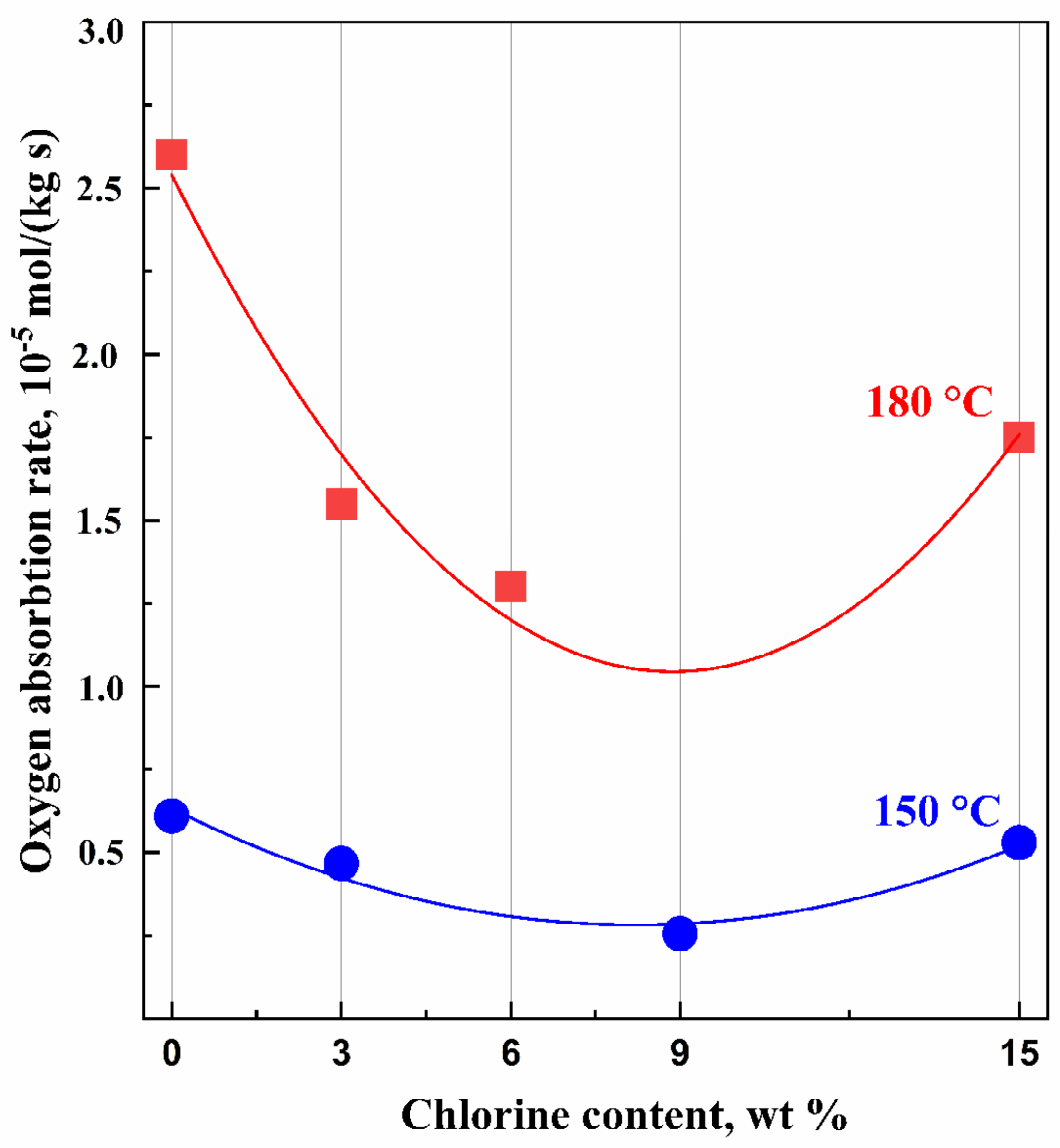
4.4. Thermal and Oxidative Stability
- The process rate (Figure 9) is quite different: for as-received IIR, it is 0.61 × 10 −5 and 2.6 × 10−5 mol/kg·s for 150 °C and 180 °C, respectively; for chlorinated IIR, it ranges from 0.46 to 0.53 × 10−5 mol/kg·s and from 1.55 to 1.75 × 10−5 mol/kg·s for 150 °C and 180 °C, respectively, depending on the chlorine content.
