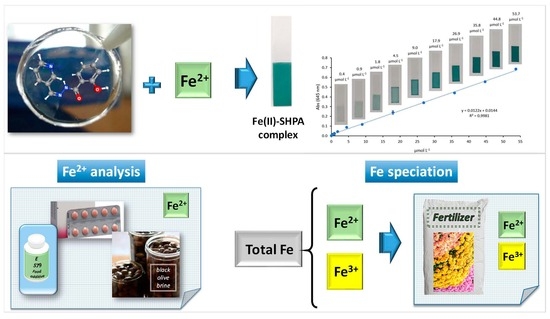
A Novel Polymer Inclusion Membrane-Based Green Optical Sensor for Selective Determination of Iron: Design, Characterization, and Analytical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
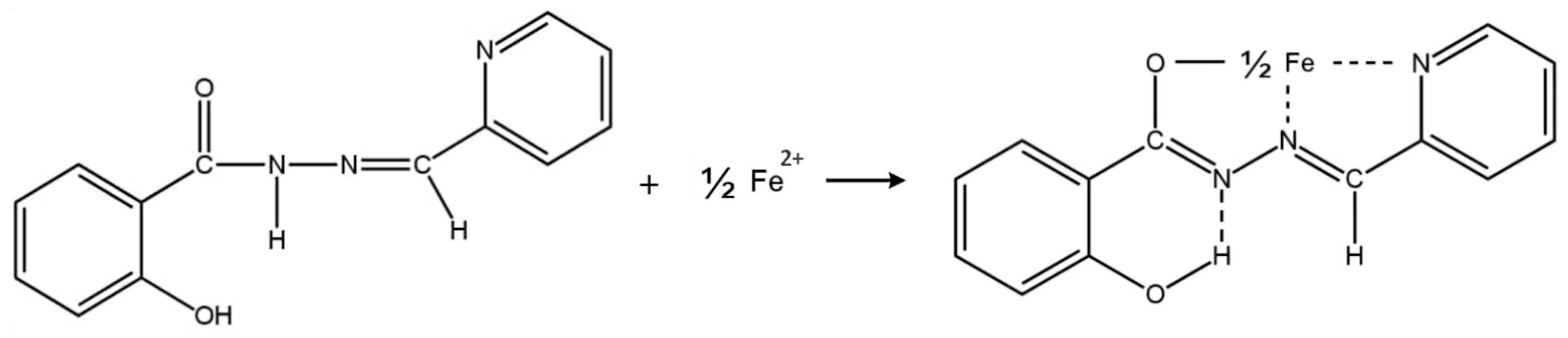
2.3. Mechanochemical Synthesis of SHPA and PIM Preparation
2.4. General Analytical Procedure for Optical Fe(II) Ion Sensing
2.5. Real Samples
3. Results and Discussion
3.1. Previous Studies
3.2. Optimization of the PIM Composition
3.3. Lifetime of the Optical Sensor
3.4. Effect of pH and Buffer Concentration on the Response of Sensor
3.5. Response Time
3.6. Curing Time of the Optical Sensor
3.7. Short-Term Stability
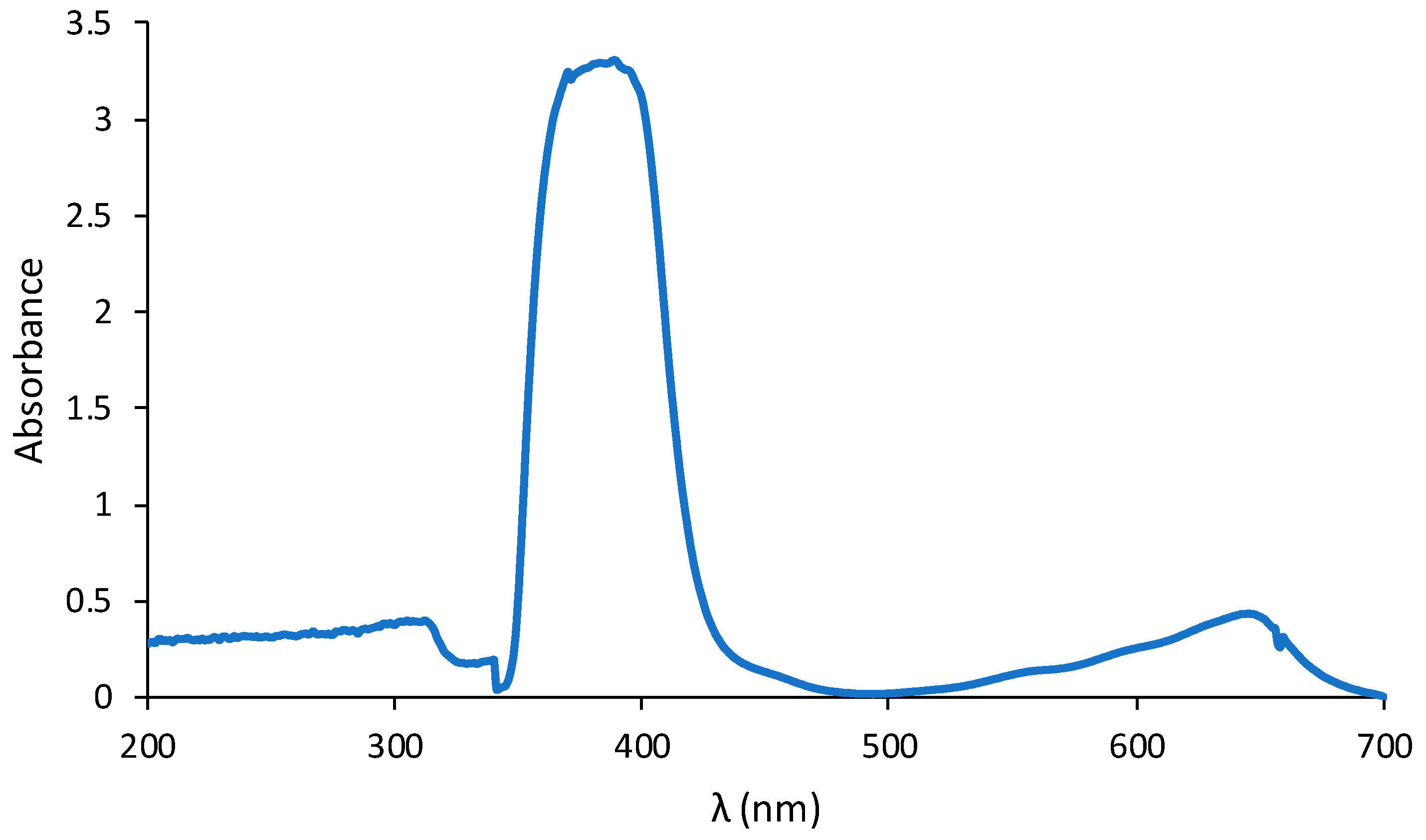
3.8. The New Polymer Inclusion Membrane-Based Optical Sensor for the Determination of Fe(II) Ions
3.9. Analytical Performance of the Method
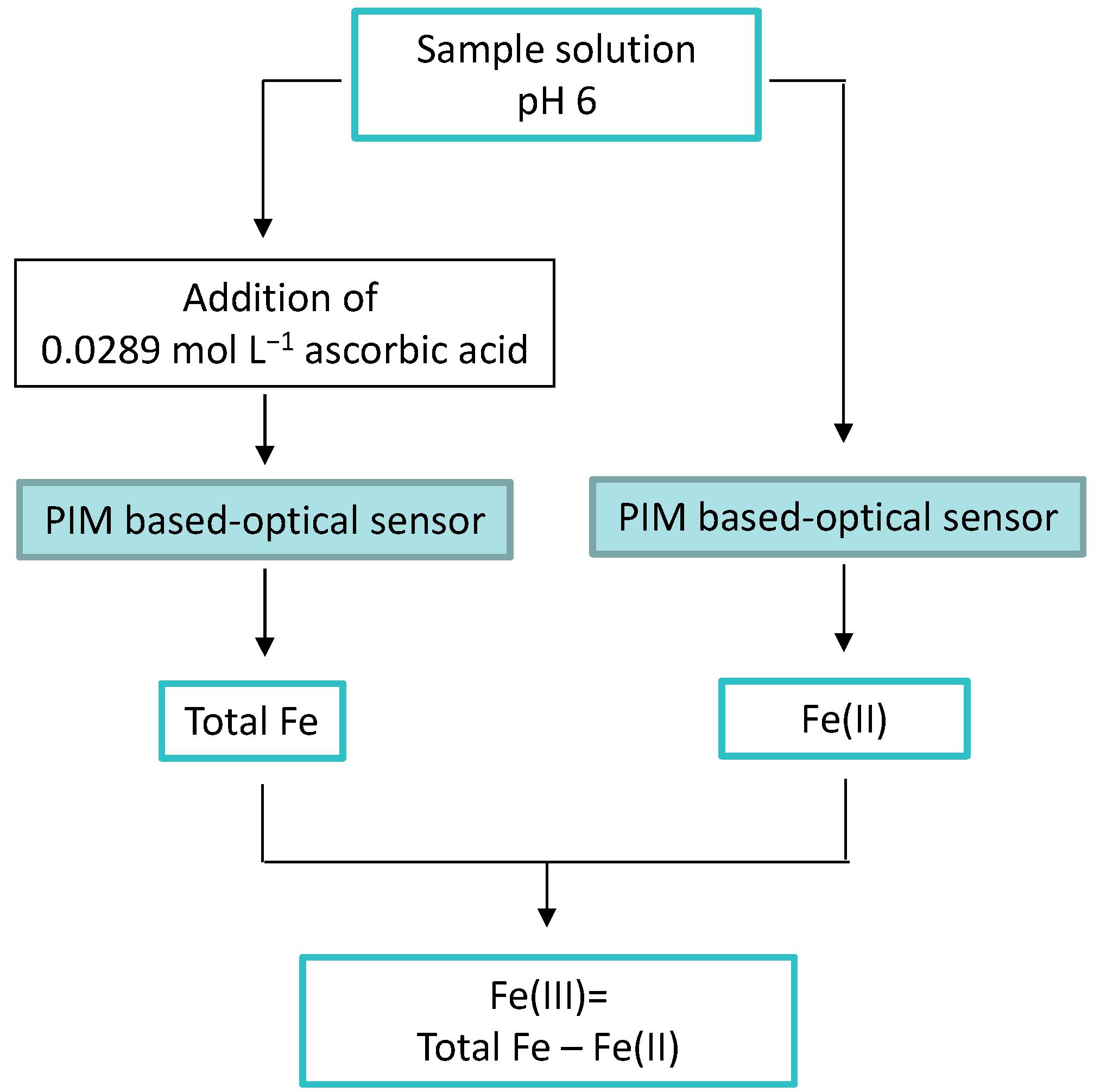
3.10. Speciation Analysis of Fe(II)/Fe(III) with the New Proposed Optical Sensor
3.11. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Hahn, Y.B.; Ahmadwa, R.; Tripathywa, N. Chemical and biological sensors based on metal oxide nanostructures. Chem. Commun. 2012, 48, 10369–10385. Available online: https://pubs.rsc.org/en/content/articlelanding/2012/CC/c2cc34706g (accessed on 15 September 2023). [CrossRef]
- Wang, W. Introductory Chapter: What is chemical sensor? In Progresses in Chemical Sensors; Wang, W., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M.; Heng, L.Y. A novel polymer inclusion membranes based optode for sensitive determination of Al3+ ions. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 2015, 144, 81–87. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Analyt. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and electrochemical sensors and biosensors for the detection of quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef]
- Suah, F.B.M. Preparation and characterization of a novel Co(II) optode based on polymer inclusion membrane. Anal. Chem. Res. 2017, 12, 40–46. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattral, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta. 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kolev, S.D.; Almeida, M.I.G.S.; Cattrall, R.W. Polymer Inclusion Membranes: Smart materials for sensing and separation. In Handbook of Smart Materials in Analytical Chemistry; de la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2019; pp. 439–461. [Google Scholar]
- Sánchez-Ponce, L.; Galindo-Riaño, M.D.; Casanueva-Marenco, M.J.; Granado-Castro, M.D.; Díaz-de-Alba, M. Sensing Cd(II) using a disposable optical sensor based on a Schiff base immobilisation on a polymer-inclusion membrane. Application in water and art paint samples. Polymers 2021, 13, 4414. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, B.; Makowka, A. Application of Plasticized Cellulose Triacetate Membranes for Recovery and Separations of Cerium(III) and Lanthanum(III). Autex Res. J. 2022, 22, 312–317. [Google Scholar] [CrossRef]
- González-Albarrán, R.; de Gyves, J.; Rodríguez de San Miguel, E. Determination of Cadmium(II) in Aqueous Solutions by In Situ MID-FTIR-PLS Analysis Using a Polymer Inclusion Membrane-Based Sensors: First Considerations. Molecules 2020, 25, 3436. [Google Scholar] [CrossRef] [PubMed]
