Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PA6 and PA6/GNPs Nanocomposites Preparation
2.3. Thermal Analyses
2.4. Morphological Analyses
3. Results
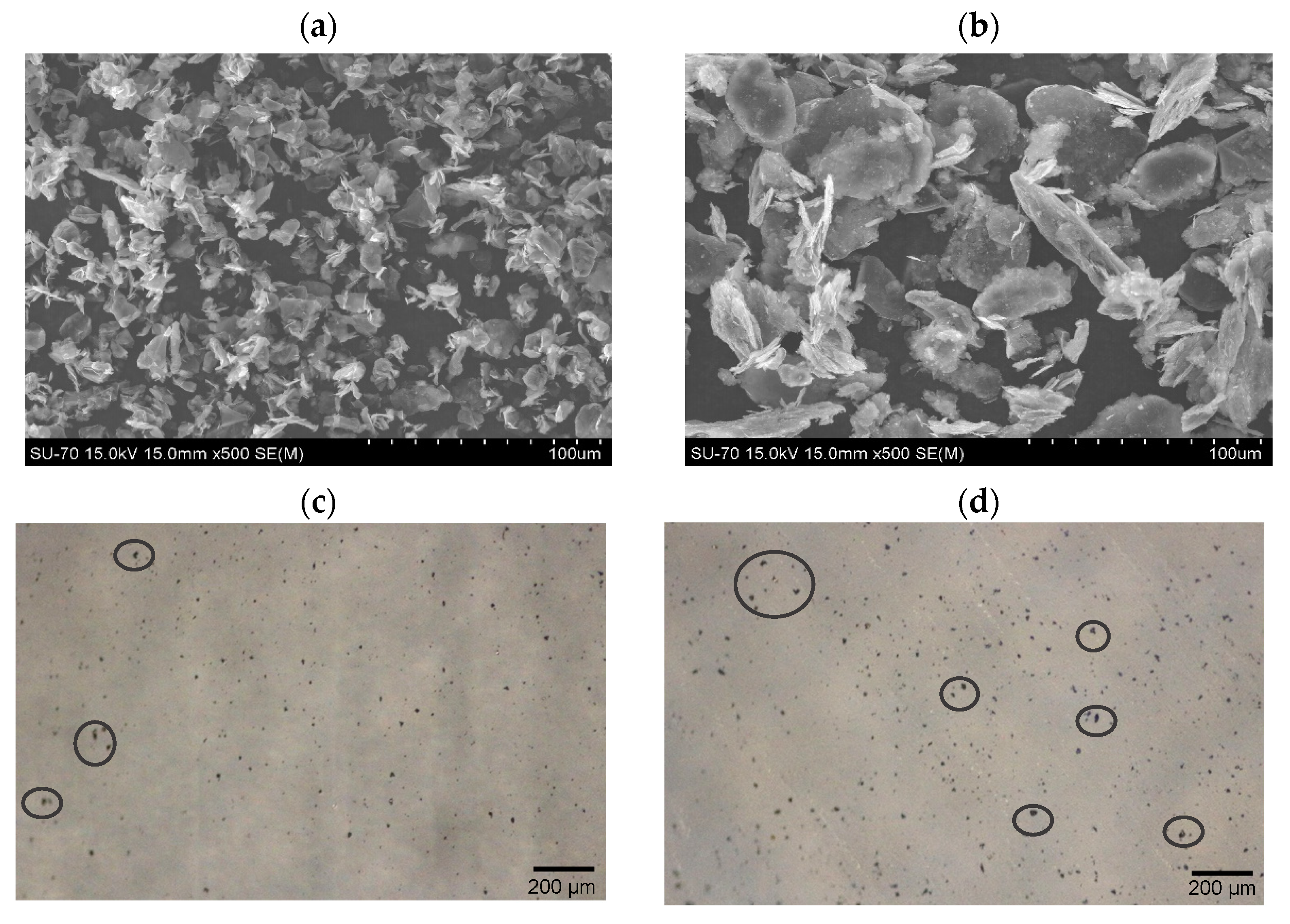
3.1. GNPs and Nanocomposites Morphology
3.2. Non-Isothermal Crystallization Behavior of PA6 and PA6/GNPs Nanocomposites
3.3. Non-Isothermal Crystallization Behavior of PA6 and PA6/GNPs Nanocomposites
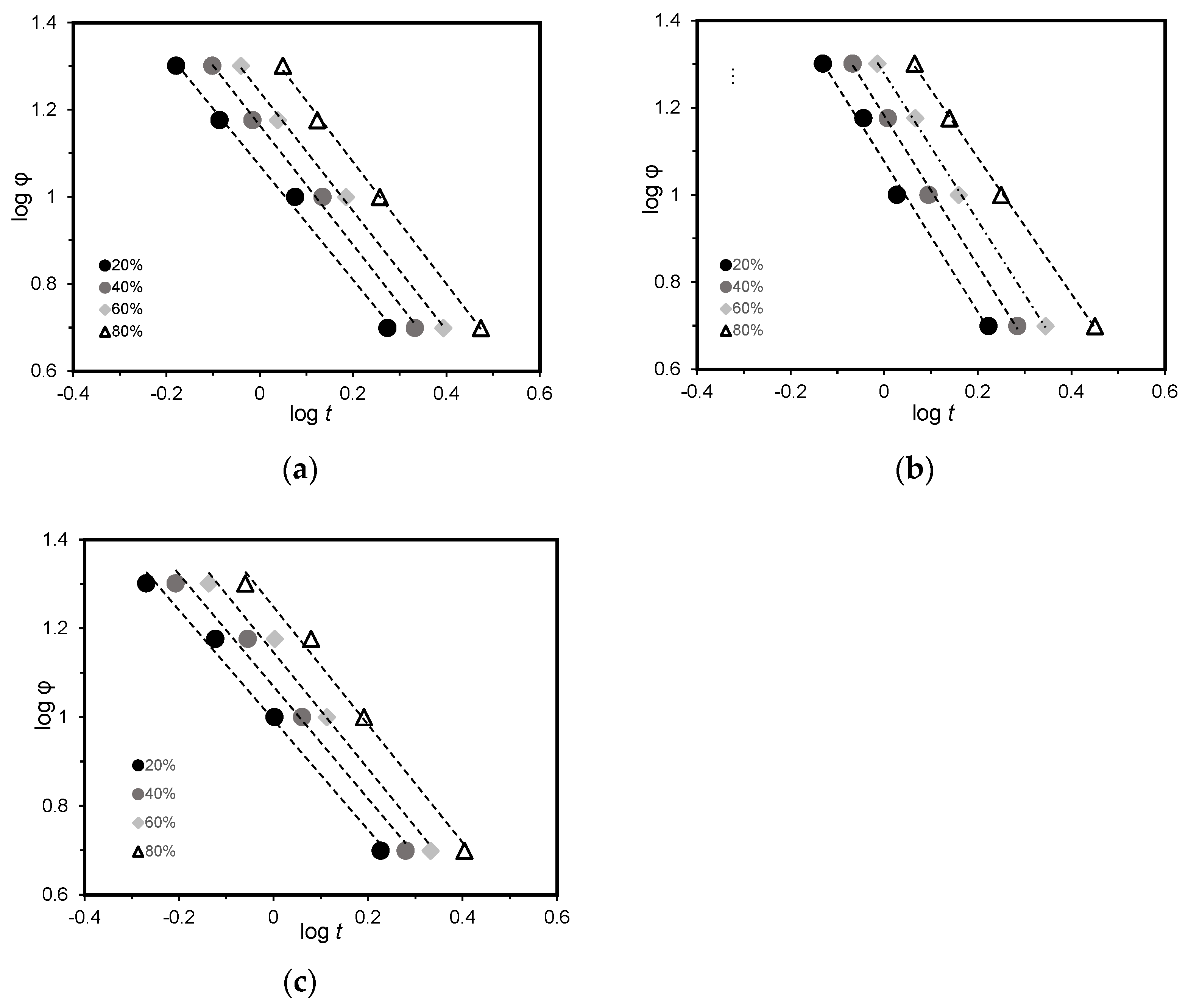
3.3.1. Avrami Model
3.3.2. Lui Model
3.3.3. Friedman Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ageyeva, T.; Sibikin, I.; Karger-Kocsis, J. Polymers and Related Composites via Anionic Ring-Opening Polymerization of Lactams: Recent Developments and Future Trends. Polymers 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Semperger, O.V.; Suplicz, A. The Effect of the Parameters of T-RTM on the Properties of Polyamide 6 Prepared by in Situ Polymerization. Materials 2020, 13, 4. [Google Scholar] [CrossRef]
- Zaldua, N.; Maiz, J.; de la Calle, A.; García-Arrieta, S.; Elizetxea, C.; Harismendy, I.; Tercjak, A.; Müller, A.J. Nucleation and Crystallization of PA6 Composites Prepared by T-RTM: Effects of Carbon and Glass Fiber Loading. Polymers 2019, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Cha, S.H.; Park, Y. Bin Ultra-High-Speed Processing of Nanomaterial-Reinforced Woven Carbon Fiber/Polyamide 6 Composites Using Reactive Thermoplastic Resin Transfer Molding. Compos. B Eng. 2018, 143, 36–46. [Google Scholar] [CrossRef]
- Ben, G.; Sakata, K. Fast Fabrication Method and Evaluation of Performance of Hybrid FRTPs for Applying Them to Automotive Structural Members. Compos. Struct. 2015, 133, 1160–1167. [Google Scholar] [CrossRef]
- Rahman, M.A.; Renna, L.A.; Venkataraman, D.; Desbois, P.; Lesser, A.J. High Crystalline, Porous Polyamide 6 by Anionic Polymerization. Polymer 2018, 138, 8–16. [Google Scholar] [CrossRef]
- Sebenda, J. Anionic Ring-Opening Polymerization: Lactams. In Comprehensive Polymer Science: The Synthesis, Characterization, Reactions & Applications of Polymers; Pergamon Press PLC: Oxford, UK, 1989; Volume 3, pp. 511–530. [Google Scholar]
- Sekiguchi, H.; Ivin, K.J.; Saegusa, T. Ring-Opening Polymerization; Ivin, K.J., Saegusa, T., Eds.; Elsevier: London, UK, 1984; Volume 2, p. 809. [Google Scholar]
- Roda, J. Polyamides. In Handbook of Ring-Opening Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 165. ISBN 3527319530. [Google Scholar]
