A Magnesium Carbonate Hydroxide Nanofiber/Poly(Vinylidene Fluoride) Composite Membrane for High-Rate and High-Safety Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Magnesium Carbonate Hydroxide Nanofibers
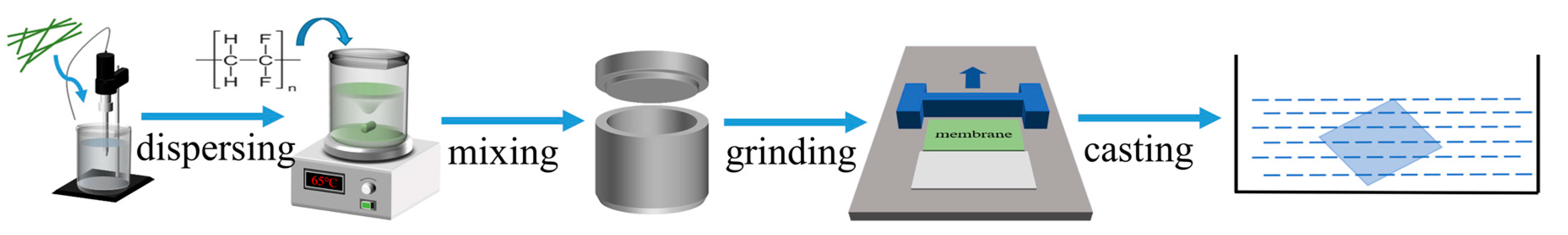
2.3. Preparation of Composite Membranes
2.4. Characterizations
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Magnesium Carbonate Hydroxide (MCH) Nanofiber
3.2. Morphology of Composite Membranes
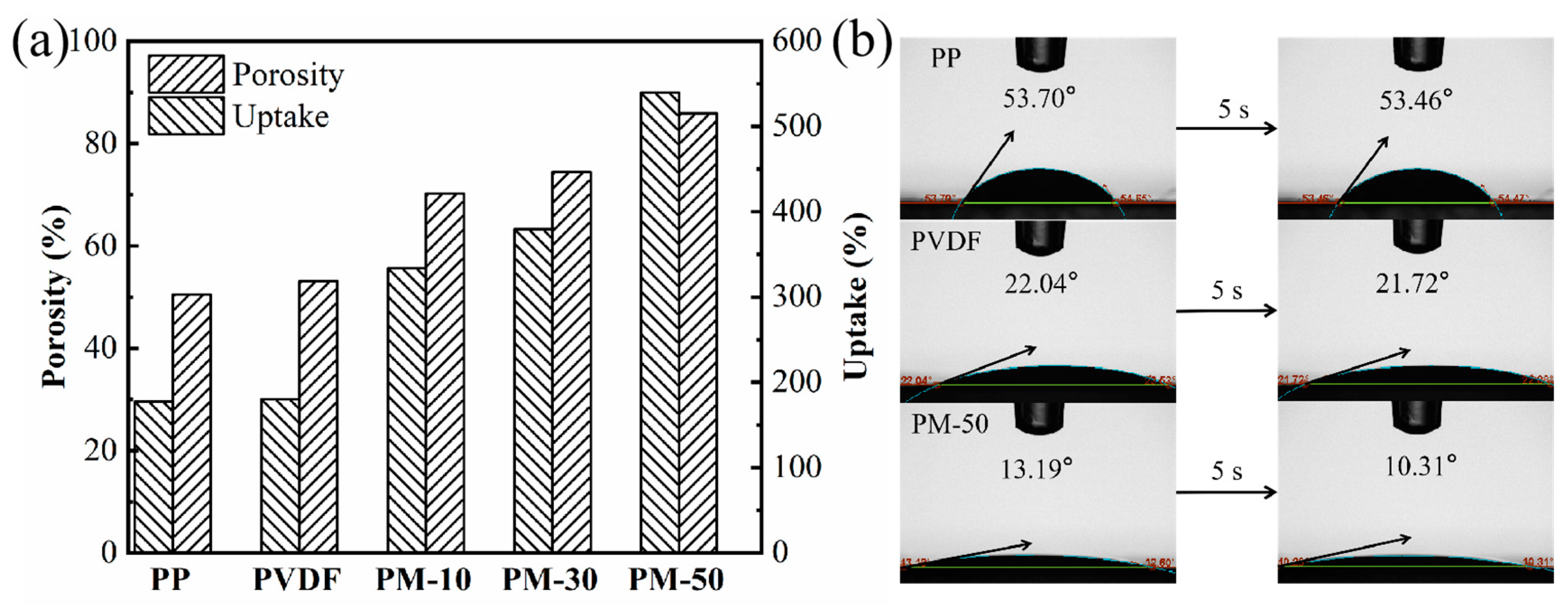
3.3. Porosity and Electrolyte Uptake of Membranes
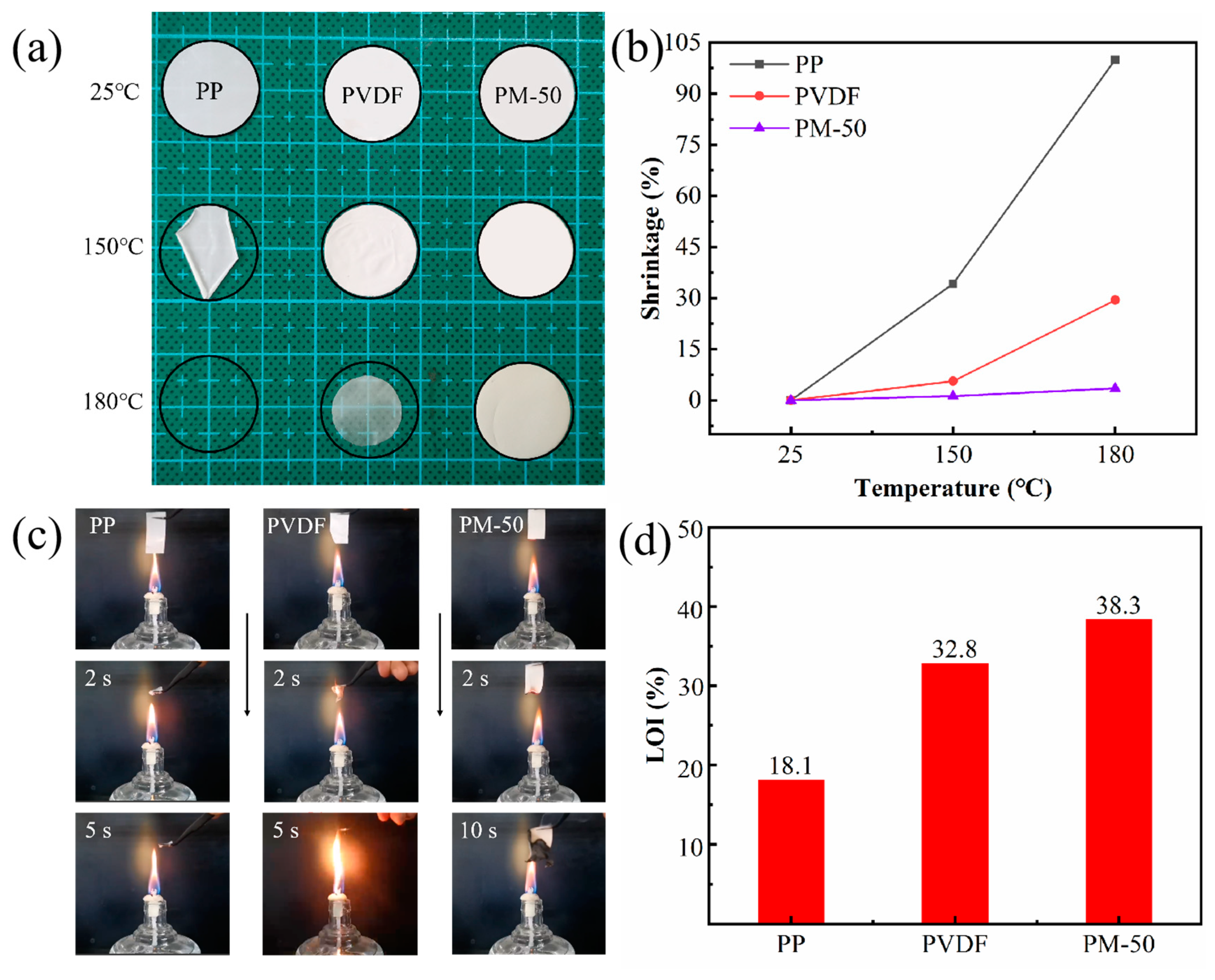
3.4. Thermal Stability and Flame-Retardant Capacity of Membranes
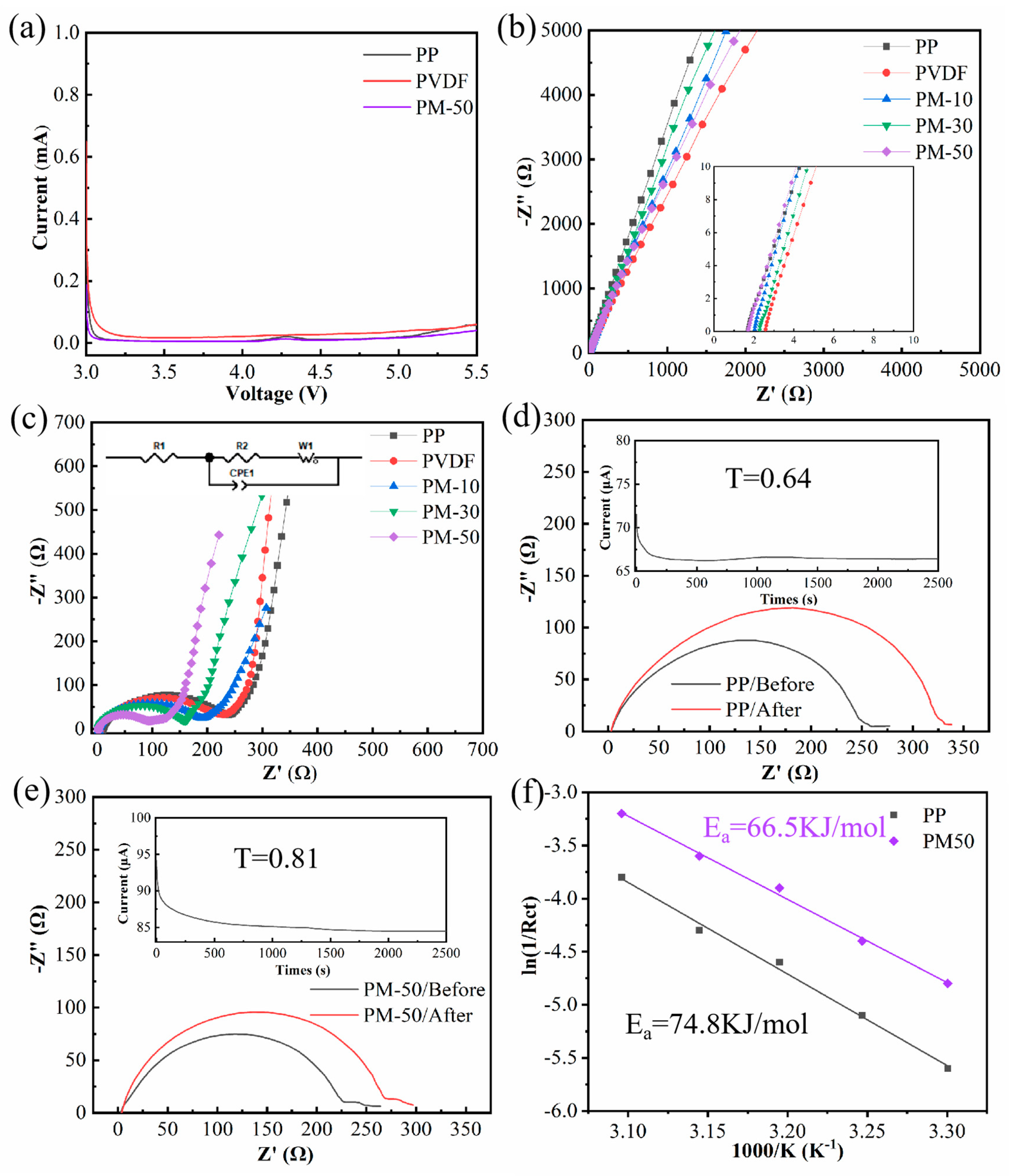
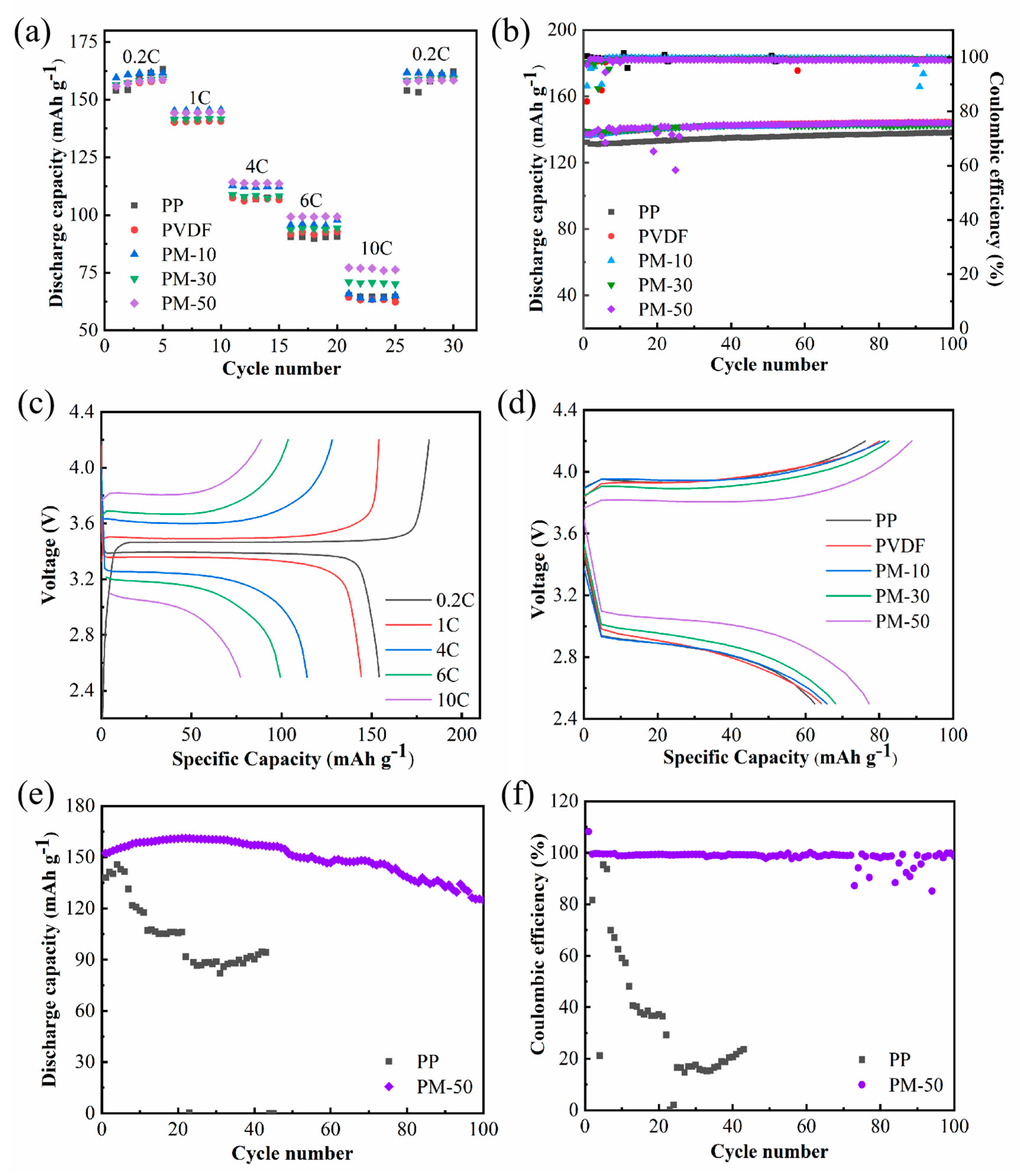
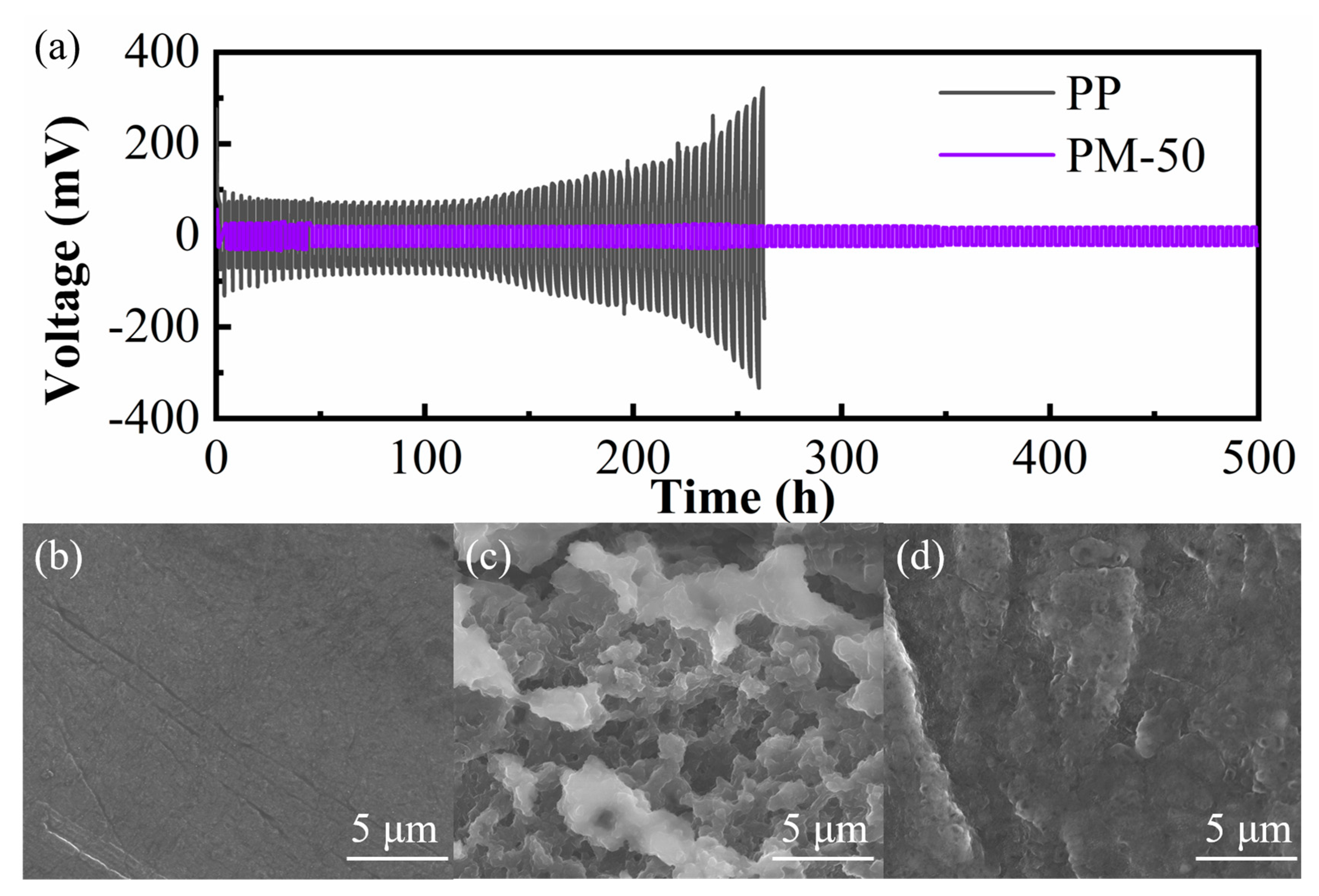
3.5. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, B.; Sun, Z.; Sun, G. Recent progress in cathodic reduction-enabled organic electrosynthesis: Trends, challenges, and opportunities. eScience 2022, 2, 243–277. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Shen, J.; Liu, F.; Chen, G.; Tao, R.; Ma, S.; Peng, Y.; Lu, Y. Anion-Sorbent Composite Separators for High-Rate Lithium-Ion Batteries. Adv. Mater. 2019, 31, 1808338. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Wang, H.; Ye, Z.; Zhou, X.; Huang, H.; Gan, Y.; Liang, C.; Zhang, J.; Zhang, W. Synthesis and electrochemical performance of poly (vinylidene fluoride)/SiO2 hybrid membrane for lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 519–527. [Google Scholar] [CrossRef]
