Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins
Abstract
1. Introduction
2. Materials and Methods
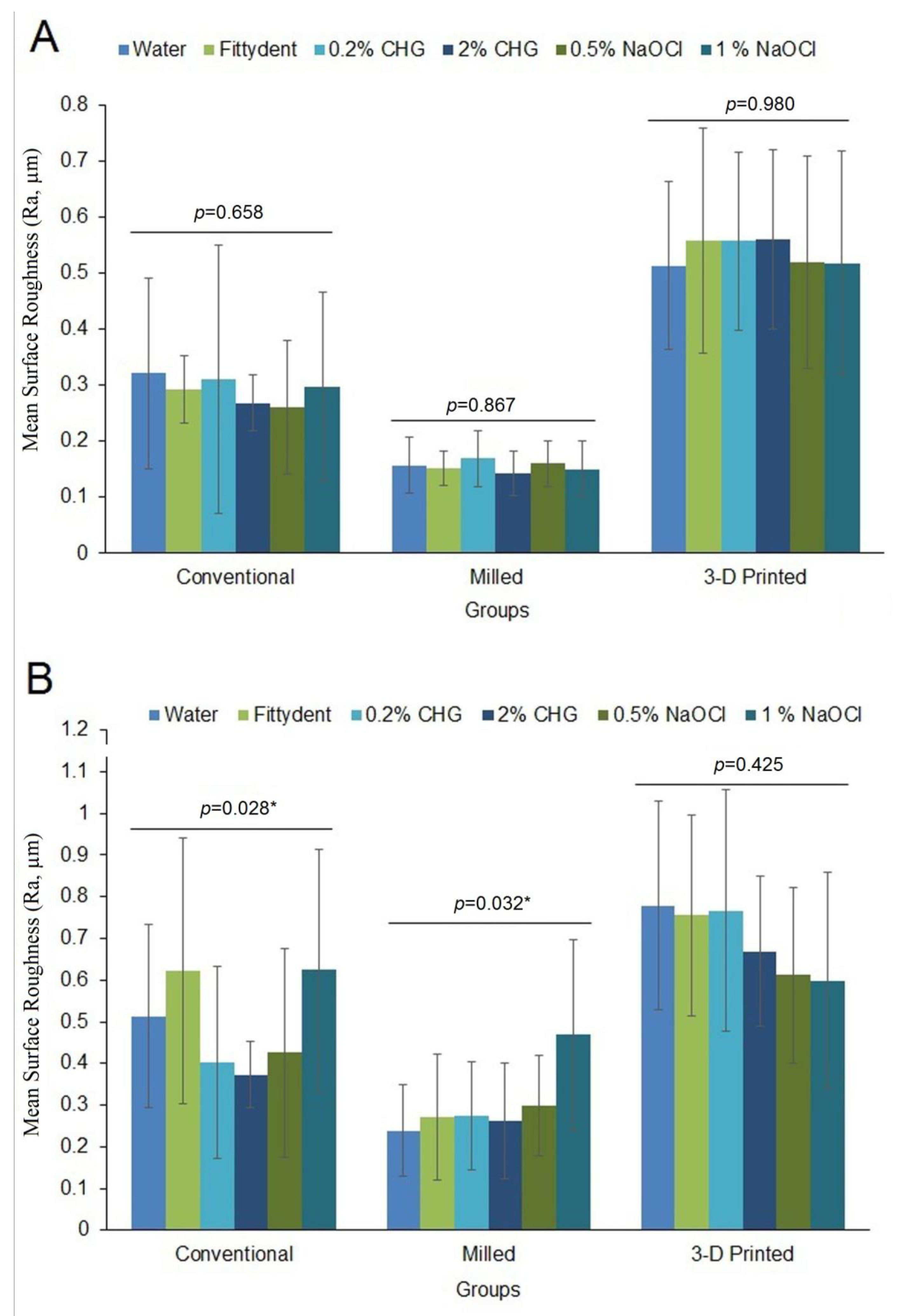
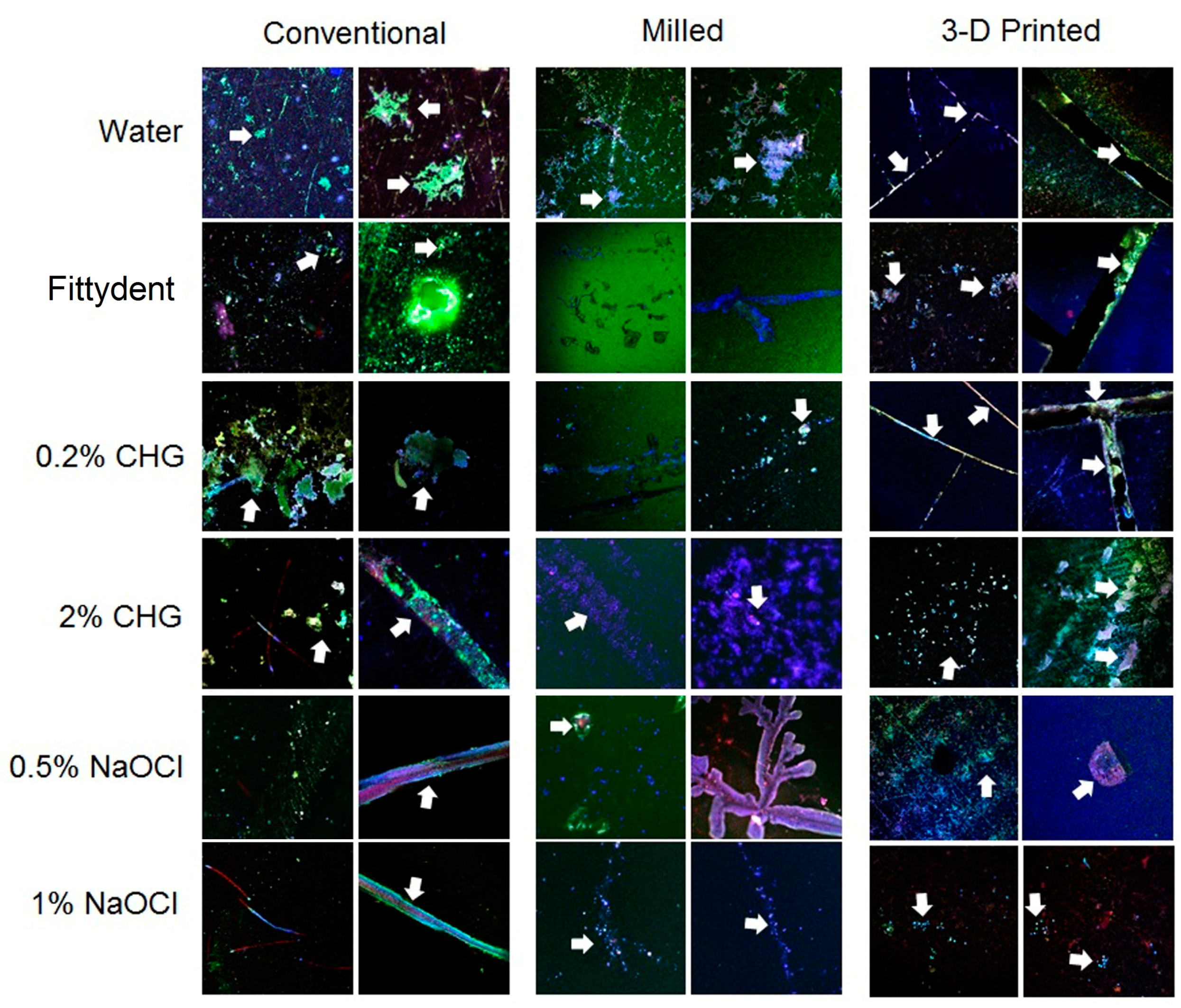
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfouzan, A.; Alotiabi, H.; Labban, N.; Al-Otaibi, H.; Al Taweel, S.; Alshehri, H. Color Stability of 3d-Printed Denture Resins: Effect of Aging, Mechanical Brushing and Immersion in Staining Medium. J. Adv. Prosthodont. 2021, 13, 160–171. [Google Scholar] [CrossRef]
- Gruber, S.; Kamnoedboon, P.; Özcan, M.; Srinivasan, M. CAD/CAM Complete Denture Resins: An in Vitro Evaluation of Color Stability. J. Prosthodont. 2020, 31, 13246. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.; Guven, M.C.; Gencel, B.; Bural, C. A Comparison of the Color Stability of Conventional and Cad/Cam Polymethyl Methacrylate Denture Base Materials. Acta. Stomatol. Croat. 2019, 53, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A Comparison of the Surface Properties of Cad/Cam and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Al-Fouzan, A.F.; Al-Mejrad, L.A.; Albarrag, A.M. Adherence of Candida to Complete Denture Surfaces in Vitro: A Comparison of Conventional and Cad/Cam Complete Dentures. J. Adv. Prosthodont. 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Bidra, A.S.; Taylor, T.D.; Agar, J.R. Computer-Aided Technology for Fabricating Complete Dentures: Systematic Review of Historical Background, Current Status, and Future Perspectives. J. Prosthet. Dent. 2013, 109, 361–366. [Google Scholar] [CrossRef]
- Chen, J.; Ahmad, R.; Suenaga, H.; Li, W.; Sasaki, K.; Swain, M.; Li, Q. Shape Optimization for Additive Manufacturing of Removable Partial Dentures—A New Paradigm for Prosthetic CAD/CAM. PLoS ONE 2015, 10, e0132552. [Google Scholar] [CrossRef]
- Kanazawa, M.; Inokoshi, M.; Minakuchi, S.; Ohbayashi, N. Trial of a Cad/Cam System for Fabricating Complete Dentures. Dent. Mater. J. 2011, 30, 93–96. [Google Scholar] [CrossRef]
- Kattadiyil, M.T.; Goodacre, C.J.; Baba, N.Z. Cad/Cam Complete Dentures: A Review of Two Commercial Fabrication Systems. J. Calif. Dent. Assoc. 2013, 41, 407–416. [Google Scholar]
- Pereyra, N.M.; Marano, J.; Subramanian, G.; Quek, S.; Leff, D. Comparison of Patient Satisfaction in the Fabrication of Conventional Dentures Vs. Dentca (Cad/Cam) Dentures: A Case Report. N. J. Dent. Assoc. 2015, 86, 26–33. [Google Scholar]
