Amino-Functionalized Silica@Resorcinol–Formaldehyde Nanocomposites for the Removal of Cr(VI) from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Reagents and Materials
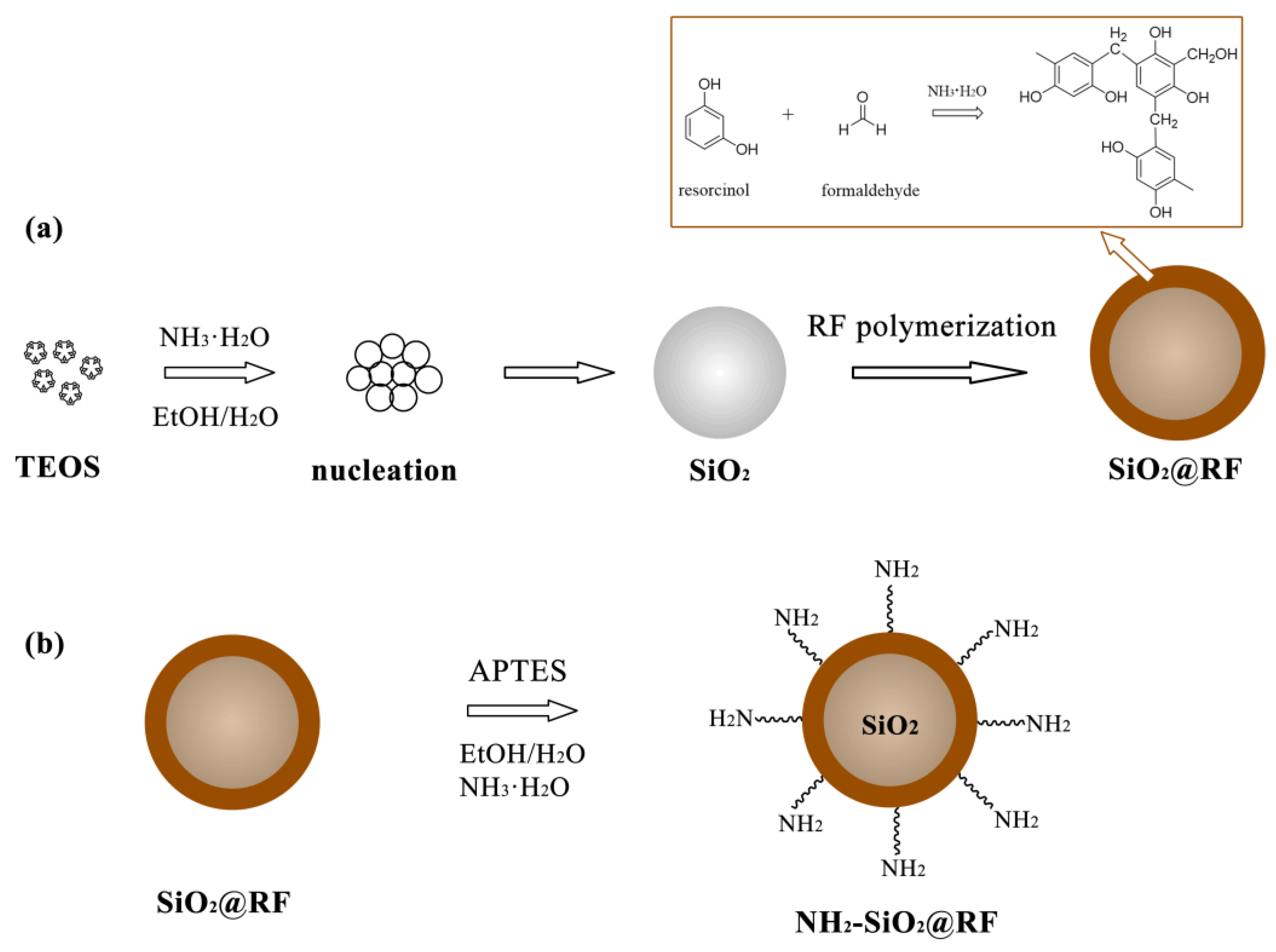
2.2. Preparation of Amino Functionalized Silica@resorcinol–Formaldehyde Nanocomposites (NH2-SiO2@RF)
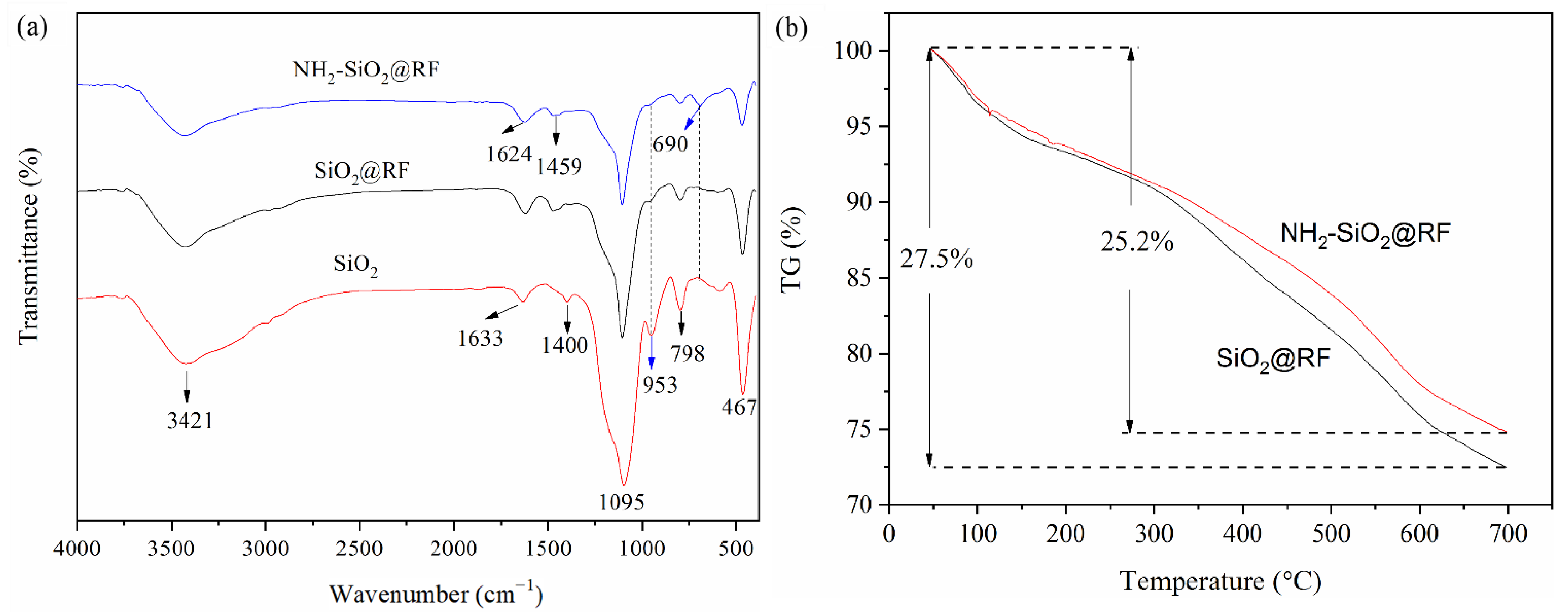
2.3. Characterization of NH2-SiO2@RF
2.4. Adsorption Studies
2.5. Desorption Studies
3. Results and Discussion
3.1. Preparation and Characterization of NH2-SiO2@RF
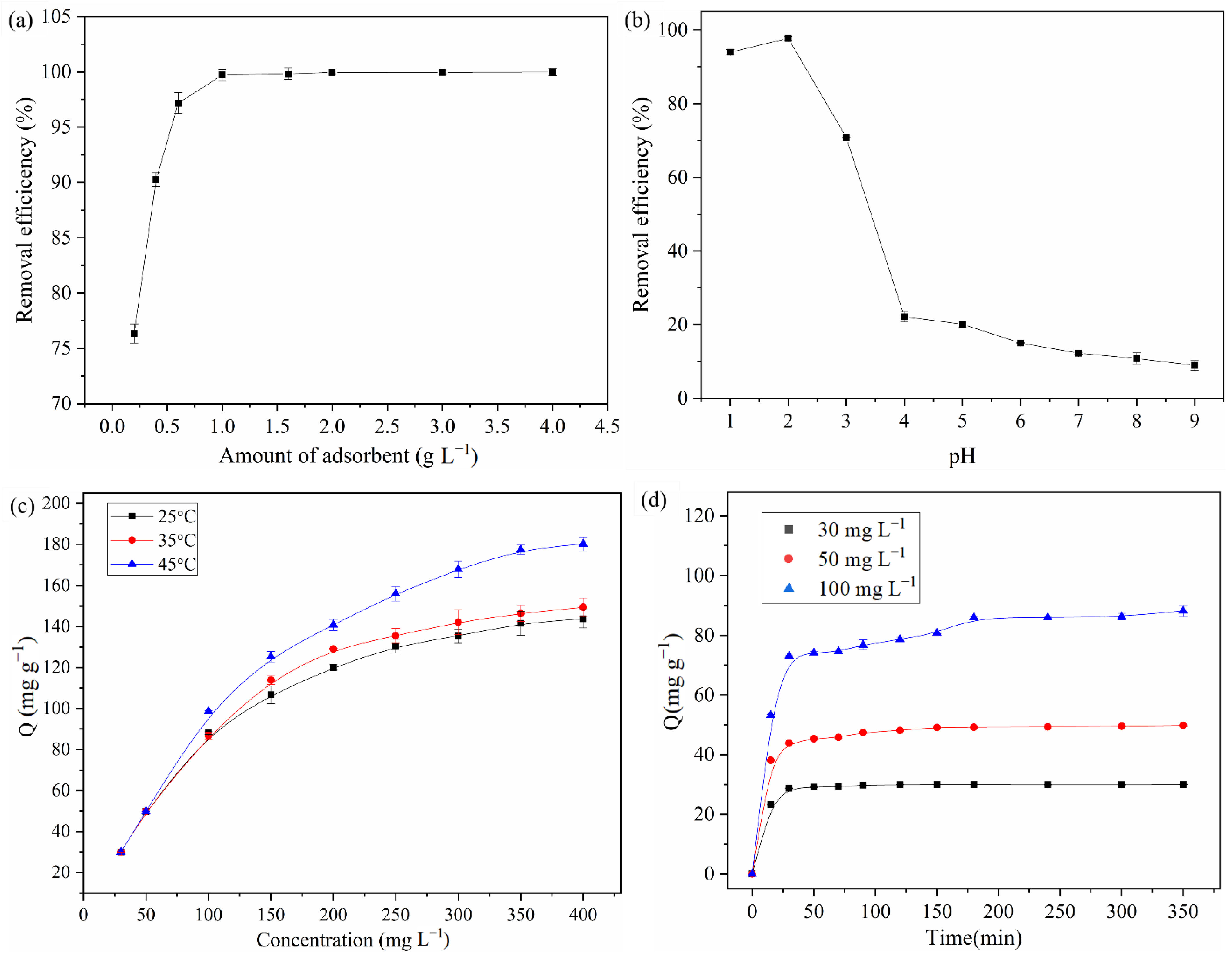
3.2. Effect of NH2-SiO2@RF Dosage
3.3. Effect of Solution pH
3.4. Effect of Initial Concentration and Temperature
3.5. Effect of Contact Time
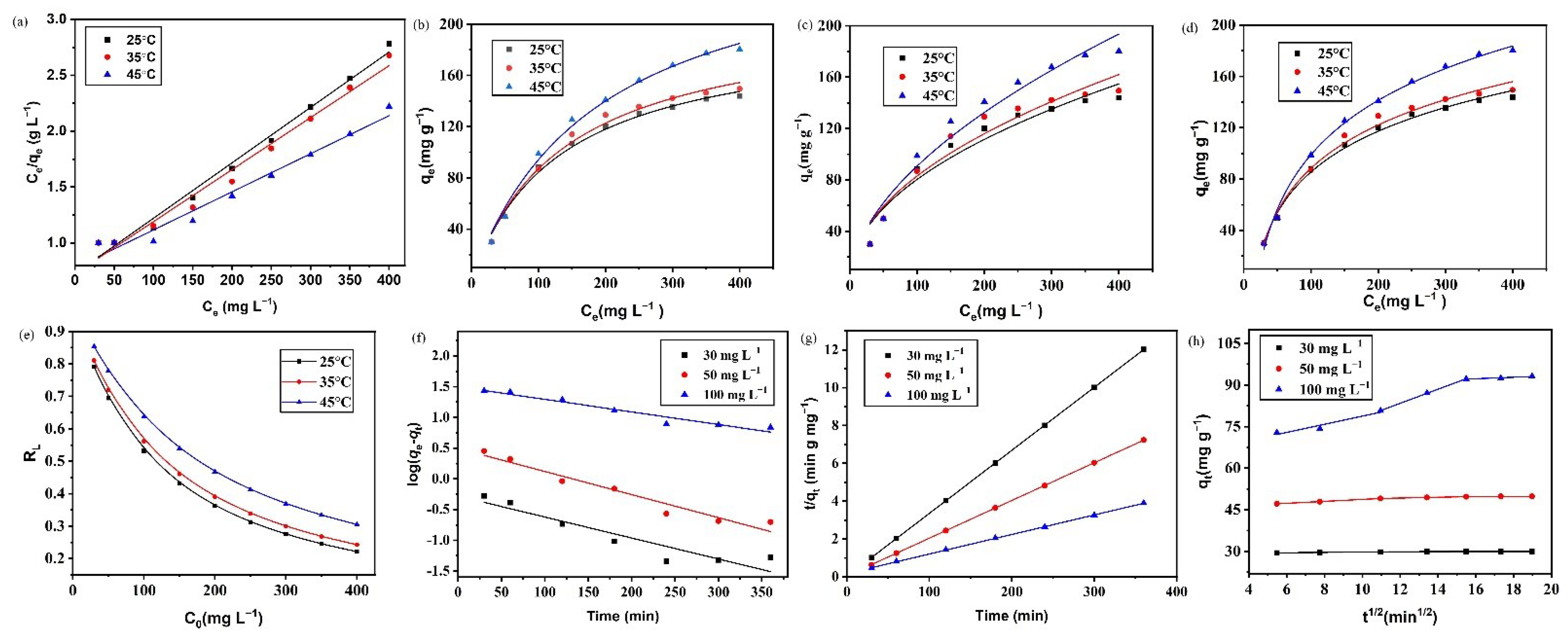
3.6. Adsorption Isotherm Analysis
3.7. Adsorption Kinetic Model
3.8. Intraparticle Diffusion Model
3.9. Thermodynamic Study
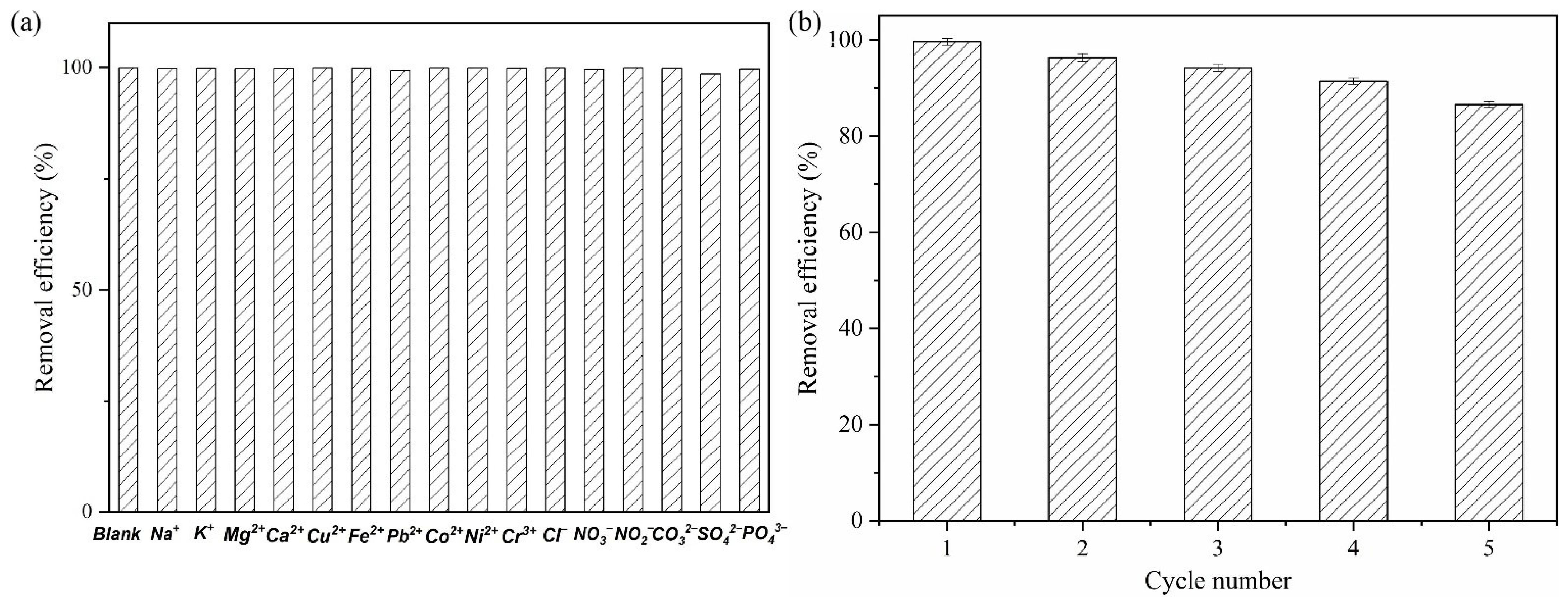
3.10. Anti-Interference Examinations
3.11. Desorption and Reusability
3.12. Comparison of Adsorption Properties
3.13. Primary Stage of Practical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Niu, W.; Sun, J.; Zhou, Q. Efficient Removal of Cr(VI) from Water by the Uniform Fiber Ball Loaded with Polypyrrole: Static Adsorption, Dynamic Adsorption and Mechanism Studies. Chemosphere 2020, 248, 126102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Zhou, S.; Gao, X. Adsorption Mechanism of Cr(VI) on Woody-Activated Carbons. Heliyon 2023, 9, e13267. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-Dimensional Titanium Carbide for Efficiently Reductive Removal of Highly Toxic Chromium(VI) from Water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef]