- Oxidation proceeds at sufficiently high rates.
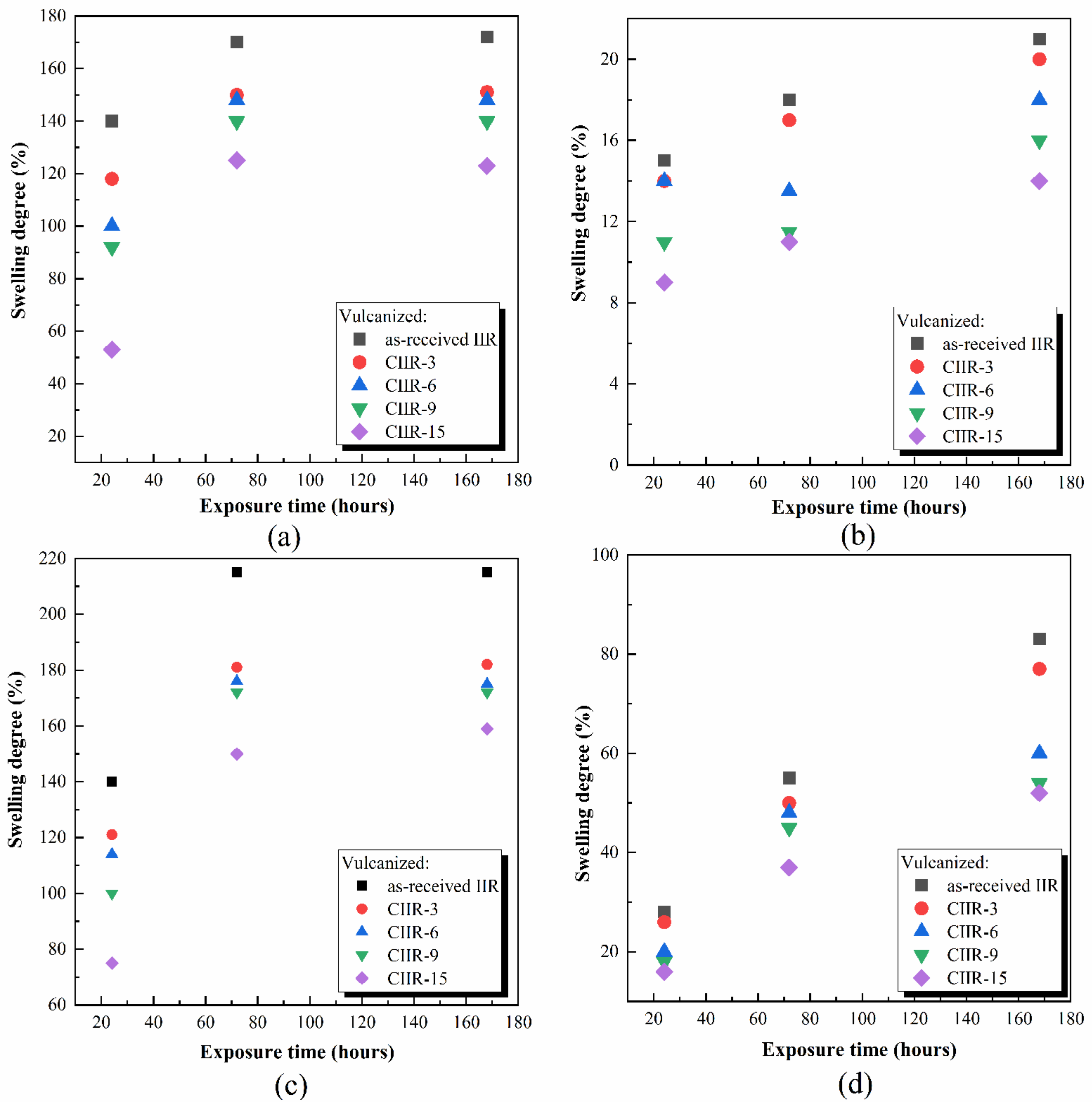
4.5. Chemical Resistance
4.6. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nihmath, A.; Ramesan, M.T. Development of Novel Elastomeric Blends Derived from Chlorinated Nitrile Rubber and Chlorinated Ethylene Propylene Diene Rubber. Polym. Test. 2020, 89, 106728. [Google Scholar] [CrossRef]
- Xiang, Y.; Shen, X.; Gao, J.; Asiri, A.M.; Marwani, H.M. Grafting Polyisoprene onto Surfaces of Nanosilica via RAFT Polymerization and Modification of Natural Rubber. Polym. Eng. Sci. 2019, 59, 1167–1174. [Google Scholar] [CrossRef]
- Promchim, J.; Kanking, S.; Niltui, P.; Wimolmala, E.; Sombatsompop, N. Swelling and Mechanical Properties of (Acrylonitrile-Butadiene Rubber)/(Hydrogenated Acrylonitrile-Butadiene Rubber) Blends with Precipitated Silica Filled in Gasohol Fuels. J. Vinyl Addit. Technol. 2016, 22, 239–246. [Google Scholar] [CrossRef]
- Bonilla-Cruz, J.; Hernández-Mireles, B.; Mendoza-Carrizales, R.; Ramírez-Leal, L.; Torres-Lubián, R.; RamosdeValle, L.; Paul, D.; Saldívar-Guerra, E. Chemical Modification of Butyl Rubber with Maleic Anhydride via Nitroxide Chemistry and Its Application in Polymer Blends. Polymers 2017, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.X.; Shao, H.Q.; Zhang, E.H.; Wang, Z.; Zhao, J.R. The Solvent-Free Modification of Butyl Rubber in the Medium of NaH. J. Ind. Eng. Chem. 2014, 20, 184–188. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, S.; Gao, S.; Wan, S.; He, X. Improving Thermal-oxidative Stability of Nitrile Butadiene Rubber Composites by Surface Modification of Zirconium Phosphate. Polym. Compos. 2023, 44, 505–514. [Google Scholar] [CrossRef]
- Fathy, E.S.; Saleh, H.H.; Elnaggar, M.Y.; Ali, Z.I. Gamma Irradiation of (Styrene Butadiene Rubber)/(Devulcanized Waste Rubber) Blends Modified by Organoclay. J. Vinyl Addit. Technol. 2018, 24, 50–57. [Google Scholar] [CrossRef]