- Choi, J.; Kim, P.J. A roadmap of battery separator development: Past and future. Curr. Opin. Electrochem. 2022, 31, 100858. [Google Scholar] [CrossRef]
- Li, S.; Zhu, W.; Tang, Q.; Huang, Z.; Yu, P.; Gui, X.; Lin, S.; Hu, J.; Tu, Y. Mini Review on Cellulose-Based Composite Separators for Lithium-Ion Batteries: Recent Progress and Perspectives. Energy Fuels 2021, 35, 12938–12947. [Google Scholar] [CrossRef]
- Bicy, K.; Gueye, A.B.; Rouxel, D.; Kalarikkal, N.; Thomas, S. Lithium-ion battery separators based on electrospun PVDF: A review. Surf. Interfaces 2022, 31, 101977. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Lu, Y. Designing safer lithium-based batteries with nonflammable electrolytes: A review. eScience 2021, 1, 163–177. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Costa, C.M.; Lanceros-Mendez, S. Recent advances on battery separators based on poly (vinylidene fluoride) and its copolymers for lithium-ion battery applications. Curr. Opin. Electrochem. 2021, 29, 100752. [Google Scholar] [CrossRef]
- Liu, K.; Zhuo, D.; Lee, H.-W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the Life of Lithium-Based Rechargeable Batteries by Reaction of Lithium Dendrites with a Novel Silica Nanoparticle Sandwiched Separator. Adv. Mater. 2017, 29, 1603987. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.-Y.; Lin, C.-E.; Zhu, B.