- Azeez, N.A.; Dash, S.S.; Gummadi, S.N.; Deepa, V.S. Nano-Remediation of Toxic Heavy Metal Contamination: Hexavalent Chromium [Cr(VI)]. Chemosphere 2021, 266, 129204. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Ooi, J.; Yu, K.L.; Tham, T.C.; Chen, W.-H.; Ok, Y.S. Engineered Macroalgal and Microalgal Adsorbents: Synthesis Routes and Adsorptive Performance on Hazardous Water Contaminants. J. Hazard. Mater. 2022, 423, 126921. [Google Scholar] [CrossRef]
- Behera, A.; Sahu, S.; Pahi, S.; Singh, S.K.; Mahapatra, B.; Patel, R.K. Polypyrrole Modified Zirconium (IV) Phosphate Nanocomposite: An Effective Adsorbent for Cr(VI) Removal by Adsorption-Reduction Mechanism. Mater. Chem. Phys. 2022, 290, 126540. [Google Scholar] [CrossRef]
- Yu, F.; Cen, L.; Lei, C.; Zhu, F.; Zhou, L.; Zhu, H.; Yu, B. Fabrication of Recyclable UiO-66-NH2/PVDF Hybrid Fibrous Membrane for Cr(VI) Removal in Wastewater. J. Ind. Eng. Chem. 2023, 123, 104–115. [Google Scholar] [CrossRef]
- Pan, L.; Wan, Z.; Feng, Q.; Wang, J.; Xiong, J.; Wang, S.; Zhu, H.; Chen, G. Biofilm Response and Removal via the Coupling of Visible-Light-Driven Photocatalysis and Biodegradation in an Environment of Sulfamethoxazole and Cr(VI). J. Environ. Sci. 2022, 122, 50–61. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Guo, J. Electrochemical Removal of Chromium (VI) from Wastewater. Appl. Sci. 2019, 9, 1156. [Google Scholar] [CrossRef]
- Jia, D.; Cai, H.; Duan, Y.; Xia, J.; Guo, J. Efficient Adsorption to Hexavalent Chromium by Iron Oxalate Modified D301: Characterization, Performance and Mechanisms. Chin. J. Chem. Eng. 2021, 33, 61–69. [Google Scholar] [CrossRef]
- Qu, J.; Yang, Q.; Gong, W.; Li, M.; Cao, B. Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-Di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics. Polymers 2022, 14, 2894. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, H.; Ali, E.; Shahab, A.; Huang, H.; Ullah, H.; Zeng, H. Synthesis of Novel Magnetic Activated Carbon for Effective Cr(VI) Removal via Synergistic Adsorption and Chemical Reduction. Environ. Technol. Innov. 2023, 30, 103092. [Google Scholar] [CrossRef]
