Process Design for Value-Added Products in a Biorefinery Platform from Agro and Forest Industrial Byproducts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Liquid Fraction Valorization
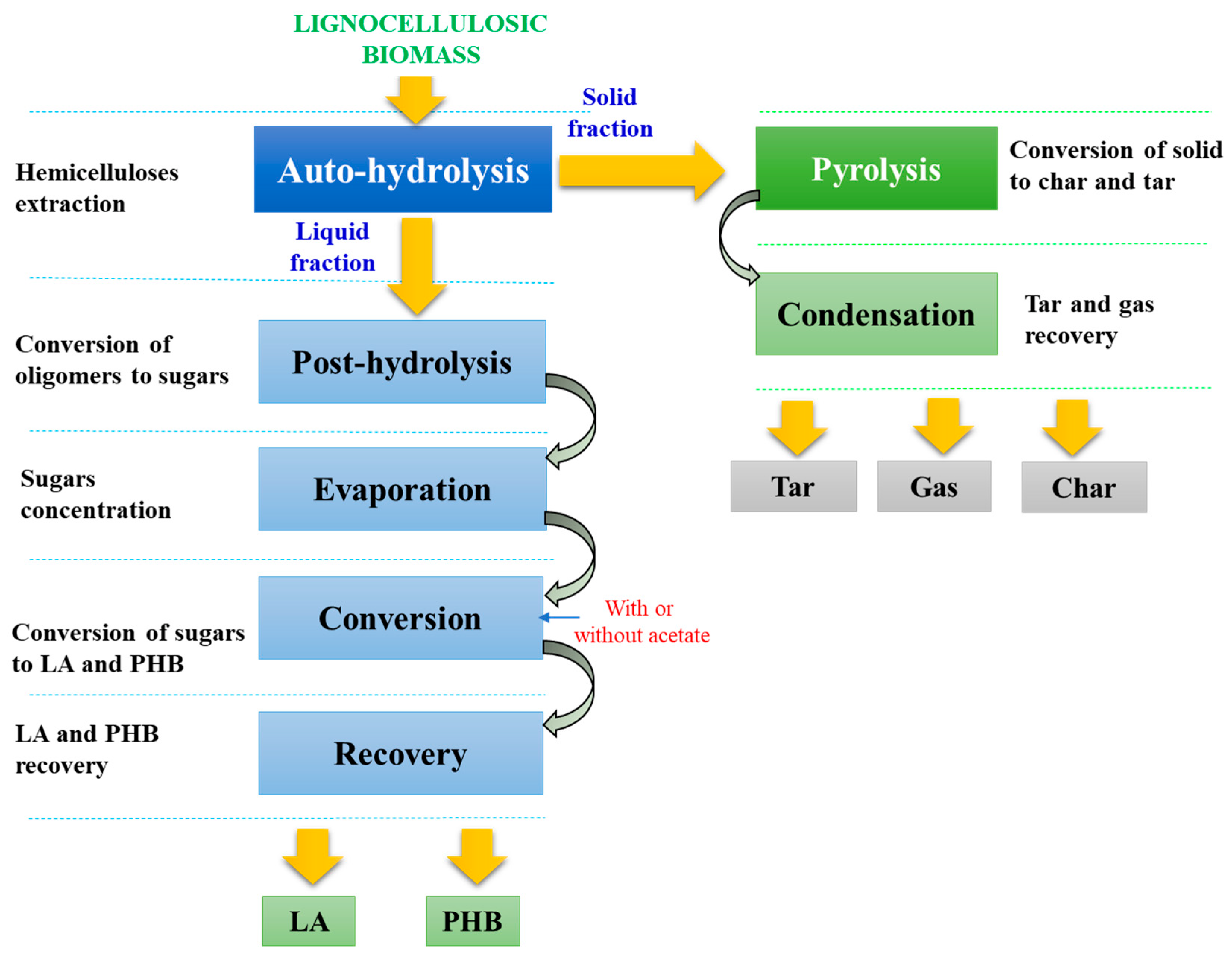
2.3. Autohydrolysis
2.4. Concentration and Post-Hydrolysis
2.5. Process Conversion