- Schubert, A.; Bürgers, R.; Baum, F.; Kurbad, O.; Wassmann, T. Influence of the Manufacturing Method on the Adhesion of Candida Albicans and Streptococcus Mutans to Oral Splint Resins. Polymers 2021, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Kalberer, N.; Mehl, A.; Schimmel, M.; Müller, F.; Srinivasan, M. Cad-Cam Milled Versus Rapidly Prototyped (3d-Printed) Complete Dentures: An In vitro Evaluation of Trueness. J. Prosthet. Dent. 2019, 121, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of Denture Tooth Movement between Cad-Cam and Conventional Fabrication Techniques. J. Prosthet. Dent. 2018, 119, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Park, J.M.; Kim, T.H.; Ahn, J.S.; Cha, H.S.; Lee, J.H. 3D Printing of Resin Material for Denture Artificial Teeth: Chipping and Indirect Tensile Fracture Resistance. Materials 2018, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Sartawi, S.; Abu-Hammad, S.; Salim, N.; Al-Omoush, S. Denture Stomatitis Revisited: A Summary of Systematic Reviews in the Past Decade and Two Case Reports of Papillary Hyperplasia of Unusual Locations. Int. J. Dent. 2021, 2021, e7338143. [Google Scholar] [CrossRef]
- Vasconcelos, L.; Sampaio, F.; Sampaio, M.; Pereira, M.; Pereira-Peixoto, M. Streptococcus Mutans in Denture Stomatitis Patients under Antifungal Therapy. Rev. Odonto. Cienc. 2009, 25, 120–125. [Google Scholar] [CrossRef]
- Karakis, D.; Akay, C.; Oncul, B.; Rad, A.Y.; Dogan, A. Effectiveness of Disinfectants on the Adherence of Candida Albicans to Denture Base Resins with Different Surface Textures. J. Oral. Sci. 2016, 58, 431–437. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida Albicans, Staphylococcus Aureus and Streptococcus Mutans Colonization in Patients Wearing Dental Prosthesis. Med. Oral. Patol. Oral. Cir. Bucal. 2005, 10 (Suppl. 1), e27–e39. [Google Scholar]
- Alfouzan, A.F.; Alnouwaisar, A.N.; Alazzam, N.F.; Al-Otaibi, H.N.; Labban, N.; Alswaidan, M.H.; Al Taweel, S.M.; Alshehri, H.A. Power Brushing and Chemical Denture Cleansers Induced Color Changes of Pre-Polymerized Cad/Cam Denture Acrylic Resins. Mater. Res. Express. 2021, 8, e085402. [Google Scholar] [CrossRef]