- Sangkarak, S.; Phetrak, A.; Kittipongvises, S.; Kitkaew, D.; Phihusut, D.; Lohwacharin, J. Adsorptive Performance of Activated Carbon Reused from Household Drinking Water Filter for Hexavalent Chromium-Contaminated Water. J. Environ. Manage 2020, 272, 111085. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Zeolite Nanoparticle as a Superior Adsorbent with High Capacity: Synthesis, Surface Modification and Pollutant Adsorption Ability from Wastewater. Microchem. J. 2019, 145, 74–83. [Google Scholar] [CrossRef]
- Jeon, C. Removal of Cr(VI) from Aqueous Solution Using Amine-Impregnated Crab Shells in the Batch Process. J. Ind. Eng. Chem. 2019, 77, 111–117. [Google Scholar] [CrossRef]
- Xie, X.; Van Huis, M.A.; Van Blaaderen, A. Single-Step Coating of Mesoporous SiO 2 onto Nanoparticles: Growth of Yolk–Shell Structures from Core–Shell Structures. Nanoscale 2021, 13, 10925–10932. [Google Scholar] [CrossRef] [PubMed]
- Vejchakul, K.; Saothayanun, T.; Phuekphong, A.; Paengjun, N.; Ogawa, M. Photocatalytic Hydrogen Evolution from Water by the Anatase Prepared on Resorcinol–Formaldehyde Resin Sphere. J. Porous Mater. 2023, 30, 303–310. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, W. Controllable Synthesis of Phenolic Resin-Based Carbon Nanospheres for Simultaneous Detection of Heavy-Metal Ions. J. Mater. Sci. Mater. Electron. 2022, 33, 1542–1554. [Google Scholar] [CrossRef]