-K. Progress in polymeric separators for lithium ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Valverde, A.; Gonçalves, R.; Silva, M.M.; Wuttke, S.; Fidalgo-Marijuan, A.; Costa, C.M.; Vilas-Vilela, J.L.; Laza, J.M.; Arriortua, M.I.; Lanceros-Méndez, S.; et al. Metal–Organic Framework Based PVDF Separators for High Rate Cycling Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 11907–11919. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Y.; Xu, C.; Shi, S.; Wang, L.; Zhang, G.; Wang, X.; Zhu, L.; Xu, D. Zirconia fiber membranes based on PVDF as high-safety separators for lithium-ion batteries using a papermaking method. J. Solid State Electrochem. 2019, 23, 269–276. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Wang, Z.; Zhu, Y.; Ma, X.; Cao, C. Poly (vinylidene fluoride)/SiO2 composite membrane separators for high-performance lithium-ion batteries to provide battery capacity with improved separator properties. J. Power Sources 2020, 451, 227759. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, M.; Liu, H.; Na, B.; Lv, R.; Wang, B.; Huang, Y. Modification and characterization of electrospun poly (vinylidene fluoride)/poly (acrylonitrile) blend separator membranes. Compos. Part B Eng. 2017, 112, 31–37. [Google Scholar] [CrossRef]
- Cai, M.; Yuan, D.; Zhang, X.; Pu, Y.; Liu, X.; He, H.; Zhang, L.; Ning, X. Lithium ion battery separator with improved performance via side-by-side bicomponent electrospinning of PVDF-HFP/PI followed by 3D thermal crosslinking. J. Power Sources 2020, 461, 228123. [Google Scholar] [CrossRef]