- Casanueva-Marenco, M.J.; Díaz-de-Alba, M.; Herrera-Armario, A.; Galindo-Riaño, M.D.; Granado-Castro, M.D. Desing and optimization of a single-use optical sensor based on a polymer inclusion membrane for zinc determination in drinks, food supplement and foot health care products. Mater. Sci. Eng. 2020, 110, 110680. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Hadadzadeh, H.; Azimi, A. Fabrication of an optical sensor based on the immobilization of Qsal on the plasticized PVC membrane for the determination of copper(II). J. Anal. Chem. 2012, 67, 687–693. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M. Preparation and characterization of polymer inclusion membrane based optode for determination of Al3+ ion. Anal. Chim. Acta 2017, 951, 133–139. [Google Scholar] [CrossRef]
- Kalyan, Y.; Pandey, A.K.; Bhagat, P.R.; Acharya, R.; Natarajan, V.; Naidu, G.R.K.; Reddy, A.V.R. Membrane optode for mercury(II) determination in aqueous samples. J. Hazard. Mater. 2009, 166, 377–382. [Google Scholar] [CrossRef]
- García-Beleño, J.; Rodriguez de San Miguel, E. Integration of response surface methodology (RSM) and principal component analysis (PCA) as an optimization tool for polymer inclusion membrane based-optodes designed for Hg(II), Cd(II), and Pb(II). Membranes 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.Y.; Sabahi, R.R.; El-Shishtawy, R.M. Chromophoric thin film based on cellulose triacetate blends for sensing metal ions. C. R. Chim. 2014, 17, 557–562. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, S.A.; Kumar, D.S.A.; Pandey, A.K.; Kumar, S.D.; Reddy, A.V.R. Development of a visual optode sensor for onsite determination of Hg(II). Sens. Actuators B Chem. 2015, 211, 346–353. [Google Scholar] [CrossRef]
- Zargoosh, K.; Babadi, F.F. Highly selective and sensitive optical sensor for determination of Pb2+ and Hg2+ ions based on the covalent immobilization of dithizone on agarose membrane. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 105–110. [Google Scholar] [CrossRef]
- Fukushima, Y.; Aikawa, S. Colorimetric detection of Ni2+ based on an anionic triphenylmethane dye and a cationic polyelectrolyte in aqueous solution. Tetrahedron Lett. 2019, 60, 675–680. [Google Scholar] [CrossRef]
- Ahmad, M.; Narayanaswami, R. Development of an optical fibre Al(III) sensor based on immobilised chrome azurol S. Talanta 1995, 42, 1337–1344. [Google Scholar] [CrossRef]
- Tavallali, H.; Vahdati, P.; Shaabanpur, E. Developing a new method of 4-(2-pyridylazo)- resorcinol immobilization on triacetylcellulose membrane for selective determination of Ga3+ in water samples. Sens. Actuators B Chem. 2011, 159, 154–158. [Google Scholar] [CrossRef]
- Tavallali, H.; Malekzadeh, H.; Karimi, M.A.; Payehghadr, M.; Pisheh, M.G. Design and evaluation of a copper (II) optode based on immobilization of 1-(2-pyridylazo)-2-naphthol (PAN) on a triacetylcellulose membrane. Int. J. Chemtech Res. 2012, 4, 284–289. Available online: https://sphinxsai.com/2012/chem/CHEM/CT=45(284-289)JM12.pdf (accessed on 7 September 2023).