- Raef, M.; Hosseini, S.M.; Nabavian Kalat, M.; Razzaghi-Kashani, M. Vulcanization Kinetics of Styrene Butadiene Rubber Reinforced by Graphenic Particles. SPE Polym. 2021, 2, 122–133. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, Z.; Yu, H.; Zhang, B. The Influences of Silane Coupling Agents on Rheological Properties of Bentonite/Nitrile Butadiene Rubber Nanocomposites during Curing Process. J. Vinyl Addit. Technol. 2019, 25, 236–242. [Google Scholar] [CrossRef]
- Sood, N.; Jha, M.K.; Bajpai, S. Studies on the Effect of Low Density Polyethylene on Ethylene Vinyl Acetate/Styrene Butadiene Styrene Blends for Yoga Mat Applications. SPE Polym. 2021, 2, 325–335. [Google Scholar] [CrossRef]
- Sementsov, Y.; Yang, W.; Ivanenko, K.; Makhno, S.; Kartel, M. Modification of Rubber Compositions by Carbon Nanotubes. Appl. Nanosci. 2022, 12, 621–628. [Google Scholar] [CrossRef]
- Grigoruk, Z.G.; Orlov, Y.N.; Levanova, S.V.; Abramova, N.V. Oxidative Bromination of Isoprenyl Links of Butyl Rubber by the System Sodium Bromide-Sodium Hypochlorite-Phosphoric Acid. Russ. J. Appl. Chem. 2010, 83, 1880–1882. [Google Scholar] [CrossRef]
- Maksimov, D.A.; Dorozhkin, V.P.; Khusainova, R.M. Non-Traditional Halogenation Methods for Butyl Rubber. Kauchuk i Rezina 2004, 3, 16–17. [Google Scholar]
- Grigoruk, Z.G.; Orlov, Y.N.; Levanova, S.V.; Abramova, N.V. Oxidative Bromination of Isoprenyl Units of Butyl Rubber by a Combination of Sodium Bromide—Sodium Hypochlorite—Phosphoric Acid. Zhurnal Prikl. Khimii 2010, 83, 1740–1742. [Google Scholar]
- Cao, R.; Zhao, X.; Zhao, X.; Wu, X.; Li, X.; Zhang, L. Bromination Modification of Butyl Rubber and Its Structure, Properties, and Application. Ind. Eng. Chem. Res. 2019, 58, 16645–16653. [Google Scholar] [CrossRef]
- Wang, H.; Wei, X.; Zou, M.; Feng, Y. The Synthesis of Bromobutyl Rubbers with Desired Allylic Bromide Structures and Mechanism Suggestion. Polym. Sci. Ser. B 2022, 64, 651–656. [Google Scholar] [CrossRef]
- Mikhailov, I.A.; Andriasyan, Y.O.; Jozwik, R.; Zaikov, G.E.; Popov, A.A. Physical Chemistry Research for Engineering and Applied Sciences, Volume Two; Pearce, E.M., Howell, B.A., Pethrick, R.A., Zaikov, G.E., Eds.; Apple Academic Press: New York, NY, USA, 2015; Volume 2, ISBN 9780429172366. [Google Scholar]