- Song, Y.; Liu, X.; Ren, D.; Liang, H.; Wang, L.; Hu, Q.; Cui, H.; Xu, H.; Wang, J.; Zhao, C.; et al. Simultaneously Blocking Chemical Crosstalk and Internal Short Circuit via Gel-Stretching Derived Nanoporous Non-Shrinkage Separator for Safe Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2106335. [Google Scholar] [CrossRef]
- Liu, J.; Cao, D.; Yao, H.; Liu, D.; Zhang, X.; Zhang, Q.; Chen, L.; Wu, S.; Sun, Y.; He, D.; et al. Hexagonal Boron Nitride-Coated Polyimide Ion Track Etched Separator with Enhanced Thermal Conductivity and High-Temperature Stability for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 8639–8649. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Xu, L.; Wang, J. Design of A High Performance Zeolite/Polyimide Composite Separator for Lithium-Ion Batteries. Polymers 2020, 12, 764. [Google Scholar] [CrossRef]
- Liu, L.; Xu, T.; Gui, X.; Gao, S.; Sun, L.; Lin, Q.; Song, X.; Wang, Z.; Xu, K. Electrospun Silsequioxane-grafted PVDF hybrid membranes for high-performance rechargeable lithium batteries. Compos. Part B Eng. 2021, 215, 108849. [Google Scholar] [CrossRef]
- Nien, Y.-H.; Chang, C.-N.; Chuang, P.-L.; Hsu, C.-H.; Liao, J.-L.; Lee, C.-K. Fabrication and Characterization of Nylon 66/PAN Nanofibrous Film Used as Separator of Lithium-Ion Battery. Polymers 2021, 13, 1984. [Google Scholar] [CrossRef]
- Meng, N.; Ren, X.; Santagiuliana, G.; Ventura, L.; Zhang, H.; Wu, J.; Yan, H.; Reece, M.J.; Bilotti, E. Ultrahigh β-phase content poly (vinylidene fluoride) with relaxor-like ferroelectricity for high energy density capacitors. Nat. Commun. 2019, 10, 4535. [Google Scholar] [CrossRef] [PubMed]