- Ren, D.; Chen, N.; Xu, J.; Ye, Z.; Li, X.; Chen, Q.; Ma, S. Resorcinol-Formaldehyde-Assisted Dissolution-Regrowth Strategy for Synthesis of Hollow Silica Nanoparticles with Tunable Morphology. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126508. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface Functionalization of Bamboo Leave Mediated Synthesized SiO2 Nanoparticles: Study of Adsorption Mechanism, Isotherms and Enhanced Adsorption Capacity for Removal of Cr (VI) from Aqueous Solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Jang, E.-H.; Pack, S.P.; Kim, I.; Chung, S. A Systematic Study of Hexavalent Chromium Adsorption and Removal from Aqueous Environments Using Chemically Functionalized Amorphous and Mesoporous Silica Nanoparticles. Sci. Rep. 2020, 10, 5558. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-K.; Lee, S.-C.; Kim, S.-B. Synthesis of Powdered and Granular N -(3-Trimethoxysilylpropyl)Diethylenetriamine-Grafted Mesoporous Silica SBA-15 for Cr(VI) Removal from Industrial Wastewater. J. Taiwan Inst. Chem. Eng. 2018, 87, 140–149. [Google Scholar] [CrossRef]
- Dinari, M.; Soltani, R.; Mohammadnezhad, G. Kinetics and Thermodynamic Study on Novel Modified–Mesoporous Silica MCM-41/Polymer Matrix Nanocomposites: Effective Adsorbents for Trace Cr VI Removal. J. Chem. Eng. Data 2017, 62, 2316–2329. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Yan, W.; Cui, S.; Wu, X.; Suo, H. Preparation of the Novel B4C–SiC Composite Aerogel with High Compressive Strength and Low Thermal Conductivity. J. Porous Mater. 2021, 28, 703–710. [Google Scholar] [CrossRef]
- Berthon-Fabry, S.; Hildenbrand, C.; Ilbizian, P.; Jones, E.; Tavera, S. Evaluation of Lightweight and Flexible Insulating Aerogel Blankets Based on Resorcinol-Formaldehyde-Silica for Space Applications. Eur. Polym. J. 2017, 93, 403–416. [Google Scholar] [CrossRef]
- Choe, J.; Ji, J.; Yu, J.; Jang, K.; Yun, J.; Choe, S.; Rim, Y.; Jo, C. Adsorption of Cr(VI) in Aqueous Solution by Polypyrrole Nanotube and Polypyrrole Nanoparticle; Kinetics, Isotherm Equilibrium, and Thermodynamics. Inorg. Chem. Commun. 2022, 145, 109981. [Google Scholar] [CrossRef]