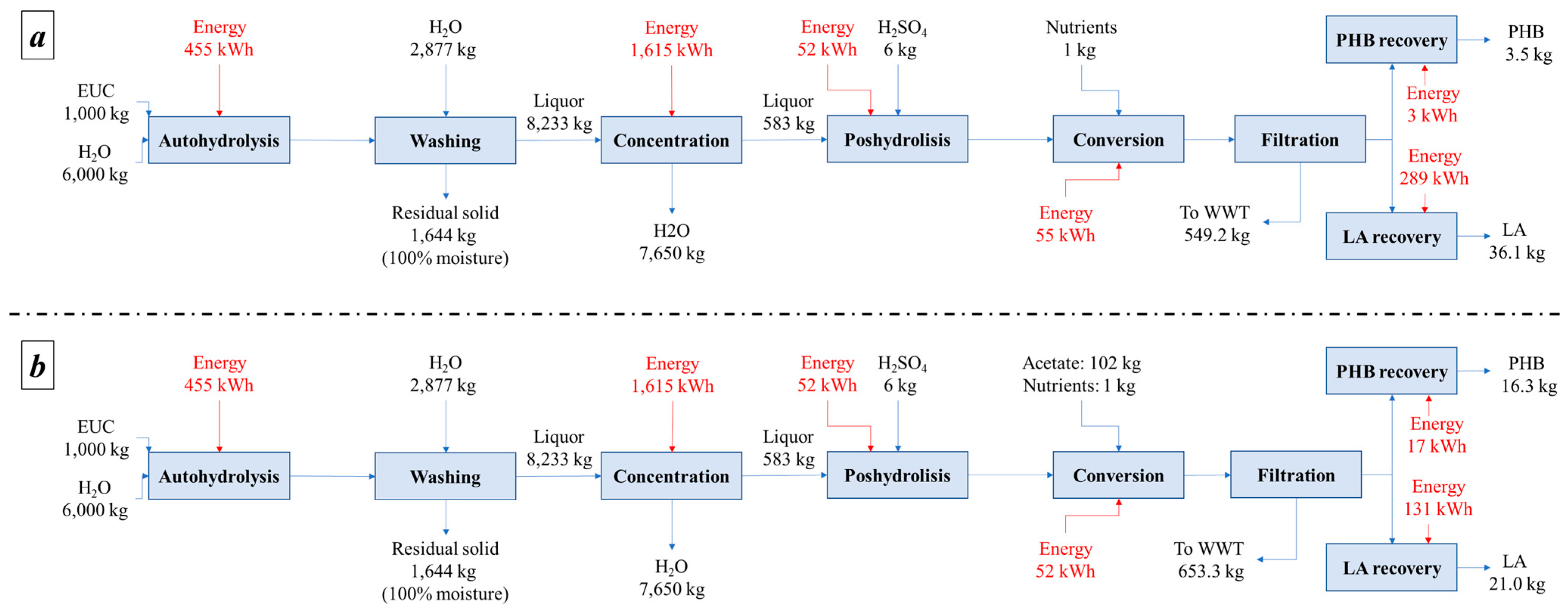
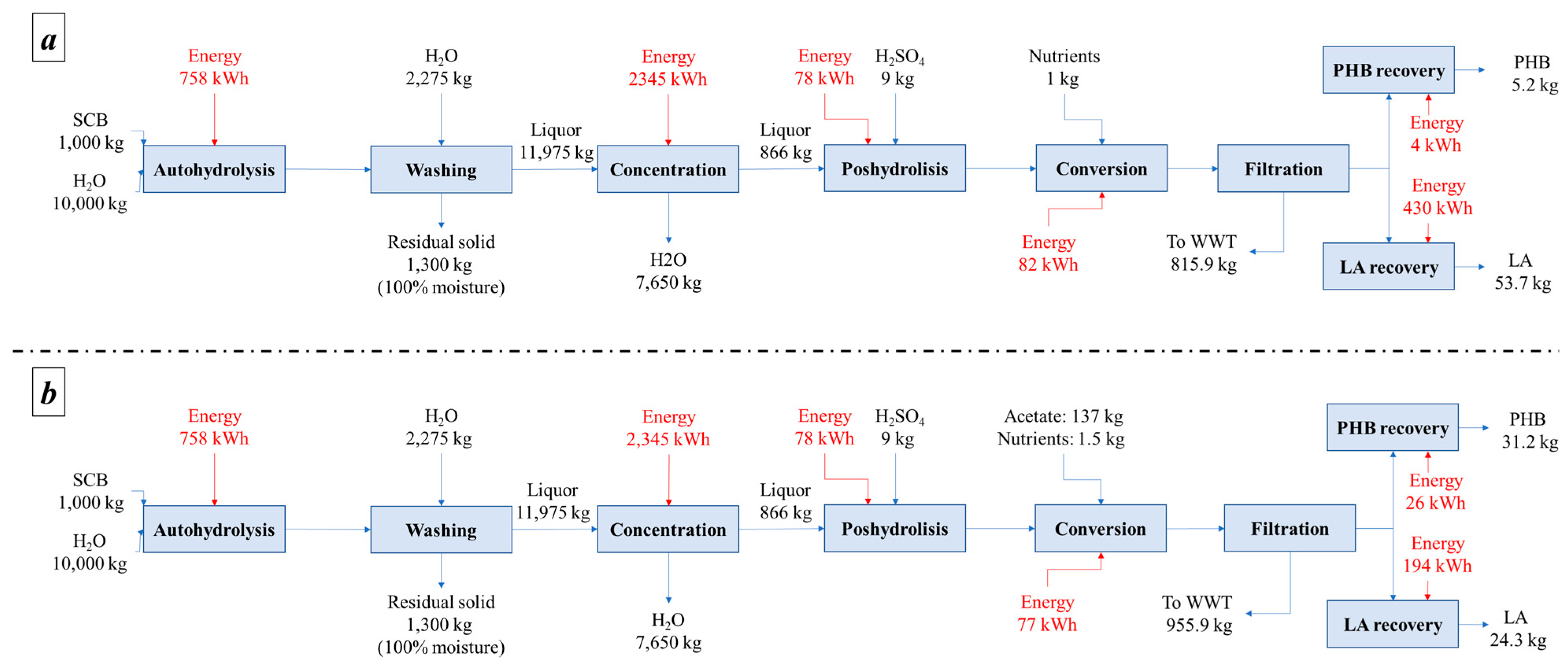
- Alternative I: the hemicellulosic fraction was sent to microbial culture. After microbial culture, LA and PHB yields were 31 and 3%, respectively, from the initial carbon source.
- Alternative II: the hemicellulosic fraction is