- Babiker, D.M.D.; Wan, C.; Mansoor, B.; Usha, Z.R.; Yu, R.; Habumugisha, J.C.; Chen, W.; Chen, X.; Li, L. Superior lithium battery separator with extraordinary electrochemical performance and thermal stability based on hybrid UHMWPE/SiO2 nanocomposites via the scalable biaxial stretching process. Compos. Part B Eng. 2021, 211, 108658. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Cai, Q.; Dong, A.; Yang, D.; Zhao, D. Hierarchically Porous Silica Membrane as Separator for High-Performance Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2107957. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Lan, Y.; Song, Z.; Luo, J.; Wei, X.; Sun, F.; Yue, Z.; Yin, C.; Zhou, L.; et al. Aqueous aluminide ceramic coating polyethylene separators for lithium-ion batteries. Solid State Ion. 2020, 345, 115188. [Google Scholar] [CrossRef]
- Rafiz, K.; Murali, D.R.L.; Lin, J.Y.S. Suppressing lithium dendrite growth on lithium-ion/metal batteries by a tortuously porous γ-alumina separator. Electrochim. Acta 2022, 421, 140478. [Google Scholar] [CrossRef]
- Han, D.-H.; Zhang, M.; Lu, P.-X.; Wan, Y.-L.; Chen, Q.-L.; Niu, H.-Y.; Yu, Z.-W. A multifunctional separator with Mg(OH)2 nanoflake coatings for safe lithium-metal batteries. J. Energy Chem. 2021, 52, 75–83. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, L.; Wen, R.; Song, X.; Gao, Z.; Zheng, Z.; Zhang, J. A multifunctional composite membrane for high-safety lithium-ion batteries. J. Mater. Chem. A 2023, 11, 1774–1784. [Google Scholar] [CrossRef]