- Chua, Y.T.; Lin, C.X.C.; Kleitz, F.; Smart, S. Synthesis of Mesoporous Carbon–Silica Nanocomposite Water-Treatment Membranes Using a Triconstituent Co-Assembly Method. J. Mater. Chem. A 2015, 3, 10480–10491. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, W.; Liu, C.; Wang, H. Facial Synthesis of Adsorbent from Hemicelluloses for Cr(VI) Adsorption. Molecules 2021, 26, 1443. [Google Scholar] [CrossRef]
- Ebrahimi-Gatkash, M.; Younesi, H.; Shahbazi, A.; Heidari, A. Amino-Functionalized Mesoporous MCM-41 Silica as an Efficient Adsorbent for Water Treatment: Batch and Fixed-Bed Column Adsorption of the Nitrate Anion. Appl. Water Sci. 2017, 7, 1887–1901. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Li, J.; Peng, X.; Ge, H.; Wu, Q.; Wang, X. Highly Stable Core-Shell Structured SiO2@C-Ag Composites for Organic Contaminants Degradation and Antibacterial Application. Colloids Surf. Physicochem. Eng. Asp. 2023, 665, 131263. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Q.; Pan, X.Y.; Yu, H.; Zhao, Z.T. Adsorption Behavior of Cr(VI) by Biomass-Based Adsorbent Functionalized with Deep Eutectic Solvents (DESs). BMC Chem. 2022, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Khankhasaeva, S.T.; Badmaeva, S.V.; Ukhinova, M.V. Adsorption of Diclofenac onto Fe2O3-Pillared Montmorillonite: Equilibrium, Kinetics and Thermodynamic Studies. J. Mol. Liq. 2023, 380, 121725. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of Gentian Violet in Aqueous Solution by Activated Carbon Equilibrium, Kinetics, and Thermodynamic Study. Adsorpt. Sci. Technol. 2019, 37, 566–589. [Google Scholar] [CrossRef]
