Ultra-High-Capacity Lithium Metal Batteries Based on Multi-Electron Redox Reaction of Organopolysulfides including Conductive Organic Moieties
Abstract
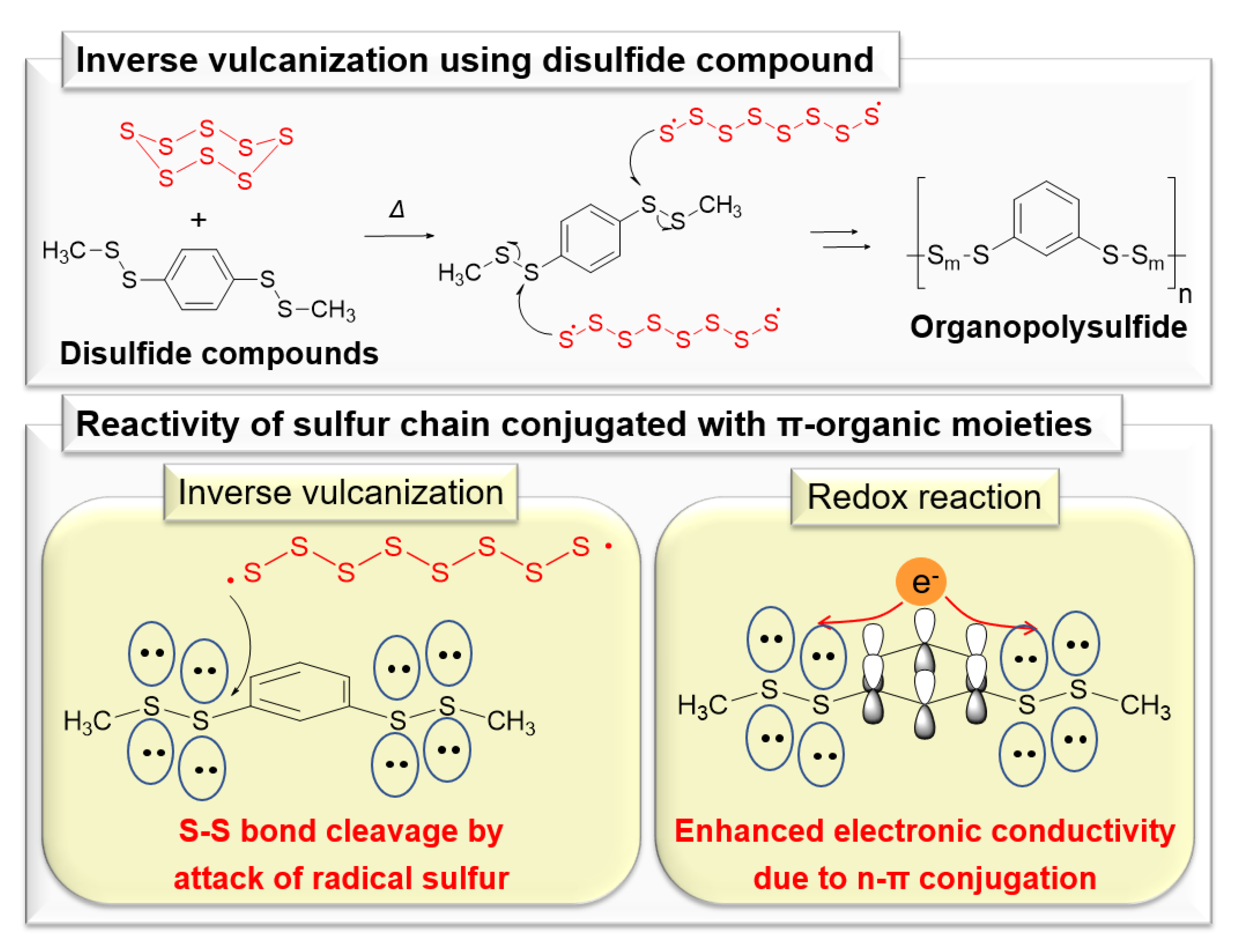
1. Introduction
2. Materials
3. Methods
3.1. Nucler Magnetic Resonance (NMR)
3.2. Synthesis
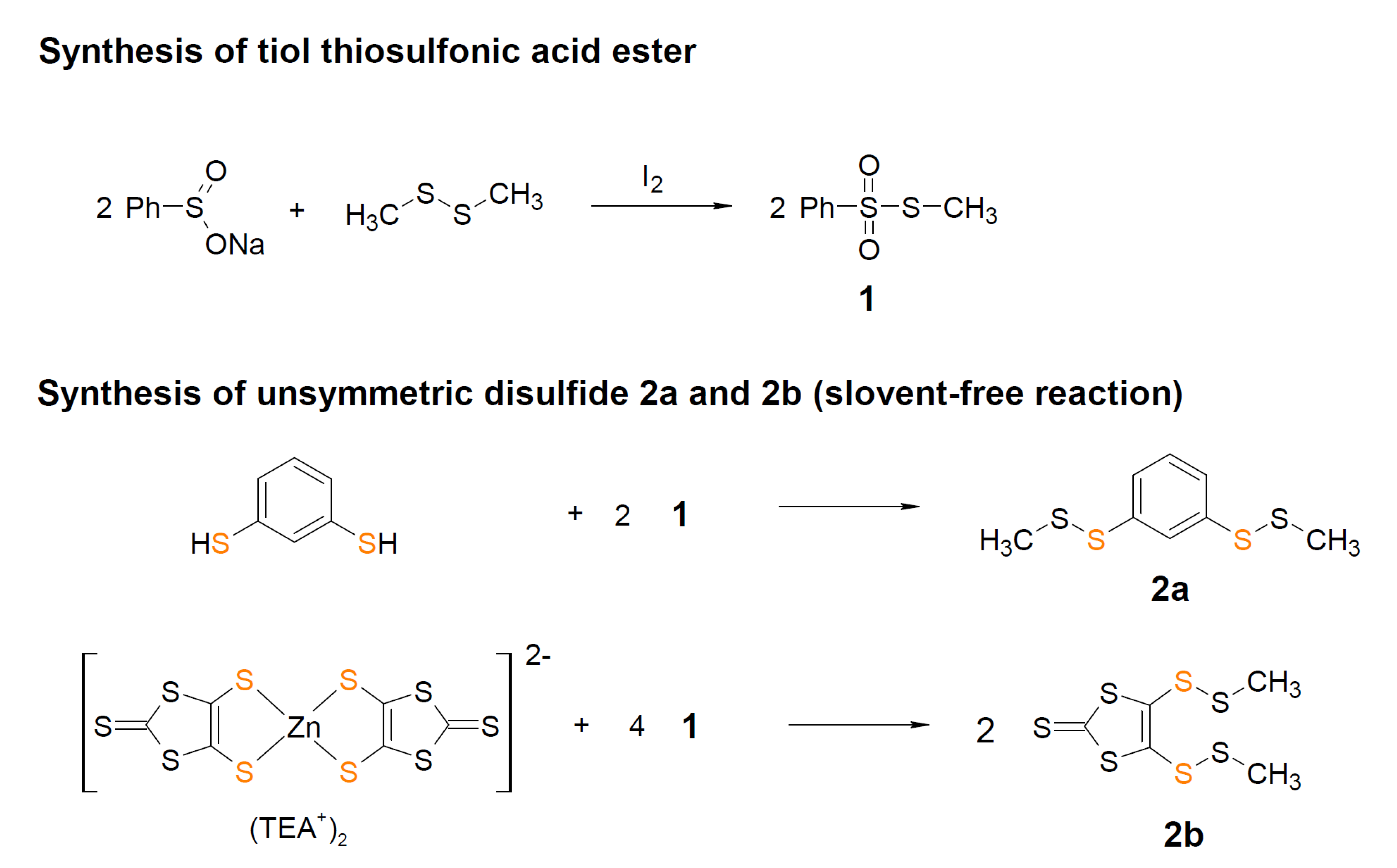
3.2.1. Syntheses of Asymmetric Organodisulfides
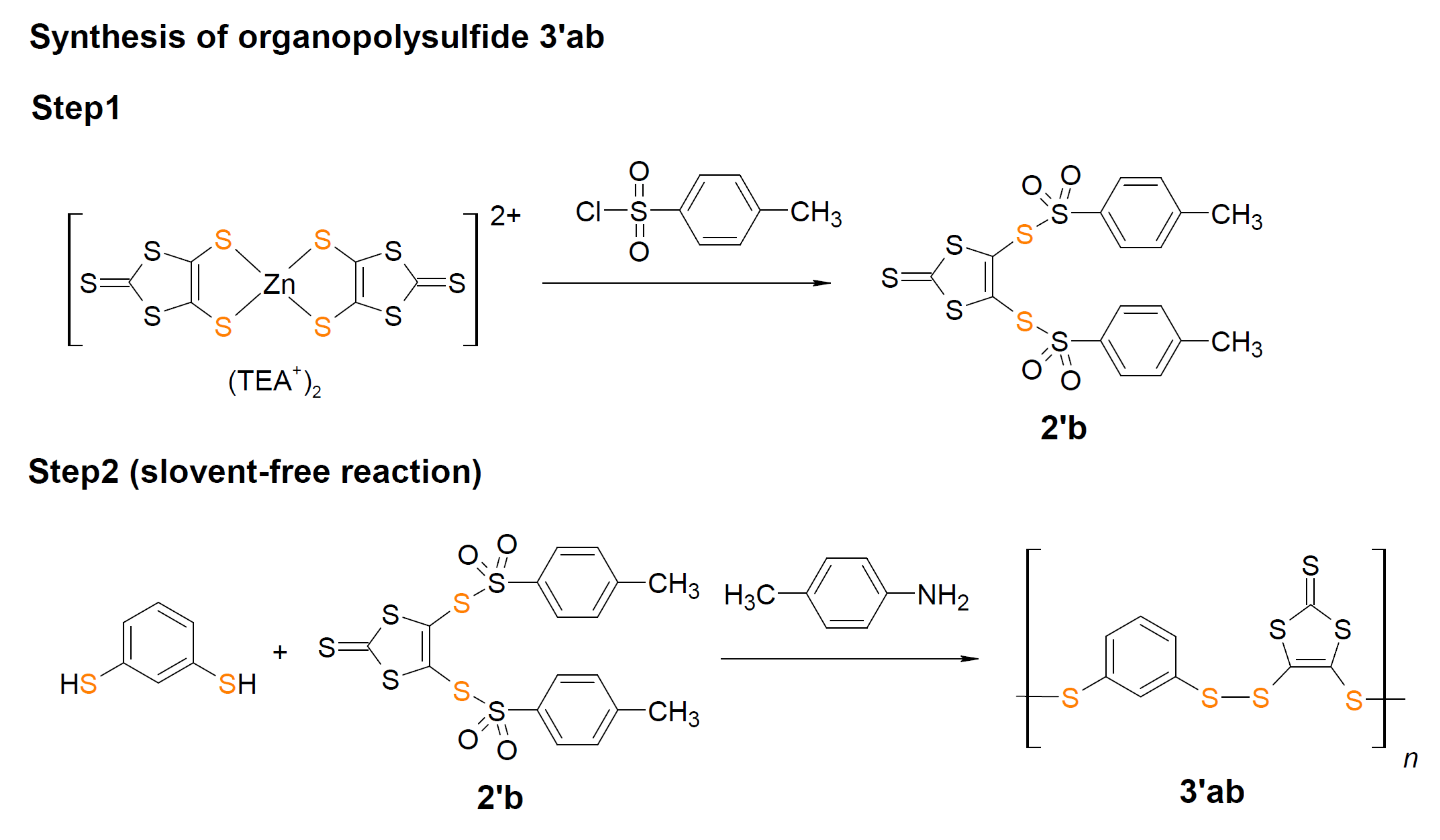
3.2.2. Syntheses of Organopolysulfides
3.2.3. Syntheses of organodisulfide polymers
3.3. Characterization of 3ab
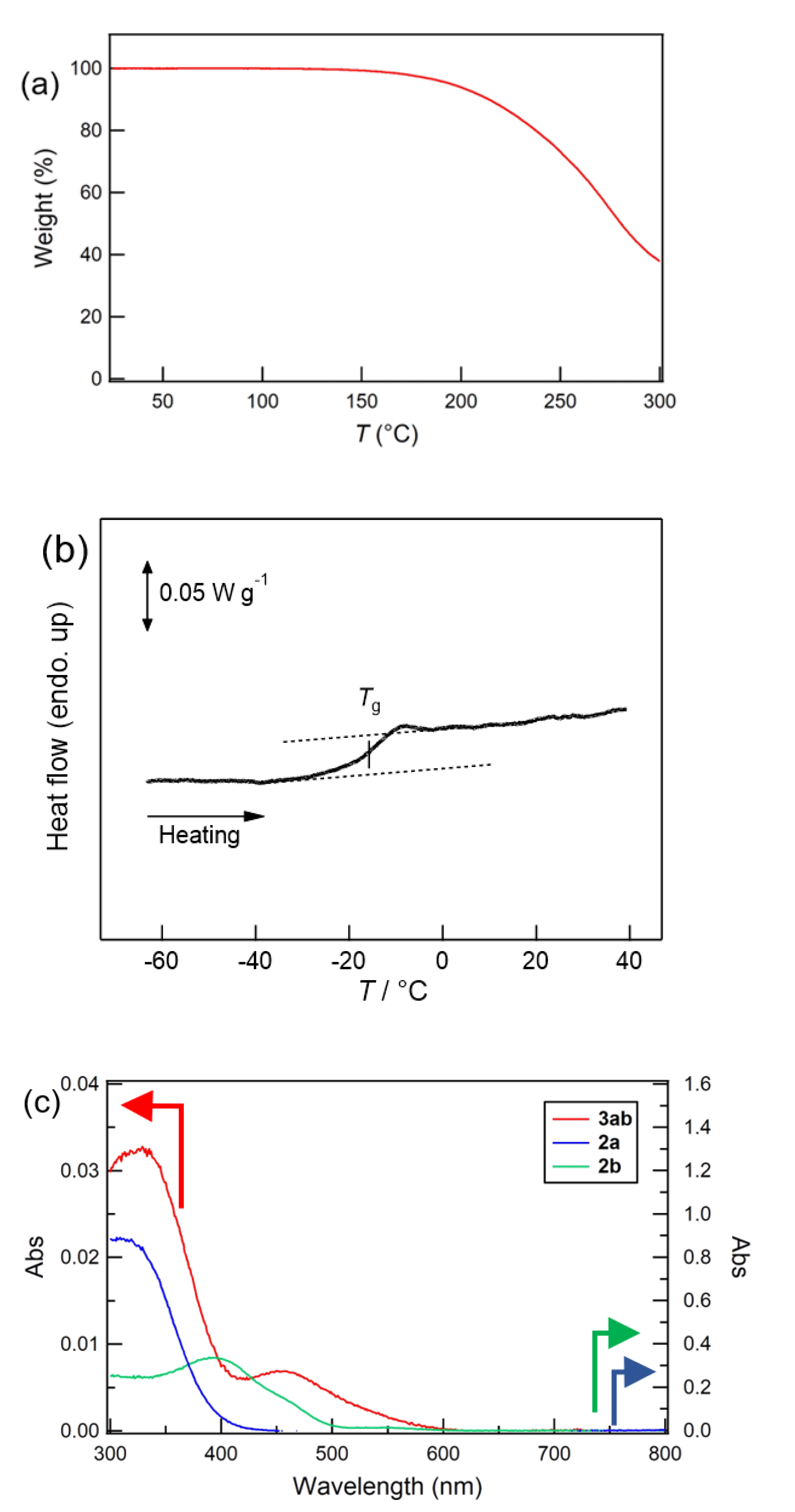
3.3.1. Thermal Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.3.2. Diffuse Reflectance Ultraviolet–Visible–Near-Infrared (UV–vis–NIR) Spectroscopy
3.4. Electrochemical Measurements
3.4.1. Battery Fabrication
3.4.2. Discharge–Charge Measurement
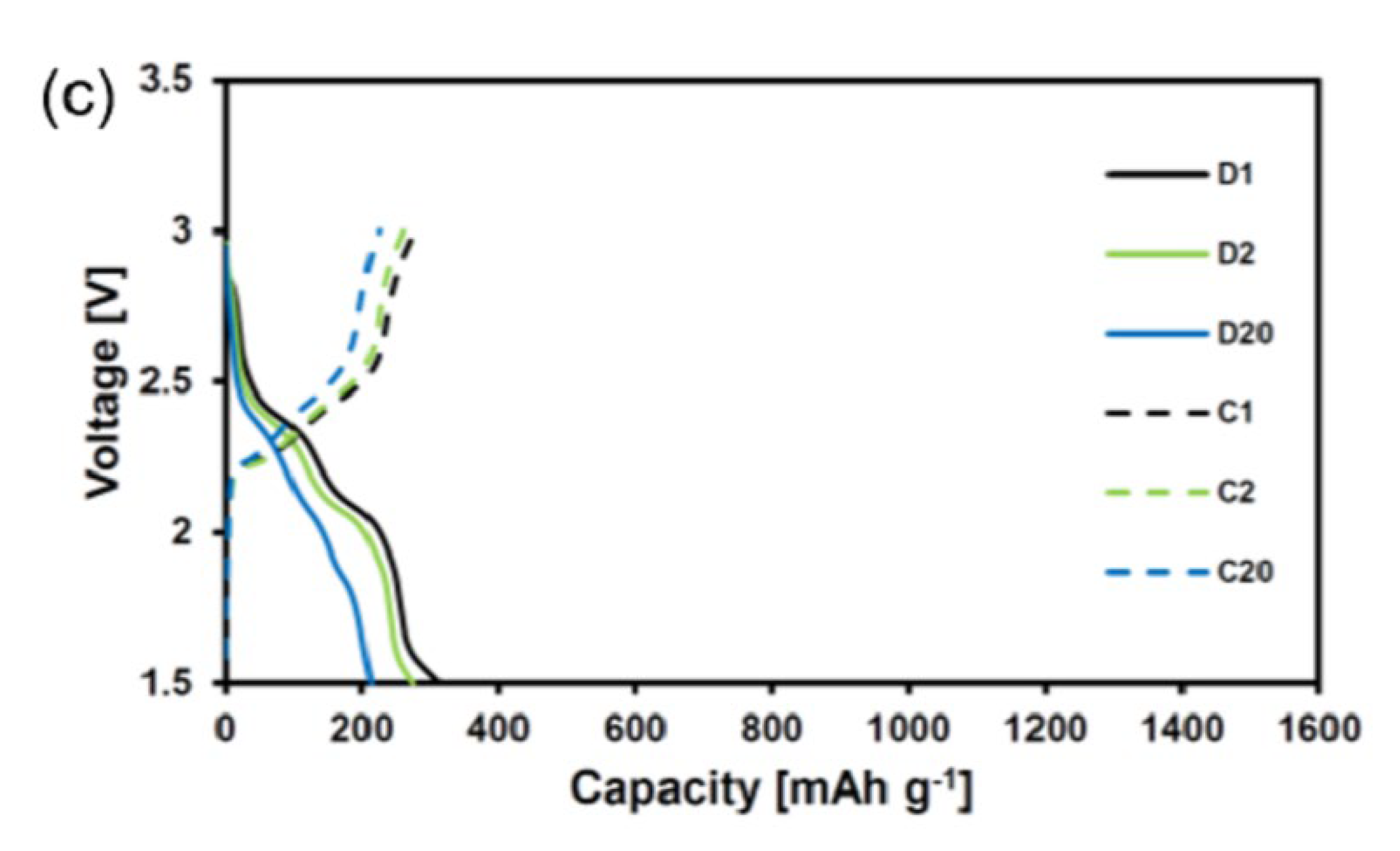
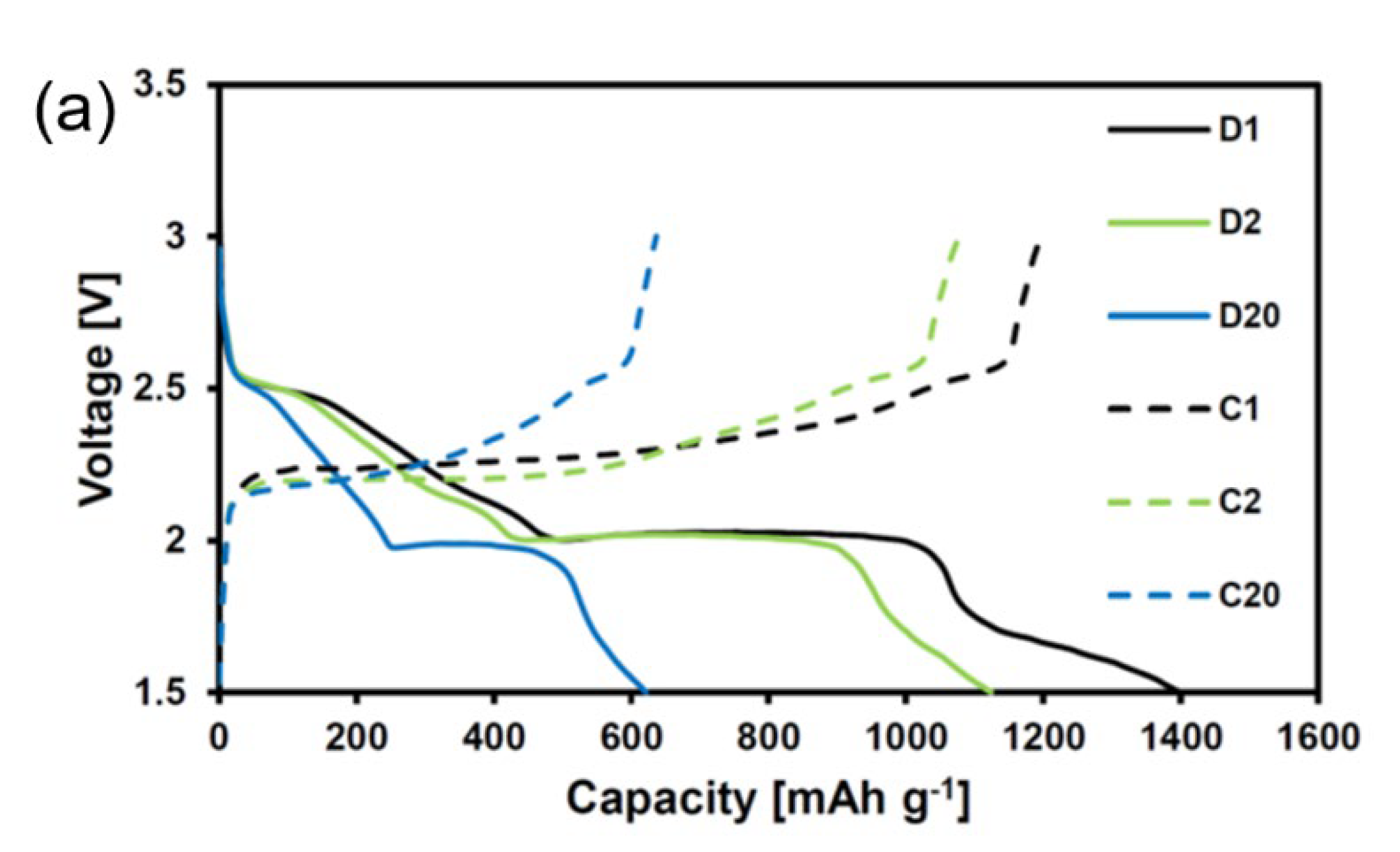
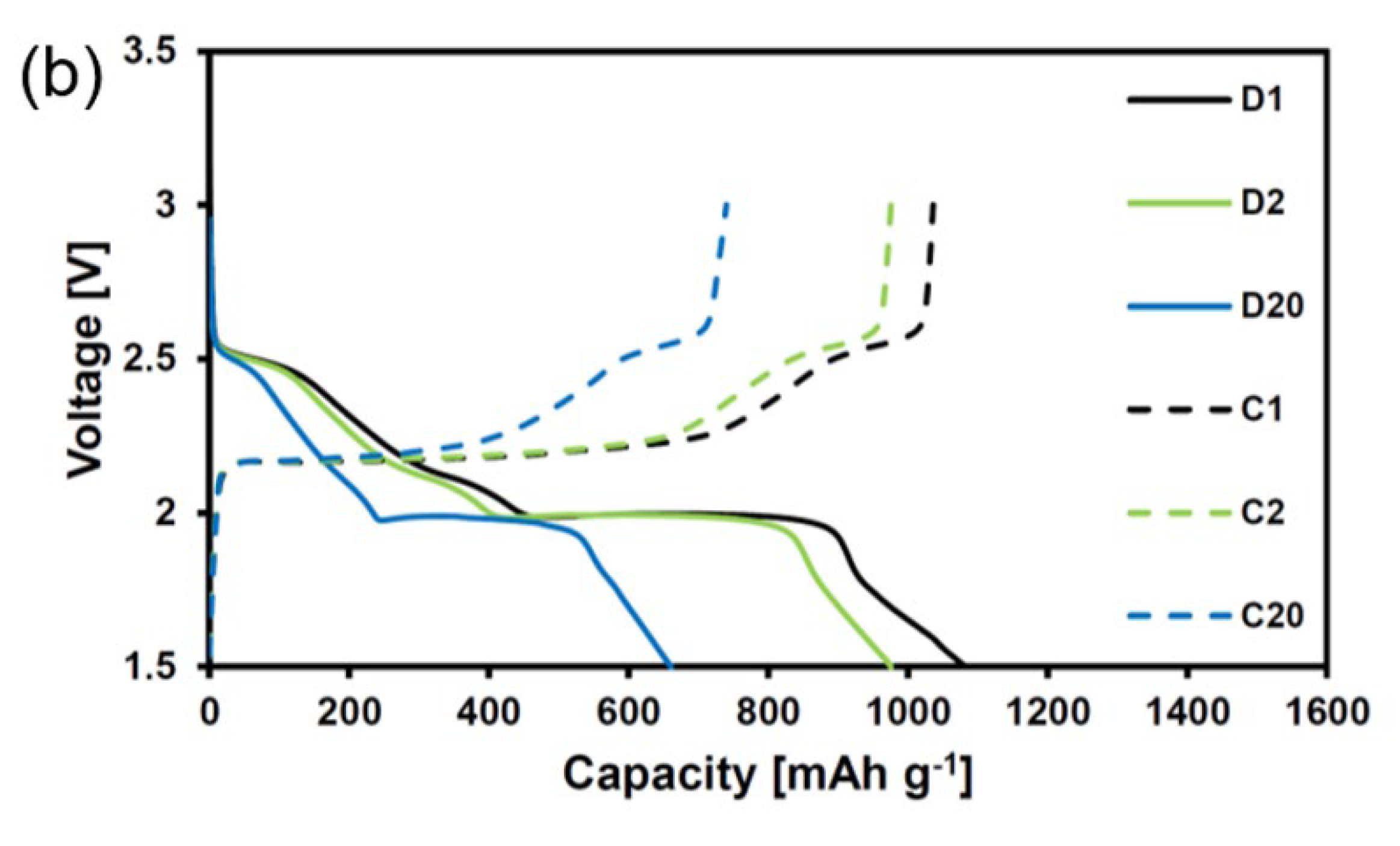