- Gao, Z.; Wen, R.; Deng, H.; Luo, L.; Cui, X.; Yang, Z.; Zheng, Z.; Zhang, J. Composite Membrane of Poly (vinylidene fluoride) and 2D Ni(OH)2 Nanosheets for High-Performance Lithium-Ion Battery. ACS Appl. Polym. Mater. 2022, 4, 960–970. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Botelho, G.; Lanceros-Méndez, S. Nonsolvent induced phase separation preparation of poly (vinylidene fluoride-co-chlorotrifluoroethylene) membranes with tailored morphology, piezoelectric phase content and mechanical properties. Mater. Des. 2015, 88, 390–397. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, B.; Ji, G.; Xu, Y. Preparation and characterization of PVDF–HFP microporous flat membranes by supercritical CO2 induced phase separation. J. Membr. Sci. 2005, 266, 102–109. [Google Scholar] [CrossRef]
- GB/T 2406.2 standard; Plastics-Determination of Burning Behavior by Oxygen Index-Part 2: Ambient-Temperature Test. GAQSIQ: Beijing, China, 2009.
- Li, X.; Jiang, H.; Liu, Y.; Guo, X.; He, G.; Chu, Z.; Yu, G. Hierarchically porous membranes for lithium rechargeable batteries: Recent progress and opportunities. EcoMat 2022, 4, e12162. [Google Scholar] [CrossRef]
- Yuennan, J.; Sukwisute, P.; Muensit, N. Effect of hydrated salts on the microstructure and phase transformation of poly (vinylidenefluoride-hexafluoropropylene) composites. Mater. Res. Express 2018, 5, 055702. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.