- Jeronimo, P.C.A.; Araujo, A.N.; Montenegro, M.C.B.S.M. Development of a sol-gel optical sensor for analysis of zinc in pharmaceuticals. Sens. Actuators B Chem. 2004, 103, 169–177. [Google Scholar] [CrossRef]
- Hu, L.; Yan, X.W.; Li, Q.; Zhang, X.J.; Shan, D. Br-PADAP embedded in cellulose acetate electrospun nanofibers: Colorimetric sensor strips for visual uranyl recognition. J. Hazard. Mater. 2017, 329, 205–210. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Isfahani, Z.N. A simple optical sensor for cadmium ions assay in water samples using spectrophotometry. J. Anal. Chem. 2011, 66, 151–157. [Google Scholar] [CrossRef]
- Darroudi, A.; Kakhki, J.F.; Mofrad, I.Z. Fabrication of a New Selective Optical Sensor for Cobalt Determination Using 1-Nitroso-2-naphthol and Monitoring of Cobalt in Water Samples. Anal. Bioanal. Chem. Res. 2022, 9, 443–449. [Google Scholar] [CrossRef]
- Gaidamauskas, E.; Saejueng, K.; Holder, A.A.; Bharuah, S.; Kashemirov, B.A.; Crans, D.C.; McKenna, C.E. Metal complexation chemistry used for phosphate and nucleotide determination: An investigation of the Yb3+-pyrocatechol violet sensor. J. Biol. Inorg. Chem. 2008, 13, 1291–1299. [Google Scholar] [CrossRef]
- Vukovic, J.; Avidad, M.A.; Capitán-Vallvey, L.F. Characterization of disposable optical sensors for heavy metal determination. Talanta 2012, 94, 123–132. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Spectrophotometric reagents. In Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Kloczko, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 53–57. [Google Scholar]
- Zhou, P.; Zhao, Y.G.; Bai, Y.; Pang, K.L.; He, C. Fluoroboration of a Schiff-base ligand through nickel tetrafluoroborate. Inorg. Chim. Acta 2007, 360, 3965–3970. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Seo, Y.K.; Baek, S.O.; Ammireddy, V.R. Review on Analytical and Biological applications of Hydrazones and their Metal Complexes. E-J. Chem. 2012, 9, 1288–1304. [Google Scholar] [CrossRef]
- Shamsipur, M.; Sadeghi, M.; Alizadeh, K.; Bencini, A.; Valtancoli, B.; Garau, A.; Lippolis, V. Novel fluorimetry bulk optode membrane based on 5,8-bis((5’-chloro-8’-hydroxy-7’-quinolinyl)methyl)-2,11-dithia-5,8-diaza-2,6-pyridinophane for selective detection of lead(II) ions. Talanta 2010, 80, 2023–2033. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, H.; Jiang, N.; Wang, L.; Wang, G. Oxadiazole-based ‘on-off’ fluorescence chemosensor for rapid recognition and detection of Fe2+ and Fe3+ in aqueous solution and in living cells. Microchem. J. 2019, 145, 435–443. [Google Scholar] [CrossRef]
- Li, Z.; Ti, M.; Yang, K.; Li, X.; Wu, L.; He, Y. Colorimetric detection of iron (II) using 4-mercaptobenzoic acid and 3,3’,5,5’-tetramethylbenzidine modified silver nanoparticles. Microchem. J. 2019, 151, 104254. [Google Scholar] [CrossRef]
- Guan, J.; Tu, Q.; Chen, L.; Yuan, M.S.; Wang, J. A benzothiazole-rhodol based luminophore: ESIPT-induced AIE and an application for detecting Fe2+ ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 220, 117114. [Google Scholar] [CrossRef]
- Xia, L.; Wu, Y.; Jiang, Z.; Li, S.; Hu, B. Speciation of Fe(III) and Fe(II) in water samples by liquid-liquid extraction combined with low-temperature electrothermal vaporization (ETV) ICP-AES. Int. J. Environ. Anal. Chem. 2003, 83, 953–962. [Google Scholar] [CrossRef]
- Lin, H.; Twining, B.S. Chemical speciation of iron in Antarctic waters surrounding free-drifting icebergs. Mar. Chem. 2012, 128–129, 81–91. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ardini, F.; Frache, R. Determination of sub-nanomolar levels of iron in sea water using reaction cell inductively coupled plasma mass spectrometry after Mg(OH)2 coprecipitation. J. Anal. Atom. Spectrom. 2009, 24, 522–527. [Google Scholar] [CrossRef]