- Amaya Arbeláez, M.I.; Vergani, C.E.; Barbugli, P.A.; Pavarina, A.C.; Sanitá, P.V.; Jorge, J.H. Long-Term Effect of Daily Chemical Disinfection on Surface Topography and Candida Albicans Biofilm Formation on Denture Base and Reline Acrylic Resins. Oral. Health. Prev. Dent. 2020, 18, 999–1010. [Google Scholar]
- De Andrade, I.M.; Cruz, P.C.; Silva-Lovato, C.H.; De Souza, R.F.; Souza-Gugelmin, M.C.; Paranhos Hde, F. Effect of Chlorhexidine on Denture Biofilm Accumulation. J. Prosthodont. 2012, 21, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Yuzugullu, B.A.O.; Cetinsahin, C.; Celik, C. Effect of Different Denture Cleansers on Surface Roughness and Microhardness of Artificial Denture Teeth. J. Adv. Prosthodont. 2016, 8, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Kimpara, E.T.; Mancini, M.N.; Balducci, I.; Jorge, A.O.; Koga-Ito, C.Y. Effectiveness of Six Different Disinfectants on Removing Five Microbial Species and Effects on the Topographic Characteristics of Acrylic Resin. J. Prosthodont. 2008, 17, 627–633. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Fernandes, F.S.; Pereira-Cenci, T.; Da Silva, W.J.; Filho, A.P.; Straioto, F.G.; Del Bel Cury, A.A. Efficacy of Denture Cleansers on Candida Spp. Biofilm Formed on Polyamide and Polymethyl Methacrylate Resins. J. Prosthet. Dent. 2011, 105, 51–58. [Google Scholar] [CrossRef]
- Felipucci, D.N.; Davi, L.R.; Paranhos, H.F.; Bezzon, O.L.; Silva, R.F.; Pagnano, V.O. Effect of Different Cleansers on the Surface of Removable Partial Denture. Braz. Dent. J. 2011, 22, 392–397. [Google Scholar] [CrossRef]
- Hayran, Y.; Sarikaya, I.; Aydin, A.; Tekin, Y.H. Determination of the Effective Anticandidal Concentration of Denture Cleanser Tablets on Some Denture Base Resins. J. Appl. Oral. Sci. 2018, 26, e20170077. [Google Scholar] [CrossRef]
- Chatzivasileiou, K.; Kotsiomiti, E.; Vyzantiadis, T.A. Effectiveness of Denture Cleansers on Removal of Adherent Candida Albicans Cells from Denture Base Acrylics of Various Roughness. Int. J. Prosthodont. 2019, 32, 196–197. [Google Scholar] [CrossRef]
- Ayaz, E.; Ustun, S. Effect of Staining Denture Cleaning on Color Stability of Differently Polymerized Denture Base Acrylic Resins. Niger. J. Clin. Pract. 2020, 23, 304–309. [Google Scholar]
- Machado, A.L.; Giampaolo, E.T.; Vergani, C.E.; Pavarina, A.C.; Salles Dda, S.; Jorge, J.H. Weight Loss and Changes in Surface Roughness of Denture Base and Reline Materials after Simulated Toothbrushing in Vitro. Gerodontology 2012, 29, 1741–2358. [Google Scholar] [CrossRef]