-W.; Fang, J.-B.; Li, X.; Yu, M.; Long, Y.-Z. Electrospun High-Thermal-Resistant Inorganic Composite Nonwoven as Lithium-Ion Battery Separator. J. Nanomater. 2020, 2020, 3879040. [Google Scholar] [CrossRef]
- Costa, C.M.; Rodrigues, H.M.; Gören, A.; Machado, A.V.; Silva, M.M.; Lanceros-Méndez, S. Preparation of Poly (vinylidene fluoride) Lithium-Ion Battery Separators and Their Compatibilization with Ionic Liquid—A Green Solvent Approach. ChemistrySelect 2017, 2, 5394–5402. [Google Scholar] [CrossRef]
- Abrha, L.H.; Nikodimos, Y.; Weldeyohannes, H.H.; Hagos, T.T.; Wang, D.-Y.; Huang, C.-J.; Jiang, S.-K.; Wu, S.-H.; Su, W.-N.; Tsai, M.-C.; et al. Effects of a Thermally Electrochemically Activated β-PVDF Fiber on Suppression of Li Dendrite Growth for Anode-Free Batteries. ACS Appl. Energy Mater. 2021, 4, 3240–3248. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, D.; Zuo, K.; Xia, Y.; Yin, J.; Liang, H.; Zeng, Y.-P. Highly dispersible silicon nitride whiskers in asymmetric porous separators for high-performance lithium-ion battery. J. Membr. Sci. 2021, 621, 119001. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Wang, X.; Peng, L.; Hu, T.; Zhang, P.; Zhao, J. Fiber-supported alumina separator for achieving high rate of high-temperature lithium-ion batteries. J. Power Sources 2020, 477, 228680. [Google Scholar] [CrossRef]
- GB/T 2406.1 standard; Plastics-Determination of Burning Behavior by Oxygen Index-Part 1: Guidance. GAQSIQ: Beijing, China, 2008.