- Andriasyan, Y.O.; Mikhailov, I.A.; Dvoryashina, T.N.; Karpova, S.G.; Popov, A.A.; Moskalev, Y.G. Studying the Properties of Elastomeric Composites of SKI-3 Isoprene Rubber and Chlorine-Containing Butyl Rubber. Int. Polym. Sci. Technol. 2011, 38, 15–18. [Google Scholar] [CrossRef]
- Metze, F.K.; Sant, S.; Meng, Z.; Klok, H.-A.; Kaur, K. Swelling-Activated, Soft Mechanochemistry in Polymer Materials. Langmuir 2023, 39, 3546–3557. [Google Scholar] [CrossRef]
- Clough, J.M.; van der Gucht, J.; Sijbesma, R.P. Mechanoluminescent Imaging of Osmotic Stress-Induced Damage in a Glassy Polymer Network. Macromolecules 2017, 50, 2043–2053. [Google Scholar] [CrossRef]
- Lee, C.K.; Diesendruck, C.E.; Lu, E.; Pickett, A.N.; May, P.A.; Moore, J.S.; Braun, P.V. Solvent Swelling Activation of a Mechanophore in a Polymer Network. Macromolecules 2014, 47, 2690–2694. [Google Scholar] [CrossRef]
- Messmer, D.; Bertran, O.; Kissner, R.; Alemán, C.; Schlüter, A.D. Main-Chain Scission of Individual Macromolecules Induced by Solvent Swelling. Chem. Sci. 2019, 10, 6125–6139. [Google Scholar] [CrossRef]
- Raukhvarger, A.B.; Solovyov, M.Y.; Irzhak, V.I. Microphase Segregation during Deformation of Elastomers. Chem. Phys. Lett. 1989, 155, 455–458. [Google Scholar] [CrossRef]
- Darabi, E.; Itskov, M. A Generalized Tube Model of Rubber Elasticity. Soft Matter 2021, 17, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, Y. Visualized Bond Scission in Mechanically Activated Polymers. Chin. J. Polym. Sci. 2017, 35, 1315–1327. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Masjuki, H.H.; Siang, C.T.; Fazal, M.A. Compatibility of Elastomers in Palm Biodiesel. Renew Energy 2010, 35, 2356–2361. [Google Scholar] [CrossRef]
- Chai, A.B.; Andriyana, A.; Verron, E.; Johan, M.R. Mechanical Characteristics of Swollen Elastomers under Cyclic Loading. Mater. Des. 2013, 44, 566–572. [Google Scholar] [CrossRef]