- Kovács, Z.; Pomázi, Á.; Toldy, A. The Flame Retardancy of Polyamide 6—Prepared by in Situ Polymerisation of ε-Caprolactam—For T-RTM Applications. Polym. Degrad. Stab. 2021, 195, 109797. [Google Scholar] [CrossRef]
- Barhoumi, N.; Maazouz, A.; Jaziri, M.; Abdelhedi, R. Polyamide from Lactams by Reactive Rotational Molding via Anionic Ring-Opening Polymerization: Optimization of Processing Parameters. Express Polym. Lett. 2013, 7, 76–87. [Google Scholar] [CrossRef]
- Murray, J.J.; Robert, C.; Gleich, K.; McCarthy, E.D.; Brádaigh, C.M.Ó. Manufacturing of Unidirectional Stitched Glass Fabric Reinforced Polyamide 6 by Thermoplastic Resin Transfer Moulding. Mater. Des. 2020, 189, 108512. [Google Scholar] [CrossRef]
- Murray, J.J.; Allen, T.; Bickerton, S.; Bajpai, A.; Gleich, K.; McCarthy, E.D.; Brádaigh, C.M.Ó. Thermoplastic RTM: Impact Properties of Anionically Polymerised Polyamide 6 Composites for Structural Automotive Parts. Energies 2021, 14, 5790. [Google Scholar] [CrossRef]
- Boros, R.; Sibikin, I.; Ageyeva, T.; Kovács, J.G. Development and Validation of a Test Mold for Thermoplastic Resin Transfer Molding of Reactive PA-6. Polymers 2020, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Miranda Campos, B.; Bourbigot, S.; Fontaine, G.; Bonnet, F. Thermoplastic Matrix-based Composites Produced by Resin Transfer Molding: A Review. Polym. Compos. 2022, 43, 2485–2506. [Google Scholar] [CrossRef]
- Höhne, C.; Wendel, R.; Käbisch, B.; Anders, T.; Henning, F.; Kroke, E. Hexaphenoxycyclotriphosphazene as FR for CFR Anionic PA6 via T-RTM: A Study of Mechanical and Thermal Properties. Fire Mater. 2016, 41, 291–306. [Google Scholar] [CrossRef]
- Stewart, R. Thermoplastic Composites—Recyclable and Fast to Process. Reinf. Plast. 2011, 55, 22–28. [Google Scholar] [CrossRef]
- Uematsu, H.; Kurita, D.; Nakakubo, S.; Yamaguchi, A.; Yamane, M.; Kawabe, K.; Tanoue, S. Mechanical Behavior of Unidirectional Carbon Fiber-Reinforced Polyamide 6 Composites under Transverse Tension and the Structure of Polyamide 6 among Carbon Fibers. Polym. J. 2020, 52, 1195–1201. [Google Scholar] [CrossRef]