- Yang, C.L.; Liu, H.Y.; Xia, Q.L.; Li, Z.H.; Xiao, Q.Z.; Lei, G.T. Effects of SiO2 Nanoparticles and Diethyl Carbonate on the Electrochemical Properties of a Fibrous Nanocomposite Polymer Electrolyte for Rechargeable Lithium Batteries. Arab. J. Sci. Eng. 2014, 39, 6711–6720. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Cai, C.; Wei, Z.; Fu, Y. 3D-cellulose acetate-derived hierarchical network with controllable nanopores for superior Li+ transference number, mechanical strength and dendrites hindrance. Carbohydr. Polym. 2021, 274, 118620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, K.; Zhang, P.; Li, H.; Mi, H. PVDF-HFP based polymer electrolytes with high Li+ transference number enhancing the cycling performance and rate capability of lithium metal batteries. Appl. Surf. Sci. 2022, 574, 151593. [Google Scholar] [CrossRef]
- Gören, A.; Mendes, J.; Rodrigues, H.M.; Sousa, R.E.; Oliveira, J.; Hilliou, L.; Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. High performance screen-printed electrodes prepared by a green solvent approach for lithium-ion batteries. J. Power Sources 2016, 334, 65–77. [Google Scholar] [CrossRef]
- Nien, Y.-H.; Carey, J.R.; Chen, J.-S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors. J. Power Sources 2009, 193, 822–827. [Google Scholar] [CrossRef]


| Sample | D (μm) | Rb (Ω) | σ (mS cm−1) | Rct (Ω) |
|---|---|---|---|---|
| PP | 25.0 | 1.7 | 0.7 | 204.7 |
| PVDF | 41.0 | 2.6 | 0.8 | 195.5 |
| PM-10 | 45.0 | 2.0 | 1.1 | 190.8 |
| PM-30 | 51.5 | 2.3 | 1.2 | 159.1 |
| PM-50 | 49.5 | 1.7 | 1.4 | 93.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Ma, K.; Song, X.; Zhao, Y.; Tang, J.; Zheng, Z.; Zhang, J. A Magnesium Carbonate Hydroxide Nanofiber/Poly(Vinylidene Fluoride) Composite Membrane for High-Rate and High-Safety Lithium-Ion Batteries. Polymers 2023, 15, 4120. https://doi.org/10.3390/polym15204120
Luo L, Ma K, Song X, Zhao Y, Tang J, Zheng Z, Zhang J. A Magnesium Carbonate Hydroxide Nanofiber/Poly(Vinylidene Fluoride) Composite Membrane for High-Rate and High-Safety Lithium-Ion Batteries. Polymers. 2023; 15(20):4120. https://doi.org/10.3390/polym15204120
Chicago/Turabian StyleLuo, Lin, Kang Ma, Xin Song, Yuling Zhao, Jie Tang, Zongmin Zheng, and Jianmin Zhang. 2023. "A Magnesium Carbonate Hydroxide Nanofiber/Poly(Vinylidene Fluoride) Composite Membrane for High-Rate and High-Safety Lithium-Ion Batteries" Polymers 15, no. 20: 4120. https://doi.org/10.3390/polym15204120
APA StyleLuo, L., Ma, K., Song, X., Zhao, Y., Tang, J., Zheng, Z., & Zhang, J. (2023). A Magnesium Carbonate Hydroxide Nanofiber/Poly(Vinylidene Fluoride) Composite Membrane for High-Rate and High-Safety Lithium-Ion Batteries. Polymers, 15(20), 4120. https://doi.org/10.3390/polym15204120