- Proch, J.; Niedzielski, P. Iron species determination by high performance liquid chromatography with plasma based optical emission detectors: HPLC–MIP OES and HPLC–ICP OES. Talanta 2021, 231, 122403. [Google Scholar] [CrossRef]
- Sanvito, F.; Monticelli, D. Exploring bufferless iron speciation in seawater by Competitive Ligand Equilibration-Cathodic Stripping Voltammetry: Does pH control really matter? Talanta 2021, 220, 122300. [Google Scholar] [CrossRef]
- Kumar, S.A.; Thakur, N.; Parab, H.J.; Pandey, S.P.; Shinde, R.N.; Pandey, A.K.; Kumar, S.D.; Reddy, A.V. A visual strip sensor for determination of iron. Anal. Chim. Acta 2014, 851, 87–94. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Rezaei, V.; Rastegarzadeh, S. Sol-gel based optical sensor for determination of Fe(II): A novel probe for iron speciation. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 2015, 136, 832–837. [Google Scholar] [CrossRef]
- Kunthadee, P.; Klinjan, P.; Saikrajang, N. N,N′-Ethylenebis(salicylimine)-based membrane sensor for the selective detection of Fe(II) ion. In Proceedings of the 35th International Conference of the Microscopy Society of Thailand, Chiang Mai, Thailand, 30 January–2 February 2018. [Google Scholar]
- Muliawati, N.T.; Siswanta, D.; Aprilita, N.H. Development of a simple Fe(II) ion colorimetric sensor from the immobilization of 1,10-phenanthroline in alginate/pectin film. Indones. J. Chem. 2021, 21, 411–420. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; Arroyo, E.; Berenguer, C.; Fernández-Ramos, M.D.; Avidad, R. Single-use optical sensor for the determination of iron in water and white wines. Fresenius J. Anal. Chem. 2001, 369, 139–144. [Google Scholar] [CrossRef]
- Zhu, Q.; Aller, R.C. Two-dimensional dissolved ferrous iron distributions in marine sediments as revealed by a novel planar optical sensor. Mar. Chem. 2012, 136–137, 14–23. [Google Scholar] [CrossRef]
- Ondigo, D.A.; Tshentu, Z.R.; Torto, N. Electrospun nanofiber based colorimetric probe for rapid detection of Fe2+ in water. Anal. Chim. Acta 2013, 804, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Gao, Z.P.; Yi, H.; Bing, Y.X.; Niu, C.G.; Guo, Q.W.; Lai, C. A facile fluorescent probe based on anthraldehyde for trade Fe(III) ion determination in neutral aqueous solution. Anal. Methods 2015, 7, 353–358. [Google Scholar] [CrossRef]
- Gallego, M.; Valcarcel, M.; Garcia-Vargas, M. Spectrofluorimetric and spectrophotometric determination of aluminium with the salicyloylhydrazones of pyridine-2-aldehyde and pyridoxal. Analyst 1983, 108, 92–98. [Google Scholar] [CrossRef]
- Gallego, M.; Garcia-Vargas, M.; Pino, F.; Valcarcel, M. Analytical applications of picolinealdehyde salicyloylhydrazone: Spectrophotometric determination of nickel and zinc. Microchem. J. 1978, 23, 353–359. [Google Scholar] [CrossRef]
- Gallego, M.; Garcia-Vargas, M. Analytical applications of picolinealdehyde salicyloylhydrazone: II. Extraction and spectrophotometric determination of vanadium(V). Microchem. J. 1979, 24, 143–149. [Google Scholar] [CrossRef]
- Gallego, M.; Garcia-Vargas, M.; Valcarcel, M. Analytical applications of picolinealdehyde salicyloylhydrazone: III. Extraction and determination of zinc by atomic absorption spectrophotometry. Microchem. J. 1982, 27, 328–338. [Google Scholar] [CrossRef]
- Gallego, M.; Garcia-Vargas, M.; Valcarcel, M. Pyridine-2-carbaldehyde 2-hydroxybenzoylhydrazone as a selective reagent for the extraction and spectrophotometric determination of iron(II). Analyst 1979, 104, 613–619. [Google Scholar] [CrossRef]