- Manfrin, J.; Gonçalves Jr, A.C.; Schwantes, D.; Tarley, C.R.T.; Schiller, A.D.P.; Klassen, G.J. Triple Activation (Thermal-Chemical-Physical) in the Development of an Activated Carbon from Tobacco: Characterizations and Optimal Conditions for Cd2+ and Pb2+ Removal from Waters. Water Pract. Technol. 2020, 15, 877–898. [Google Scholar] [CrossRef]
- Dong, F.-X.; Yan, L.; Zhou, X.-H.; Huang, S.-T.; Liang, J.-Y.; Zhang, W.-X.; Guo, Z.-W.; Guo, P.-R.; Qian, W.; Kong, L.-J.; et al. Simultaneous Adsorption of Cr(VI) and Phenol by Biochar-Based Iron Oxide Composites in Water: Performance, Kinetics and Mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, Y.; Yao, J.; Liu, Q.; Kong, F.-G. Facile Synthesis of Polyethylene Glycol@Tannin-Amine Microsphere towards Cr(VI) Removal. Polymers 2021, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Ding, G.; You, Y. Tannin-Based Biosorbent Encapsulated into Calcium Alginate Beads for Cr(VI) Removal. Water Sci. Technol. 2020, 81, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Fellenz, N.; Perez-Alonso, F.J.; Martin, P.P.; García-Fierro, J.L.; Bengoa, J.F.; Marchetti, S.G.; Rojas, S. Chromium (VI) Removal from Water by Means of Adsorption-Reduction at the Surface of Amino-Functionalized MCM-41 Sorbents. Microporous Mesoporous Mater. 2017, 239, 138–146. [Google Scholar] [CrossRef]
- Chang, J.H.; Kim, J.; Lee, H. PNIPAm Grafted Amino-Functionalized Mesoporous Silica for Thermo-Responsive Chromium Elimination. Appl. Surf. Sci. 2017, 424, 115–121. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Thoravat, S.S.; Patil, V.S.; Shinde, P.S.; Burungale, S.H.; Patil, P.S. Synthesis and Testing of Functional Mesoporous Silica Nanoparticles for Removal of Cr(VI) Ions From Water. Biointerface Res. Appl. Chem. 2020, 11, 8599–8607. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.-H.; Choi, K.; Kim, H.-G.; Park, J.-A.; Cho, S.-H.; Hong, S.W.; Lee, J.