4. Results and Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, L.; Li, Y.; An, Y.; Zhu, M.; Dai, B. Mechanism Studies of LiFePO4 Cathode Material: Lithiation/Delithiation Process, Electrochemical Modification and Synthetic Reaction. RSC Adv. 2014, 4, 54576–54602. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and Fuel Cells for Emerging Electric Vehicle Markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Xue, L.J.; Li, J.X.; Hu, S.Q.; Zhang, M.X.; Zhou, Y.H.; Zhan, C.M. Anthracene Based Organodisulfide Positive Active Materials for Lithium Secondary Battery. Electrochem. Commun. 2003, 5, 903–906. [Google Scholar] [CrossRef]
- Zeng, F.L.; Li, N.; Shen, Y.Q.; Zhou, X.Y.; Jin, Z.Q.; Yuan, N.Y.; Ding, J.N.; Wang, A.B.; Wang, W.K.; Yang, Y.S. Improve the Electrodeposition of Sulfur and Lithium Sulfide in Lithium-Sulfur Batteries with a Comb-like Ion-Conductive Organo-Polysulfide Polymer Binder. Energy Storage Mater. 2019, 18, 190–198. [Google Scholar] [CrossRef]
- Liu, M.; Visco, S.J.; De Jonghe, L.C. Electrochemical Properties of Organic Disulfide/Thiolate Redox Couples. J. Electrochem. Soc. 1989, 136, 2570–2575. [Google Scholar] [CrossRef]
- Hong, H.; Che Mohamad, N.A.R.; Chae, K.; Marques Mota, F.; Kim, D.H. The Lithium Metal Anode in Li-S Batteries: Challenges and Recent Progress. J. Mater. Chem. A 2021, 9, 10012–10038. [Google Scholar] [CrossRef]
- Cameron, J.M.; Holc, C.; Kibler, A.J.; Peake, C.L.; Walsh, D.A.; Newton, G.N.; Johnson, L.R. Molecular Redox Species for Next-Generation Batteries. Chem. Soc. Rev. 2021, 50, 5863–5883. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic Inverse Vulcanization. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li–S Batteries. Small Methods 2018, 2, 1–34. [Google Scholar] [CrossRef]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li-S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griebel, J.J.; Dirlam, P.T.; Nguyen, N.A.; Glass, R.S.; Mackay, M.E.; Char, K.; Pyun, J. Inverse vulcanization of elemental sulfur and styrene for polymeric cathodes in Li-S batteries. J. Polym. Sci. Part A: Polym. Chem. 2017, 55, 107–116. [Google Scholar] [CrossRef]