- Şanli, S.; Durmus, A.; Ercan, N. Effect of Nucleating Agent on the Nonisothermal Crystallization Kinetics of Glass Fiber- and Mineral-Filled Polyamide-6 Composites. J. Appl. Polym. Sci. 2012, 125, E268–E281. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Zhang, T.; Niu, K.; Wang, Y.; Zhao, Y.; Zhang, B. Interfacial Adhesion and Shear Behaviors of Aramid Fiber/Polyamide 6 Composites under Different Thermal Treatments. Polym. Test. 2020, 81, 106209. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.; Wang, X.; Lu, L. Non-Isothermal Crystallization Kinetics of Nylon 6/Attapulgite Nanocomposites. Polym. Test. 2010, 29, 596–602. [Google Scholar] [CrossRef]
- Pan, B.; Yue, Q.; Ren, J.; Wang, H.; Jian, L.; Zhang, J.; Yang, S. Non-Isothermal Crystallization Kinetics of PA6/Attapulgite Composites Prepared by Melt Compounding. J. Macromol. Sci. Part B 2006, 45, 1025–1037. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, G.; Justice, R.; Schaefer, D.W.; Zhang, S.; Yang, M.; Han, C.C. Synthesis and Characterization of Multi-Walled Carbon Nanotubes Reinforced Polyamide 6 via in Situ Polymerization. Polymer 2005, 46, 5125–5132. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical Properties and Electrical Conductivity of Carbon-Nanotube Filled Polyamide-6 and Its Blends with Acrylonitrile/Butadiene/Styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-Manchado, M.A. Graphene Filled Polymer Nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef]
- Keledi, G.; Hari, J.; Pukanszky, B. Polymer Nanocomposites: Structure, Interaction, and Functionality. Nanoscale 2012, 4, 1919–1938. [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 1260132315. [Google Scholar]
- Mago, G.; Kalyon, D.M.; Jana, S.C.; Fisher, F.T. Polymer Nanocomposite Processing, Characterization, and Applications. J. Nanomater. 2010, 2010, 325807. [Google Scholar] [CrossRef]
- Van Rijswijk, K.; Bersee, H.E.N.; Jager, W.F.; Picken, S.J. Optimisation of Anionic Polyamide-6 for Vacuum Infusion of Thermoplastic Composites: Choice of Activator and Initiator. Compos. Part A Appl. Sci. Manuf. 2006, 37, 949–956. [Google Scholar] [CrossRef]
- Van Rijswijk, K.; Bersee, H.E.N.; Beukers, A.; Picken, S.J.; Van Geenen, A.A. Optimisation of Anionic Polyamide-6 for Vacuum Infusion of Thermoplastic Composites: Influence of Polymerisation Temperature on Matrix Properties. Polym. Test. 2006, 25, 392–404. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Askeland, P.; Drzal, L.T. The Nucleating Effect of Exfoliated Graphite Nanoplatelets and Their Influence on the Crystal Structure and Electrical Conductivity of Polypropylene Nanocomposites. J. Mater. Sci. 2008, 43, 2895–2907. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Xue, C.; Young, R.J. The Mechanisms of Reinforcement of Polypropylene by Graphene Nanoplatelets. Mater. Sci. Eng. B 2017, 216, 2–9. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Gomez, J.; Villaro, E.; Karagiannidis, P.G.; Elmarakbi, A. Effects of Chemical Structure and Morphology of Graphene-Related Materials (GRMs) on Melt Processing and Properties of GRM/Polyamide-6 Nanocomposites. Results Mater. 2020, 7, 100105. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-Based Composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent Advances in the Synthesis and Applications of Graphene–Polymer Nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Elmarakbi, A.; Azoti, W. State of the Art on Graphene Lightweighting Nanocomposites for Automotive Applications. In Experimental Characterization, Predictive Mechanical and Thermal Modeling of Nanostructures and Their Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene-Based Polymer Composites and Their Applications. Polym. Plast. Technol. Eng. 2013, 52, 319–331. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Pavlidou, E.; Vouroutzis, N.; Palatzoglou, P.; Karayannidis, G.P. Preparation by Melt Mixing and Characterization of Isotactic Polypropylene/SiO2 Nanocomposites Containing Untreated and Surface-Treated Nanoparticles. J. Appl. Polym. Sci. 2006, 100, 2684–2696. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Zhao, X.; Zhao, D.; Yang, G. A Commercial Production Route to Prepare Polymer-based Nanocomposites by Unmodified Multilayer Graphene. J. Appl. Polym. Sci. 2015, 132, 42742. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, H.; Liang, M. Non-Isothermal Crystallization Study of in-Situ Exfoliated Graphite Filled Nylon 6 Composites. J. Polym. Res. 2014, 21, 417. [Google Scholar] [CrossRef]
- Valente, M.; Rossitti, I.; Biblioteca, I.; Sambucci, M. Thermoplastic Composite Materials Approach for More Circular Components: From Monomer to In Situ Polymerization, a Review. J. Compos. Sci. 2022, 6, 132. [Google Scholar] [CrossRef]
- Beatrice, C.A.G.; Branciforti, M.C.; Alves, R.M.V.; Bretas, R.E.S. Rheological, Mechanical, Optical, and Transport Properties of Blown Films of Polyamide 6/Residual Monomer/Montmorillonite Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 3581–3592. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal Melt and Cold Crystallization Kinetics of Poly (Aryl Ether Ether Ketone Ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Lagarinhos, J.; Santos, L.; Oliveira, J. Effect of Catalyst and Activator on Properties of Polyamide 6 Prepared by Thermoplastic Resin Transfer Molding Technology. J. Mater. Eng. Perform. 2022, 31, 7098–7103. [Google Scholar] [CrossRef]