- Williams, D.W.; Lewis, M.A.O. Oral Microbiology: Isolation and Identification of Candida from the Oral Cavity. Oral. Dis. 2000, 6, 3–11. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Orsi, I.A.; Villabona, C.A. Effects of the Peracetic Acid and Sodium Hypochlorite on the Colour Stability and Surface Roughness of the Denture Base Acrylic Resins Polymerised by Microwave and Water Bath Methods. Gerodontology 2013, 30, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Paranhos Hde, F.; Peracini, A.; Pisani, M.X.; Oliveira Vde, C.; De Souza, R.F.; Silva-Lovato, C.H. Color Stability, Surface Roughness and Flexural Strength of an Acrylic Resin Submitted to Simulated Overnight Immersion in Denture Cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Pavarina, A.C.; Pizzolitto, A.C.; Machado, A.L.; Vergani, C.E.; Giampaolo, E.T. An Infection Control Protocol: Effectiveness of Immersion Solutions to Reduce the Microbial Growth on Dental Prostheses. J. Oral. Rehabil. 2003, 30, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Pinto Lde, R.; Acosta, E.J.; Távora, F.F.; Da Silva, P.M.; Porto, V.C. Effect of Repeated Cycles of Chemical Disinfection on the Roughness and Hardness of Hard Reline Acrylic Resins. Gerodontology 2010, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-S.; Cha, H.-S.; Kim, T.-H.; Ahn, J.-S.; Lee, J.-H. Color Stability of Three Dimensional-Printed Denture Teeth Exposed to Various Colorants. J. Korean Acad. Prosthodont. 2020, 58, 1–6. [Google Scholar] [CrossRef]
- Alfouzan, A.F.; Alotiabi, H.M.; Labban, N.; Al-Otaibi, H.N.; Al Taweel, S.M.; Alshehri, H.A. Effect of Aging and Mechanical Brushing on Surface Roughness of 3d Printed Denture Resins: A Profilometer and Scanning Electron Microscopy Analysis. Technol. Health Care 2022, 30, 161–173. [Google Scholar] [CrossRef]
- Maart, R.; Grobler, S.R.; Kruijsse, H.W.; Osman, Y.; Patel, N.; Moodley, D. The Whitening Effect of Four Different Commercial Denture Cleansers on Stained Acrylic Resin. S. Afr. Dent. J. 2016, 71, 106–111. [Google Scholar]