- Gardner, D.M.; Fraenkel, G.K. Paramagnetic Resonance of Liquid Sulfur: Determination of Molecular Properties. J. Am. Chem. Soc. 1956, 78, 3279–3288. [Google Scholar] [CrossRef]
- Tanifuji, N. Syntheses of Organopolysulfides. Jpn. Kokai Tokkyo Koho JP2015-54834A, 23 March 2015. [Google Scholar]
- Shirahata, T.; Ikeda, M.; Watadzu, H.; Fujiwara, H.; Maruyama, T.; Yamabe, T.; Misaki, Y. Organic Conductors with Narrow Bandwidth Based on 2-(Pyran-4-Ylidene)-1,3-Dithiole. Bull. Chem. Soc. Jpn. 2021, 94, 1331–1339. [Google Scholar] [CrossRef]
- Horiuchi, H.; Misaki, Y. Synthesis and Properties of 1,3-Dithiole[5]Dendralenes with Two Thiophene Spacers. Chem. Lett. 2010, 39, 989–991. [Google Scholar] [CrossRef]
- Kawasaki, N.; Wang, H.; Nakanishi, R.; Hamanaka, S.; Kitaura, R.; Shinohara, H.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. Nanohybridization of Polyoxometalate Clusters and Single-Wall Carbon Nanotubes: Applications in Molecular Cluster Batteries. Angew. Chem. Ed. 2011, 50, 3471–3474. [Google Scholar] [CrossRef]