- Lagarinhos, J.N.; Oliveira, M. The Effect of Graphene-Based Materials in Polyamide 6 Obtained by in Situ Thermoplastic Resin Transfer Moulding (T-RTM) Polymerization. In Proceedings of the 20th European Conference on Composite Materials—Composites Meet Sustainability (Vol 1–6), Lausanne, Switzerland, 26–30 June 2022; Vassilopoulos, A., Michaud, V., Eds.; EPFL Lausanne, Composite Construction Laboratory: Lausanne, Switzerland, 2022; pp. 152–159. [Google Scholar]
- Xu, Z.; He, S.; Zhang, J.; Huang, S.; Chen, A.; Fu, X.; Zhang, P. Relationship between the Structure and Thermal Properties of Polypropylene/Graphene Nanoplatelets Composites for Different Platelet-Sizes. Compos. Sci. Technol. 2019, 183, 107826. [Google Scholar] [CrossRef]
- Lagarinhos, J.; Oliveira, M. Nucleation Activity of Graphene in Polyamide 6-Based Nanocomposites Prepared by In Situ Polymerization. Mater. Proc. 2022, 8, 83. [Google Scholar]
- Li, J.; Wong, P.-S.; Kim, J.-K. Hybrid Nanocomposites Containing Carbon Nanotubes and Graphite Nanoplatelets. Mater. Sci. Eng. A 2008, 483–484, 660–663. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q. Non-Isothermal Crystallization Behaviors of Polyamide 6/Clay Nanocomposites. Eur. Polym. J. 2002, 38, 1383–1389. [Google Scholar] [CrossRef]
- Alvaredo, Á.; Martín, M.; Castell, P.; Guzmán de Villoria, R.; Fernández-Blázquez, J. Non-Isothermal Crystallization Behavior of PEEK/Graphene Nanoplatelets Composites from Melt and Glass States. Polymers 2019, 11, 124. [Google Scholar] [CrossRef]
- Hou, X.; Hu, Y.; Hu, X.; Jiang, D. Poly (Ether Ether Ketone) Composites Reinforced by Graphene Oxide and Silicon Dioxide Nanoparticles. High Perform. Polym. 2018, 30, 406–417. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Atiqullah, M.; Al-Harthi, M.A.; Abdelaal, A.F.; Pradhan, D.R. Non-isothermal Crystallization of Ziegler Natta I-PP-graphene Nanocomposite: DSC and New Model Prediction. Can. J. Chem. Eng. 2020, 98, 1398–1410. [Google Scholar] [CrossRef]