- Valentín, J.L.; Carretero-González, J.; Mora-Barrantes, I.; Chassé, W.; Saalwächter, K. Uncertainties in the Determination of Cross-Link Density by Equilibrium Swelling Experiments in Natural Rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Monte Carlo and Molecular Dynamics Simulations of Polymers. Phys. Scr. 1991, T35, 61–65. [Google Scholar] [CrossRef]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Jensen, E.V.; Urry, W.H. Reactions of Atoms and Free Radicals in Solution. X. The Addition of Polyhalomethanes to Olefins. J. Am. Chem. Soc. 1947, 69, 1100–1105. [Google Scholar] [CrossRef]
- Mikhaylov, I.A.; Sukhareva, K.V.; Andriasyan, Y.O.; Popov, A.A.; Vorontsov, N.V. Mechanochemical Modification of Natural Rubber. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 19–23 September 2016; p. 020153. [Google Scholar]
- Sukhareva, K.V.; Mikhailov, I.A.; Andriasyan, Y.O.; Popov, A.A. Thermal Mechano-Chemical Modification of Butyl Rubber in the Presence of Chlorine Containing Reagents. Gummi Fasern Kunststoffe 2016, 69, 374–376. [Google Scholar]
- Mohd-Setapar, S.H.; Nian-Yian, L.; Mohd-Sharif, N.S. Extraction of Rubber (Hevea Brasiliensis) Seed Oil Using Soxhlet Method. Malays. J. Fundam. Appl. Sci. 2014, 10, 1–6. [Google Scholar] [CrossRef]
- Goyal, K.; Singh, N.; Jindal, S.; Kaur, R.; Goyal, A.; Awasthi, R. Oxygen Flask Combustion Method. In Advanced Techniques of Analytical Chemistry: Volume 1; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 113–119. [Google Scholar]
- Klyuchnikov, O.R.; Klyuchnikov, Y.O. Vulcanization of Elastomers with Quinol Ethers of P-Benzoquinone Dioxime. Key Eng. Mater. 2021, 899, 479–485. [Google Scholar] [CrossRef]
- Sukhareva, K.; Mastalygina, E.; Mikhailov, I.; Andriasyan, Y.; Popov, A. Novel Technology of Butyl Rubber Chlorination and Investigation of Chlorinated Modifier Content Influence on Vulcanizing Characteristics of Pure-Gum Compound. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 22–25 November 2017; p. 020015. [Google Scholar]
- ISO 37-2017; International Standard. Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ASTM D2240-05(2010); STMfRPDH. ASTM International: West Conshohocken, PA, USA, 2010. Available online: www.astm.org (accessed on 15 June 2021).