- Kume, K.; Kawasaki, N.; Wang, H.; Yamada, T.; Yoshikawa, H.; Awaga, K. Enhanced Capacitor Effects in Polyoxometalate/Graphene Nanohybrid Materials: A Synergetic Approach to High Performance Energy Storage. J. Mater. Chem. A 2014, 2, 3801–3807. [Google Scholar] [CrossRef]
- Tanifuji, N. Syntheses of Disulfide Polymer. Jpn. Kokai Tokkyo Koho JP2012-229329A, 22 November 2012. [Google Scholar]
- Nakashima, N.; Tomonari, Y.; Murakami, H. Water-Soluble Single-Walled Carbon Nanotubes via Noncovalent Sidewall-Functionalization with a Pyrene-Carrying Ammonium Ion. Chem. Lett. 2002, 31, 638–639. [Google Scholar] [CrossRef]
- Tao, A.; Zhang, K.; Ma, X.; Song, X.; Liang, J.; Wang, Y.; Liu, Y.; Jin, L.; Tie, Z.; Jin, Z. Building Lithium-Polycarbonsulfide Batteries with High Energy Density and Long Cycling Life. ACS Energy Lett. 2022, 79–89. [Google Scholar] [CrossRef]
- Diez, S.; Hoefling, A.; Theato, P.; Pauer, W. Mechanical and Electrical Properties of Sulfur-Containing Polymeric Materials Prepared via Inverse Vulcanization. Polymers 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Shi Chao, Z.; Lan, Z.; Jinhua, Y. Preparation and Electrochemical Properties of Polysulfide Polypyrrole. J. Power Sources 2011, 196, 10263–10266. [Google Scholar] [CrossRef]
- Dai, S.; Feng, Y.; Wang, P.; Wang, H.; Liang, H.; Wang, R.; Linkov, V.; Ji, S. Highly Conductive Copolymer/Sulfur Composites with Covalently Grafted Polyaniline for Stable and Durable Lithium-Sulfur Batteries. Electrochim. Acta 2019, 321, 134678. [Google Scholar] [CrossRef]
- Zeng, S.; Li, L.; Zhao, D.; Liu, J.; Niu, W.; Wang, N.; Chen, S. Polymer-Capped Sulfur Copolymers as Lithium-Sulfur Battery Cathode: Enhanced Performance by Combined Contributions of Physical and Chemical Confinements. J. Phys. Chem. C 2017, 121, 2495–2503. [Google Scholar] [CrossRef]