-H.; Lee, J.H.; Lee, S.; et al. Investigation of the Mechanism of Chromium Removal in (3-Aminopropyl)Trimethoxysilane Functionalized Mesoporous Silica. Sci. Rep. 2018, 8, 12078. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B. Preparation of Amino-Functionalized Magnetic Biochar with Excellent Adsorption Performance for Cr(VI) by a Mild One-Step Hydrothermal Method from Peanut Hull. Colloids Surf. Physicochem. Eng. Asp. 2019, 563, 102–111. [Google Scholar] [CrossRef]


| Nanocomposite | Surface Area/m2·g−1 | Pore Volumes/mL·g−1 | BJH Pore Volumes/nm |
|---|---|---|---|
| SiO2@RF | 32.46 | 0.217 | 2.61 |
| NH2-SiO2@RF | 29.42 | 0.109 | 2.48 |
| Models | T/K | Linear | Nonlinear | ||||
|---|---|---|---|---|---|---|---|
| Langmuir | qm/mg·g−1 | KL/L·mg−1 | R2 | qm/mg·g−1 | KL/L·mg−1 | R2 | |
| 298 | 189.6 | 0.0088 | 0.9887 | 196.4 | 0.0075 | 0.9918 | |
| 308 | 198 | 0.0078 | 0.9822 | 208.2 | 0.0072 | 0.9864 | |
| 318 | 212.2 | 0.0057 | 0.9727 | 272.6 | 0.0053 | 0.9919 | |
| Freundlich | 1/n | Kf/mg(1−1/n)·L1/n·g−1 | R2 | 1/n | Kf/mg(1−1/n)·L1/n·g−1 | R2 | |
| 298 | 0.599 | 4.735 | 0.9330 | 0.473 | 9.075 | 0.948 | |
| 308 | 0.587 | 4.973 | 0.9230 | 0.483 | 8.971 | 0.9301 | |
| 318 | 0.59 | 5.063 | 0.8860 | 0.547 | 7.301 | 0.9552 | |
| Temkin | bT/J·mol−1 | KT/L·g−1 | R2 | bT/J·mol−1 | KT/L·g−1 | R2 | |
| 298 | 54.13 | 0.063 | 0.9914 | 54.15 | 0.065 | 0.9917 | |
| 308 | 52.48 | 0.060 | 0.9860 | 52.58 | 0.061 | 0.9862 | |
| 318 | 43.27 | 0.051 | 0.9919 | 43.28 | 0.052 | 0.9921 | |
| D-R | qm/mg·g−1 | KDR/mol2·J−2 | R2 | qm/mg·g−1 | KDR/mol2·J−2 | R2 | |
| 298 | 126.5 | 236.7 | 0.8821 | 134.4 | 424.4 | 0.9002 | |
| 308 | 129.0 | 228.6 | 0.8736 | 141.6 | 444.7 | 0.9055 | |
| 318 | 148.4 | 237.1 | 0.8556 | 169.5 | 584.6 | 0.9012 | |
| C /mg·L−1 | Qexp /mg·g−1 | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qcal /mg·g−1 | K1 /min−1 | R2 | Qcal /mg·g−1 | K2 /g·mg−1·min−1 | R2 | ki /mg·g−1·min−1/2 | C | R2 | ||