- Smolentseva, I.I.; Mashukov, V.I.; Korotkova, E.I. Determination of Unsaturation Level, Halogen Content, and Main Forms of Isoprene Units in Halogenated Butyl Rubbers by 1H NMR Spectroscopy. J. Appl. Spectrosc. 2015, 82, 465–469. [Google Scholar] [CrossRef]
- Ding, N.X.; Zuo, P.Y.; Jia, Y.; Wang, L.L.; Wang, H.T. Microstructure and Basic Properties of Domestic Butyl Rubber 1751. Adv. Mat. Res. 2014, 912–914, 235–242. [Google Scholar] [CrossRef]
- McNeish, J.R.; Parent, J.S.; Whitney, R.A. Halogenated Poly(Isobutylene-Co-Isoprene): Influence of Halogen Leaving-Group and Polymer Microstructure on Chemical Reactivity. Can. J. Chem. 2013, 91, 420–427. [Google Scholar] [CrossRef]
- Makhiyanov, N. 1H NMR Spectra (600 MHz) and Structure of an Industrial Isobutylene-Isoprene Copolymer (Butyl Rubber). Polym. Sci. Ser. A 2014, 56, 241–255. [Google Scholar] [CrossRef]
- Pazur, R.J.; Petrov, I. The Thermo-Oxidation of Chlorinated and Brominated Isobutylene-Co-Isoprene Polymers: Activation Energies and Reactions from Room Temperature to 100 °C. Polym. Degrad. Stab. 2015, 121, 311–320. [Google Scholar] [CrossRef]
- Ashok, N.; Balachandran, M.; Lawrence, F.; Sebastian, N. EPDM-Chlorobutyl Rubber Blends in γ-Radiation and Hydrocarbon Environment: Mechanical, Transport, and Ageing Behavior. J. Appl. Polym. Sci. 2017, 134, 45195. [Google Scholar] [CrossRef]
- Abdullin, M.I.; Glazyrin, A.B.; Asfandiyarov, R.N.; Muslukhov, R.R. Chlorinated Polymers Based on Low-Molecular-Mass 1,2-Polybutadiene. Polym. Sci. Ser. B 2009, 51, 303–308. [Google Scholar] [CrossRef]
- He, X.R.; Yu, H.; Wang, X.; Rong, Y.Q.; Zhang, R. The Influence of Vulcanization Agents on Vulcanization Kinetics of Chloride Butyl Rubber. Int. Polym. Process. 2013, 28, 398–414. [Google Scholar] [CrossRef]



| Parameter | IIR | CIIR |
|---|---|---|
| Mooney viscosity | 45–56 | 34–44 |
| Non-saturation level, mol% | 1.4–1.8 | - |
| Tg, °C | −69 | −42 |
| Chlorine content, % wt. | - | 1.1–1.3 |
| Sample | IIR (g) | CP (g) | Calculated Cl Content (w/w%) |
|---|---|---|---|
| CIIR-3 | 10 | 0.209 | 3 w/w% |
| CIIR-6 | 10 | 0.417 | 6 w/w% |
| CIIR-9 | 10 | 0.626 | 9 w/w% |
| CIIR-15 | 10 | 1.043 | 15 w/w% |
| Materials | Compound (phr) * |
|---|---|
| Rubber | 100 |
| Quinol ester of p-quinone dioxime | 6 |
| Dithiophosphate accelerator | 2 |
| Sample | Total Chlorine Content (Weight (%)) | |
|---|---|---|
| Before Extraction | After Extraction | |
| CIIR-3 | 3.0 | 2.8 |
| CIIR-6 | 6.0 | 5.7 |
| CIIR-9 | 9.0 | 8.3 |
| CIIR-15 | 15.0 | 14.6 |
| Sample | Element | Before Extraction | After Extraction | ||||
|---|---|---|---|---|---|---|---|
| Atoms (%) | Weight (%) | Error (±) | Atoms (%) | Weight (%) | Error (±) | ||
| CIIR-6 | Cl | 5.93 | 14.98 | 0.50 | 3.23 | 8.97 | 0.46 |
| C | 94.37 | 85.02 | 0.74 | 96.77 | 91.03 | 0.90 | |
| CIIR-9 | Cl | 9.01 | 22.62 | 0.77 | 5.28 | 14.12 | 0.80 |
| C | 90.99 | 77.38 | 0.83 | 94.72 | 85.88 | 1.11 | |
| CIIR-15 | Cl | 12.14 | 29.98 | 0.92 | 7.70 | 19.77 | 0.66 |
| C | 87.86 | 71.02 | 0.80 | 92.3 | 80.23 | 0.79 | |