- Deng, D.R.; Yuan, R.M.; Yu, P.K.; Xue, F.; Fan, X.X.; Lei, J.; Zhang, J.L.; Lin, X.D.; Wu, Q.H.; Fan, J.M.; et al. An Enhanced Electrode via Coupling with a Conducting Molecule to Extend Interfacial Reactions. Adv. Energy Mater. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Shimizu, T.; Wakamatsu, K.; Yamada, Y.; Toyoda, Y.; Akine, S.; Yoza, K.; Yoshikawa, H. Application of μ-Nitrido- and μ-Carbido-Bridged Iron Phthalocyanine Dimers as Cathode-Active Materials for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40612–40617. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, T.; Tanifuji, N.; Nishio, K.; Tanaka, Y.; Tsukaguchi, Y.; Tsubouchi, K.; Nakamura, F.; Shokura, N.; Noguchi, M.; Fujimori, H.; et al. Ultra-High-Capacity Lithium Metal Batteries Based on Multi-Electron Redox Reaction of Organopolysulfides including Conductive Organic Moieties. Polymers 2023, 15, 335. https://doi.org/10.3390/polym15020335
Shimizu T, Tanifuji N, Nishio K, Tanaka Y, Tsukaguchi Y, Tsubouchi K, Nakamura F, Shokura N, Noguchi M, Fujimori H, et al. Ultra-High-Capacity Lithium Metal Batteries Based on Multi-Electron Redox Reaction of Organopolysulfides including Conductive Organic Moieties. Polymers. 2023; 15(2):335. https://doi.org/10.3390/polym15020335
Chicago/Turabian StyleShimizu, Takeshi, Naoki Tanifuji, Kosuke Nishio, Yuma Tanaka, Yuta Tsukaguchi, Kentaro Tsubouchi, Fumiya Nakamura, Naoko Shokura, Mariko Noguchi, Hiroki Fujimori, and et al. 2023. "Ultra-High-Capacity Lithium Metal Batteries Based on Multi-Electron Redox Reaction of Organopolysulfides including Conductive Organic Moieties" Polymers 15, no. 2: 335. https://doi.org/10.3390/polym15020335
APA StyleShimizu, T., Tanifuji, N., Nishio, K., Tanaka, Y., Tsukaguchi, Y., Tsubouchi, K., Nakamura, F., Shokura, N., Noguchi, M., Fujimori, H., Kimura-Suda, H., Date, Y., Aoki, K., & Yoshikawa, H. (2023). Ultra-High-Capacity Lithium Metal Batteries Based on Multi-Electron Redox Reaction of Organopolysulfides including Conductive Organic Moieties. Polymers, 15(2), 335. https://doi.org/10.3390/polym15020335