- Kiziltas, A.; Liu, W.; Tamrakar, S.; Mielewski, D. Graphene Nanoplatelet Reinforcement for Thermal and Mechanical Properties Enhancement of Bio-Based Polyamide 6, 10 Nanocomposites for Automotive Applications. Compos. Part C Open Access 2021, 6, 100177. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Tensile and Thermomechanical Properties of Short Carbon Fiber Reinforced Polyamide 6 Composites. Compos. B Eng. 2013, 51, 270–275. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Fang, P.; Wang, S.; Qi, X.; Pan, C.; Xie, G.; Liew, K.M. Functionalization of Multi-Walled Carbon Nanotubes Grafted with Self-Generated Functional Groups and Their Polyamide 6 Composites. Carbon 2010, 48, 721–729. [Google Scholar] [CrossRef]
- Jin, J.; Rafiq, R.; Gill, Y.Q.; Song, M. Preparation and Characterization of High Performance of Graphene/Nylon Nanocomposites. Eur. Polym. J. 2013, 49, 2617–2626. [Google Scholar] [CrossRef]
- Caamaño, C.; Grady, B.; Resasco, D.E. Influence of Nanotube Characteristics on Electrical and Thermal Properties of MWCNT/Polyamide 6,6 Composites Prepared by Melt Mixing. Carbon 2012, 50, 3694–3707. [Google Scholar] [CrossRef]
- Faghihi, M.; Shojaei, A.; Bagheri, R. Characterization of Polyamide 6/Carbon Nanotube Composites Prepared by Melt Mixing-Effect of Matrix Molecular Weight and Structure. Compos. B Eng. 2015, 78, 50–64. [Google Scholar] [CrossRef]
- Cebe, P.; Hong, S.-D. Crystallization Behaviour of Poly(Ether-Ether-Ketone). Polymer 1986, 27, 1183–1192. [Google Scholar] [CrossRef]
- De Melo, C.C.N.; Beatrice, C.A.G.; Pessan, L.A.; de Oliveira, A.D.; Machado, F.M. Analysis of Nonisothermal Crystallization Kinetics of Graphene Oxide—Reinforced Polyamide 6 Nanocomposites. Thermochim. Acta 2018, 667, 111–121. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G. Non-Isothermal Crystallization Kinetics of Polyamide-6/Graphite Oxide Nanocomposites. Thermochim. Acta 2010, 500, 13–20. [Google Scholar] [CrossRef]
- Hemlata; Maiti, S.N. Nonisothermal Crystallization Kinetics of PA6 and PA6/SEBS-g-MA Blends. J. Polym. Res. 2012, 19, 9926. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters Characterizing the Kinetics of the Non-Isothermal Crystallization of Poly(Ethylene Terephthalate) Determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Allen, J.L.; Jow, T.R.; Wolfenstine, J. Kinetic Study of the Electrochemical FePO4 to LiFePO4 Phase Transition. Chem. Mater. 2007, 19, 2108–2111. [Google Scholar] [CrossRef]
- Balamurugan, G.P.; Maiti, S.N. Nonisothermal Crystallization Kinetics of Polyamide 6 and Ethylene-co-butyl Acrylate Blends. J. Appl. Polym. Sci. 2008, 107, 2414–2435. [Google Scholar] [CrossRef]