| Assignment | Chemical Shift, ppm | Multiplicity a | IIR | CIIR (c.g) | CIIR-3 | CIIR-6 | CIIR-9 | CIIR-15 | |
|---|---|---|---|---|---|---|---|---|---|
| CDCl3 | δ 7.26 | s | v.st. | v.st | v.st | v.st | v.st | v.st | |
| Residual signal (chlorinated paraffins) | δ 6.8 | d | N/A | N/A | v.w. | v.w. | v.w. | v.w. | |
| δ 5.99 | d | N/A | N/A | v.w. | v.w. | v.w. | v.w. | ||
| -CH2-C(=CH2)-CHCl-CH2 | δ 5.36 | d | N/A | w | w | st | st | st | |
| Olefinic protons of isoprene units in the 1,4 linkage =CH of isoprene -CH2-C(CH3)=CH-CH2- | δ 5.07 (δ 5.05) | t | st | N/A (o/l) | N/A (o/l) | N/A (o/l) | N/A (o/l) | N/A (o/l) | |
| -CH2-C(=CH2)-CHCl-CH2- | δ 5.01 | s | N/A | w | st | st | st | st | |
| Olefinic protons of “branched” isoprene units | δ 4.93 δ 4.98 | d | w | v.w. | w | w | w | w | |
| -CH2-C(=CH2)-CHCl-CH2- | δ 4.20 | t | N/A | v.w. | w | st | st | st | |
| CH2 of isoprene | δ 1.94 | s | st | N/A | N/A | N/A | N/A | N/A | |
| CH3 of isoprene | δ 1.65 | s | st | N/A | N/A | N/A | N/A | N/A | |
| Methyl and methylene protons of the polyisobutylene units of butyl rubber | CH2 of isobutylene | δ 1.41 | s | v.st | v.st | v.st | v.st | v.st | v.st |
| CH3 of isobutylene | δ 1.11 | s | v.st | v.st | v.st | v.st | v.st | v.st | |
| Sample | δ 1.5–0.67 | δ 4.20 (4.23–4.16) | δ 5.01 (5.05–4.98) | δ 5.36 (5.4–5.29) | δ 5.49 (5.5–5.46) |
|---|---|---|---|---|---|
| CIIR (commercial grade) | 100 | 0.14 | 0.13 | 0.17 | 0.001 |
| CIIR-3 | 100 | 0.13 | 0.16 | 0.17 | 0.01 |
| CIIR-6 | 100 | 0.15 | 0.17 | 0.18 | 0.02 |
| CIIR-9 | 100 | 0.14 | 0.19 | 0.18 | 0.02 |
| CIIR-15 | 100 | 0.18 | 0.26 | 0.16 | 0.02 |
| Samples | Tensile Strength (MPa) | SD * (MPa) | Elongation at Break (%) | SD * (%) | Rebound, (%) | SD * (%) | Hardness (c.u.) | SD * (c.u.) |
|---|---|---|---|---|---|---|---|---|
| IIR | 0.91 | 0.07 | 370 | 25 | 21 | 1 | 23 | 2 |
| CIIR-3 | 1.08 | 0.04 | 300 | 21 | 18 | 2 | 25 | 1 |
| CIIR-6 | 1.07 | 0.05 | 300 | 23 | 21 | 1 | 26 | 1 |
| CIIR-9 | 1.04 | 0.11 | 350 | 34 | 22 | 1 | 24 | 2 |
| CIIR-15 | 1.25 | 0.09 | 360 | 29 | 20 | 2 | 29 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhareva, K.V.; Sukharev, N.R.; Levina, I.I.; Offor, P.O.; Popov, A.A. Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties. Polymers 2023, 15, 4137. https://doi.org/10.3390/polym15204137
Sukhareva KV, Sukharev NR, Levina II, Offor PO, Popov AA. Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties. Polymers. 2023; 15(20):4137. https://doi.org/10.3390/polym15204137
Chicago/Turabian StyleSukhareva, Ksenia Valeriyevna, Nikita Romanovich Sukharev, Irina Ivanovna Levina, Peter Ogbuna Offor, and Anatoly Anatolyevich Popov. 2023. "Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties" Polymers 15, no. 20: 4137. https://doi.org/10.3390/polym15204137
APA StyleSukhareva, K. V., Sukharev, N. R., Levina, I. I., Offor, P. O., & Popov, A. A. (2023). Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties. Polymers, 15(20), 4137. https://doi.org/10.3390/polym15204137