- Singh, R.B.; Jain, P.; Singh, R.P. Hydrazones as analytical reagents: A review. Talanta 1982, 29, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, I.; Alwedian, F.; Ababneh, T.; Shdefat, A.; Tashtoush, H. Synthesis, characterization, computational, antioxidant and fluorescence properties of novel 1,3,5-trimesic hydrazones derivatives. Heliyon 2021, 7, e08074. [Google Scholar] [CrossRef] [PubMed]
- Welcher, F.J. Standard Methods of Chemical Analysis, 6th ed.; Van Nostrand Reinhold: New York, NY, USA, 1984. [Google Scholar]
- Feldkamp, C.S.; Watkins, R.; Baginski, E.S.; Zak, B. Essential serum trace metals: I. Determination of iron. Microchem. J. 1977, 22, 335–346. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; the “Gold Book”, McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; Online version (2019-) created by Chalk, S.J.; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]






| Membrane | Polymer | Plasticizer | Additive | Solvent | pH | Abs 389 nm | Abs 645 nm | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| 1 | PVC | TBP | - | THF | 4.6 | 0.2730 | 0.0343 | 0.0256 | 0.0048 |
| (2.5014 g) | (3 mL) | (19.1 mL) | |||||||
| 2 | PVC | NPOE | NaTPB | THF | 4.6 | 0.0268 | 0.1233 | 0.0212 | 0.0343 |
| (0.8013 g) | (1.54 mL) | (0.0205 g) | (20 mL) | ||||||
| 3 | CTA | DBP | - | DCM | 4.6 | 0.4986 | 0.0036 | 0.0500 | 0.0050 |
| (1.0024 g) | (4 mL) | (35 mL) | |||||||
| 4 | CTA | DBP | - | DCM | 2.5 | 0.0003 | 0.0096 | - | - |
| (1.0024 g) | (4 mL) | (35 mL) | |||||||
| 5 | CTA | DBP | - | DCM | 9 | 0.1055 | 0.0153 | 0.0124 | 0.0103 |
| (1.0024 g) | (4 mL) | (35 mL) | |||||||
| Vertex | CTA (g) | SHPA (g) | DBP (mL) | DCM (mL) | Abs (abs. Unit) | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| 1 * | 1 | 0.02 | 4 | 35 | 0.0528 | 0.0016 |
| 2 * | 2 | 0.02 | 4 | 35 | 0.0128 | 0.0039 |
| 3 * | 1 | 0.06 | 4 | 35 | 0.0892 | 0.0088 |
| 4 * | 1 | 0.02 | 8 | 35 | 0.0263 | 0.0001 |
| 5 * | 1 | 0.02 | 4 | 70 | 0.000 | 0.000 |
| 6 | 1.5 | 0.04 | 6 | 0.05 | Rejected | Rejected |
| 7 | 1.1 | 0.03 | 4.5 | 52 | 0.0132 | 0.0062 |
| 8 | 0.06 | 0.04 | 6.2 | 44 | Rejected | Rejected |
| 9 | 1.5 | 0.03 | 4.6 | 37 | 0.0398 | 0.0027 |
| 10 | 1.1 | 0.04 | 5.8 | 19 | Rejected | Rejected |
| 11 | 1.1 | 0.03 | 4.8 | 44 | 0.0664 | 0.0016 |
| 12 | 1.3 | 0.05 | 0.7 | 41 | Rejected | Rejected |
| 13 | 1.1 | 0.03 | 6.2 | 36 | 0.0518 | 0.0025 |
| 14 | 0.59 | 0.04 | 4.9 | 38 | 0.0891 | 0.0026 |
| 15 | 0.78 | 0.05 | 2.7 | 40 | 0.0443 | 0.0007 |
| 16 | 1 | 0.03 | 5.3 | 37 | 0.0528 | 0.0007 |
| 17 | 0.86 | 0.06 | 5.5 | 42 | 0.0607 | 0.0012 |
| Added Metal Concentration (µmol L−1) | Presence of Ascorbic Acid | Determined Fe (II) Concentration (µmol L−1) | |
|---|---|---|---|
| Fe(III) | Fe (II) | ||
| 17.9 | 0 | No | <LD |
| 17.9 | 17.9 | No | 18.30 ± 0.04 |
| 17.9 | 0 | Yes | 18.4 ± 1.6 |
| Sample | Fe(II) ± SD (µmol L−1) | tcalc | |
|---|---|---|---|
| Optical Sensor | Ortho-Phenanthroline Method | ||
| Black olive brine (Karina) | 13.25 ± 0.35 | 12.98 ± 0.24 | 0.883 |
| Black olive brine (Ifa Eliges) | 4.25 ± 0.43 | 4.17 ± 0.13 | 0.231 |
| Food additive E579 | 21.61 ± 0.35 | 20.94 ± 0.08 | 2.661 |
| Tardyferon | 20.73 ± 1.47 | 20.79 ± 0.27 | 0.062 |
| Immobilized Reagent | Polymer Matrix | pH of Sol. | V Sample (mL) | Linear Range (µmol L−1) | Sensitivity * (Abs. Unit/(µmol L−1)) | LOD (µmol L−1) | RSD (%) ([Fe(II)] (µmol L−1)) | Metal Interferences (Ratio Fe(II):Metal) | Application to Real Samples | Remark | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferrozine | D4 polyurethane hydrogel membrane | 2.5 | 100 | 0.73–5.37 | 0.0195 | 0.21 | 4.9 (2.7 µmol L−1) | Cu(II) (1:1.2) Co(II) (1:2) | Waters and wines | Measures in non-equilibrium steady state; interference of wine colorants. | [46] |