- Weng, W.; Chen, G.; Wu, D. Crystallization Kinetics and Melting Behaviors of Nylon 6/Foliated Graphite Nanocomposites. Polymer 2003, 44, 8119–8132. [Google Scholar] [CrossRef]
- Hay, J.N.; Mills, P.J. The Use of Differential Scanning Calorimetry to Study Polymer Crystallization Kinetics. Polymer 1982, 23, 1380–1384. [Google Scholar] [CrossRef]
- Tarani, E.; Wurm, A.; Schick, C.; Bikiaris, D.N.; Chrissafis, K.; Vourlias, G. Effect of Graphene Nanoplatelets Diameter on Non-Isothermal Crystallization Kinetics and Melting Behavior of High Density Polyethylene Nanocomposites. Thermochim. Acta 2016, 643, 94–103. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, D.; Qiu, Y.; Chen, J.; Yao, X.; Ding, K.; Wei, N. Crystallization of Poly(ϵ-Caprolactone) Composites with Graphite Nanoplatelets: Relations between Nucleation and Platelet Thickness. Thermochim. Acta 2015, 612, 25–33. [Google Scholar] [CrossRef]
- Liu, B.; Hu, G.; Zhang, J.; Wang, Z. The Non-Isothermal Crystallization Behavior of Polyamide 6 and Polyamide 6/HDPE/MAH/L-101 Composites. J. Polym. Eng. 2019, 39, 124–133. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Isoconversional Analysis of Combined Melt and Glass Crystallization Data. Macromol. Chem. Phys. 2006, 207, 20–25. [Google Scholar] [CrossRef]
- Ferreira, C.I.; Dal Castel, C.; Oviedo, M.A.S.; Mauler, R.S. Isothermal and Non-Isothermal Crystallization Kinetics of Polypropylene/Exfoliated Graphite Nanocomposites. Thermochim. Acta 2013, 553, 40–48. [Google Scholar] [CrossRef]
- Run, M.; Song, H.; Yao, C.; Wang, Y. Crystal Morphology and Nonisothermal Crystallization Kinetics of Short Carbon Fiber/Poly(Trimethylene Terephthalate) Composites. J. Appl. Polym. Sci. 2007, 106, 868–877. [Google Scholar] [CrossRef]




| Sample | φ (°C/min) | Tm (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|---|
| PA6 | 5 | 218.9 ± 0.1 | 180.7 ± 0.4 | 38.6 ± 4.1 |
| 10 | 219.1 ± 0.2 | 175.4 ± 0.3 | 39.4 ± 3.0 | |
| 15 | 218.9 ± 0.1 | 170.9 ± 0.7 | 40.8 ± 3.9 | |
| 20 | 218.8 ± 0.3 | 166.3 ± 0.5 | 41.6 ± 2.3 | |
| PA6/GN | 5 | 219.1 ± 0.0 | 185.8 ± 0.6 | 39.6 ± 3.8 |
| 10 | 219.2 ± 0.1 | 180.1 ± 0.4 | 40.9 ± 3.5 | |
| 15 | 219.0 ± 0.1 | 177.9 ± 0.4 | 41.5 ± 2.7 | |
| 20 | 218.9 ± 0.2 | 172.3 ± 0.3 | 42.4 ± 1.8 | |
| PA6/GP | 5 | 218.8 ± 0.3 | 182.2 ± 0.8 | 39.0 ± 3.1 |
| 10 | 218.8 ± 0.5 | 177.4 ± 0.7 | 40.6 ± 4.3 | |