- Salles, M.M.; Badaró, M.M.; Arruda, C.N.; Leite, V.M.; Silva, C.H.; Watanabe, E.; Oliveira Vde, C.; Paranhos Hde, F. Antimicrobial Activity of Complete Denture Cleanser Solutions Based on Sodium Hypochlorite and Ricinus Communis—A Randomized Clinical Study. J. Appl. Oral. Sci. 2015, 23, 637–642. [Google Scholar] [CrossRef]
- Salles, M.M.; Oliveira Vde, C.; Souza, R.F.; Silva, C.H.; Paranhos Hde, F. Antimicrobial Action of Sodium Hypochlorite and Castor Oil Solutions for Denture Cleaning—In Vitro Evaluation. Braz. Oral. Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Rodríguez Acosta, E.J.; Da Silva, P.M.; Jacobina, M.; Lara, V.S.; Neppelenbroek, K.H.; Porto, V.C. Candida Albicans Adherence to Denture Base Material: Chemical Disinfection and the Effect of Acquired Salivary Pellicle Formation. J. Prosthodont. 2015, 24, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Chau, V.B.; Saunders, T.R.; Pimsler, M.; Elfring, D.R. In-Depth Disinfection of Acrylic Resins. J. Prosthet. Dent. 1995, 74, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Abbott, P.V. The Properties and Applications of Chlorhexidine in Endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, H.J.; Kim, J.E.; Park, J.E.; Yoo, J.H.; Park, Y.D.; Hwang, K.S. Safety and Efficacy of Fittydent Mega Denture Clean Agents: In Vitro. Int. J. Clin. Prev. Dent. 2014, 10, 147–156. [Google Scholar] [CrossRef]
- Fiore, A.D.; Meneghello, R.; Brun, P.; Rosso, S.; Gattazzo, A.; Stellini, E.; Yilmaz, B. Comparison of the Flexural and Surface Properties of Milled, 3d-Printed, and Heat Polymerized Pmma Resins for Denture Bases: An in Vitro Study. J. Prosthodont. Res. 2022, 66, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Radford, D.R.; Sweet, S.P.; Challacombe, S.J.; Walter, J.D. Adherence of Candida Albicans to Denture-Base Materials with Different Surface Finishes. J. Dent. 1998, 26, 577–583. [Google Scholar] [CrossRef]
- Verran, J.; Maryan, C.J. Retention of Candida Albicans on Acrylic Resin and Silicone of Different Surface Topography. J. Prosthet. Dent. 1997, 77, 535–539. [Google Scholar] [CrossRef]


| Material | Lot No. | Composition | Manufacturer |
|---|---|---|---|
| IvoBase CAD | UP0897 | Industrially polymerized blocks containing >90% polymethylmethacrylate | Wieland Digital Denture (Danbury, CT, USA) |
| Denture 3D+ | WY032N01 | 90% methacrylic oligomers, methacrylate monomer, phosphine oxides, and pigment | NextDent (Soesterberg, The Netherlands) |