| 0.07–0.90 | 0.2595 | 0.02 | 7.6 (0.5 µmol L−1) | ||||||||
| Ferrozine | D4 polyurethane hydrogel membrane | 4.0 | 10 | 17.9–179 | 0.0011 | 0.34 | 12.12 (35.8 µmol L−1) 4.77 (89.6 µmol L−1) | Not studied | n.m. | Measures in non-equilibrium steady state. | [29] |
| Ferrozine | poly(vinyl alcohol) membrane | 5.5 | n.m. | 5–200 | n.m. | 4.5 | 4.6 (50 µmol L−1) 2.3 (150 µmol L−1) | None Zn(II), Cu(II), Pb(II), Al(III), Fe(III) (100:1) Co(II), Ni(II) (1000:1) | Seawater and marine sediment | Measures in non-equilibrium steady state. | [47] |
| 2-(2-pyridyl) imidazole | Nanofibers of poly(vinyl benzyl chloride) | 6 | n.m. | n.m. | n.m. | 35.8 | n.m. | Ni(II), Cu(II), Co(II) (1:1) | n.m. for sensing probe | Probe analytical parameters not studied. | [48] |
| 1,10-phenan-throline | Poly(acrylamide) grafted poly(propylene) membrane | 3 | 25 | 0.36–35.8 | 0.0082 | 0.36 | 8 (n.m.) | Co(II) (n.m.) | Ground waters and fruit juice | Equilibrium steady state at 2 h, but sensing at 15 min. Visual/instru-mental detection. | [42] |
| 2,4,6-tri(2-pyridyl)-s-triazine | Silica sol-gel film | 3 | 5 | 0.09–2.1 | 0.117 | 0.03 | 3.5 (0.17 µmol L−1) 1.27 (1.61 µmol L−1) | None | Tap, well and river water | Fe speciation | [43] |
| N,N′- Ethylene bis(salicyl-imine) | Poly(vinyl chloride) membrane | n.m. | n.m. | 1–1000 | n.m. | n.m. | n.m. | n.m. | Tap and mineral waters | Few analytical parameters studied. | [44] |
| 1,10-phenan-throline | Alginate/pectin film | 2 | 2 | 0–179 | 0.0008 | 7.99 | 0.7–3.13 (n.m.) | Fe(III) (1:1) | Spiked tap water | Visual/instru-mental detection. | [45] |
| Picolin-aldehyde salicyloyl-hydrazone | Cellulose triacetate membrane | 6 | 20 | 0.48–54 (645 nm) 0.21–18 (389 nm) | 0.0122 (645 nm) 0.1558 (389 nm) | 0.48 (645 nm) 0.21 (389 nm) | 1.73 (17.9 µmol L−1) 1.42 (45 µmol L−1) | None (1:1) | Black olive brines, dietary supplement, food additive, fertilizer | Measures in equilibrium steady state. Fe speciation | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ponce, L.; Casanueva-Marenco, M.J.; Díaz-de-Alba, M.; Galindo-Riaño, M.D.; Granado-Castro, M.D. A Novel Polymer Inclusion Membrane-Based Green Optical Sensor for Selective Determination of Iron: Design, Characterization, and Analytical Applications. Polymers 2023, 15, 4082. https://doi.org/10.3390/polym15204082
Sánchez-Ponce L, Casanueva-Marenco MJ, Díaz-de-Alba M, Galindo-Riaño MD, Granado-Castro MD. A Novel Polymer Inclusion Membrane-Based Green Optical Sensor for Selective Determination of Iron: Design, Characterization, and Analytical Applications. Polymers. 2023; 15(20):4082. https://doi.org/10.3390/polym15204082
Chicago/Turabian StyleSánchez-Ponce, Lorena, María José Casanueva-Marenco, Margarita Díaz-de-Alba, María Dolores Galindo-Riaño, and María Dolores Granado-Castro. 2023. "A Novel Polymer Inclusion Membrane-Based Green Optical Sensor for Selective Determination of Iron: Design, Characterization, and Analytical Applications" Polymers 15, no. 20: 4082. https://doi.org/10.3390/polym15204082
APA StyleSánchez-Ponce, L., Casanueva-Marenco, M. J., Díaz-de-Alba, M., Galindo-Riaño, M. D., & Granado-Castro, M. D. (2023). A Novel Polymer Inclusion Membrane-Based Green Optical Sensor for Selective Determination of Iron: Design, Characterization, and Analytical Applications. Polymers, 15(20), 4082. https://doi.org/10.3390/polym15204082