| 15 | 218.6 ± 0.2 | 173.8 ± 0.7 | 41.3 ± 4.1 | |
| 20 | 218.1 ± 0.4 | 168.7 ± 0.9 | 41.9 ± 3.2 |
| Sample | φ (°C/min) | t1/2 (min) | Avrami | ||
|---|---|---|---|---|---|
| n | Zc (min−1) | R2 | |||
| PA6 | 5 | 2.33 ± 0.04 | 4.92 ± 0.06 | 0.68 ± 0.04 | 0.98 |
| 10 | 1.48 ± 0.08 | 5.32 ± 0.12 | 0.89 ± 0.06 | 0.98 | |
| 15 | 1.04 ± 0.04 | 4.84 ± 0.09 | 0.98 ± 0.01 | 0.98 | |
| 20 | 0.84 ± 0.02 | 4.73 ± 0.04 | 0.99 ± 0.01 | 0.99 | |
| PA6/GN | 5 | 2.01 ± 0.04 | 4.66 ± 0.07 | 0.82 ± 0.02 | 0.98 |
| 10 | 1.20 ± 0.03 | 4.71 ± 0.05 | 0.92 ± 0.03 | 0.97 | |
| 15 | 0.95 ± 0.03 | 4.52 ± 0.05 | 1.01 ± 0.01 | 0.97 | |
| 20 | 0.67 ± 0.02 | 4.37 ± 0.04 | 1.02 ± 0.01 | 0.97 | |
| PA6/GP | 5 | 2.04 ± 0.07 | 4.28 ± 0.10 | 0.73 ± 0.03 | 0.98 |
| 10 | 1.38 ± 0.04 | 4.09 ± 0.09 | 0.90 ± 0.03 | 0.98 | |
| 15 | 1.14 ± 0.03 | 3.97 ± 0.04 | 0.97 ± 0.01 | 0.99 | |
| 20 | 0.86 ± 0.03 | 3.81 ± 0.03 | 1.00 ± 0.02 | 0.99 | |
| Sample | φ (°C/min) | Liu | |||
|---|---|---|---|---|---|
| Xt (%) | b | F(T) | R2 | ||
| PA6 | 5 | 20 | 1.31 ± 0.03 | 11.80 ± 0.11 | 0.99 |
| 10 | 40 | 1.37 ± 0.01 | 14.58 ± 0.09 | 1.00 | |
| 15 | 60 | 1.37 ± 0.02 | 17.43 ± 0.14 | 1.00 | |
| 20 | 80 | 1.40 ± 0.03 | 22.93 ± 0.12 | 1.00 | |
| PA6/GN | 5 | 20 | 1.43 ± 0.02 | 10.93 ± 0.16 | 0.99 |
| 10 | 40 | 1.42 ± 0.02 | 13.18 ± 0.11 | 1.00 | |
| 15 | 60 | 1.46 ± 0.03 | 16.98 ± 0.17 | 1.00 | |
| 20 | 80 | 1.49 ± 0.01 | 21.91 ± 0.10 | 1.00 | |
| PA6/GP | 5 | 20 | 1.47 ± 0.03 | 11.47 ± 0.21 | 0.99 |
| 10 | 40 | 1.49 ± 0.02 | 14.01 ± 0.11 | 0.99 | |
| 15 | 60 | 1.51 ± 0.01 | 18.09 ± 0.12 | 0.99 | |
| 20 | 80 | 1.59 ± 0.02 | 21.52 ± 0.13 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagarinhos, J.; Magalhães da Silva, S.; Oliveira, J.M. Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size. Polymers 2023, 15, 4109. https://doi.org/10.3390/polym15204109
Lagarinhos J, Magalhães da Silva S, Oliveira JM. Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size. Polymers. 2023; 15(20):4109. https://doi.org/10.3390/polym15204109
Chicago/Turabian StyleLagarinhos, Joana, Sara Magalhães da Silva, and José Martinho Oliveira. 2023. "Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size" Polymers 15, no. 20: 4109. https://doi.org/10.3390/polym15204109
APA StyleLagarinhos, J., Magalhães da Silva, S., & Oliveira, J. M. (2023). Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size. Polymers, 15(20), 4109. https://doi.org/10.3390/polym15204109