| 30 | 30.0 | 0.52 | 0.0079 | 0.8751 | 30.01 | 0.048 | 1 | 0.031 | 29.48 | 0.9781 |
| 50 | 49.8 | 3.13 | 0.0087 | 0.9500 | 50.15 | 0.0080 | 1 | 0.142 | 47.52 | 0.9964 |
| 100 | 93.1 | 31.5 | 0.0047 | 0.9457 | 97.09 | 6.1 × 10−4 | 0.9998 | 2.538 | 52.94 | 0.9995 |
| T/K | ΔG/kJ·mol−1 | ΔH/kJ·mol−1 | ΔS/J·mol−1·K−1 |
|---|---|---|---|
| 298 | −9.788 | 0.02008 | 32.91 |
| 308 | −10.12 | ||
| 318 | −10.45 |
| Adsorbent | Amine Functionalization | Adsorption Capacity/mg·g−1 | Reference |
|---|---|---|---|
| NH2-SiO2@RF | APTES | 272.6 | this study |
| NH2-MCM-41/PMNCs | APTES | 20 | [23] |
| NH2-MCM-41 | APTES | 86.4 | [38] |
| NH2-PNIPAm | APTES | 123.8 | [39] |
| NH2-pSiO2 | APTES | 50 | [40] |
| NH2-MSNs | APTES | 42.2 | [21] |
| NH2-MPS | APTMS | 83.5 | [41] |
| NH2-SBA-15 | DAEAPTS | 330.9 | [22] |
| NH2-MPHC | HDA | 142.9 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Lu, W.; Zhu, D. Amino-Functionalized Silica@Resorcinol–Formaldehyde Nanocomposites for the Removal of Cr(VI) from Aqueous Solutions. Polymers 2023, 15, 4094. https://doi.org/10.3390/polym15204094
Li N, Lu W, Zhu D. Amino-Functionalized Silica@Resorcinol–Formaldehyde Nanocomposites for the Removal of Cr(VI) from Aqueous Solutions. Polymers. 2023; 15(20):4094. https://doi.org/10.3390/polym15204094
Chicago/Turabian StyleLi, Nan, Wenhui Lu, and Deyi Zhu. 2023. "Amino-Functionalized Silica@Resorcinol–Formaldehyde Nanocomposites for the Removal of Cr(VI) from Aqueous Solutions" Polymers 15, no. 20: 4094. https://doi.org/10.3390/polym15204094
APA StyleLi, N., Lu, W., & Zhu, D. (2023). Amino-Functionalized Silica@Resorcinol–Formaldehyde Nanocomposites for the Removal of Cr(VI) from Aqueous Solutions. Polymers, 15(20), 4094. https://doi.org/10.3390/polym15204094