| Meliodent heat cure acrylic resin | K010028 | Powder: Polymethylmethacrylate, ethyl hexyl acrylate, N-octyl methacrylate Liquid: methyl methacrylate, glycol dimethacrylate, dimethyl p-touludine | Heraeus Kulzer GmbH (Hanau, Germany) |
| Denture Cleansers | Conventional | CAD/CAM | 3D-Printed | p Value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| C. albicans | Water | 14.3 ± 13.1 | 7.7 ±5.8 | 5.0 ± 5.8 | 0.20 |
| Fittydent | 17.2 ± 13.6 | 2.2 ±1.6 a | 32.1 ± 22.6 b | 0.003 * | |
| 0.2% CHG | 0.00 ± 0.00 | 0.00 ± 0.00 | 4 ± 4.2 | n/a † | |
| 2% CHG | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † | |
| 0.5% NaOCl | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † | |
| 1% NaOCl | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † | |
| S. mutans | Water | 288.4 ± 64.4 | 327.9 ± 47 | 245.4 ± 577.7 | 0.29 |
| Fittydent | 35.7 ± 25.6 | 16.3 ± 19.5 | 92.3 ± 126.2 a | 0.017 * | |
| 0.2% CHG | 79.1 ± 101.3 | 16.5 ± 12 | 44.8 ± 49.7 | 0.74 | |
| 2% CHG | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † | |
| 0.5% NaOCl | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † | |
| 1% NaOCl | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | n/a † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfouzan, A.F.; Tuwaym, M.; Aldaghri, E.N.; Alojaymi, T.; Alotiabi, H.M.; Taweel, S.M.A.; Al-Otaibi, H.N.; Ali, R.; Alshehri, H.; Labban, N. Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins. Polymers 2023, 15, 460. https://doi.org/10.3390/polym15020460
Alfouzan AF, Tuwaym M, Aldaghri EN, Alojaymi T, Alotiabi HM, Taweel SMA, Al-Otaibi HN, Ali R, Alshehri H, Labban N. Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins. Polymers. 2023; 15(2):460. https://doi.org/10.3390/polym15020460
Chicago/Turabian StyleAlfouzan, Afnan F., Malath Tuwaym, Ebtihal N. Aldaghri, Tagreed Alojaymi, Hadeel Minife Alotiabi, Sara M. Al Taweel, Hanan N. Al-Otaibi, Rizwan Ali, Huda Alshehri, and Nawaf Labban. 2023. "Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins" Polymers 15, no. 2: 460. https://doi.org/10.3390/polym15020460
APA StyleAlfouzan, A. F., Tuwaym, M., Aldaghri, E. N., Alojaymi, T., Alotiabi, H. M., Taweel, S. M. A., Al-Otaibi, H. N., Ali, R., Alshehri, H., & Labban, N. (2023). Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins. Polymers, 15(2), 460. https://doi.org/10.3390/polym15020460