enriched with acetate to reach 50% the total composition. LA and PHB yields after microbial culture were 31 and 3%, respectively, of the initial carbon source.
2.6. Process Recovery
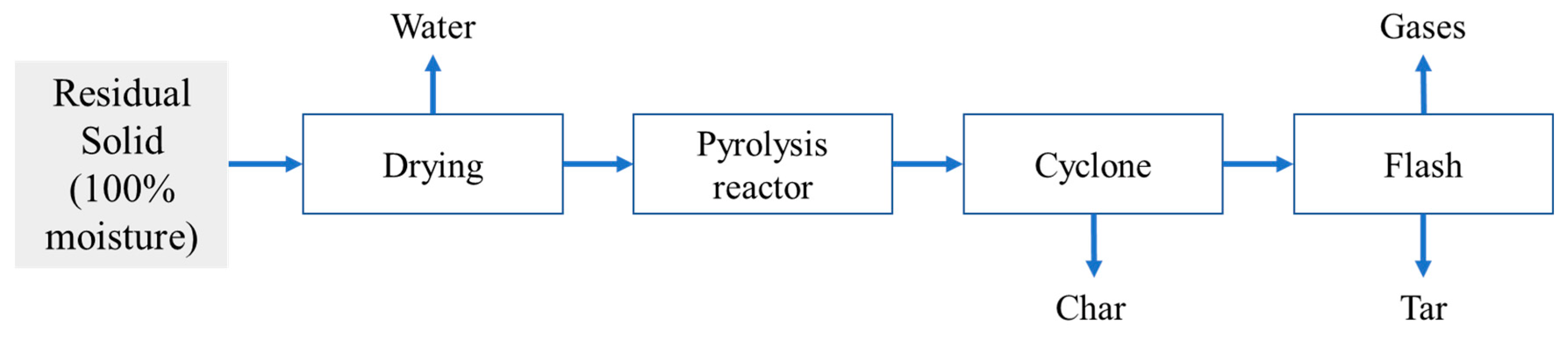
2.7. Valorization of the Residual Solid
Mass and Energy Balances
2.8. Economic Assessment
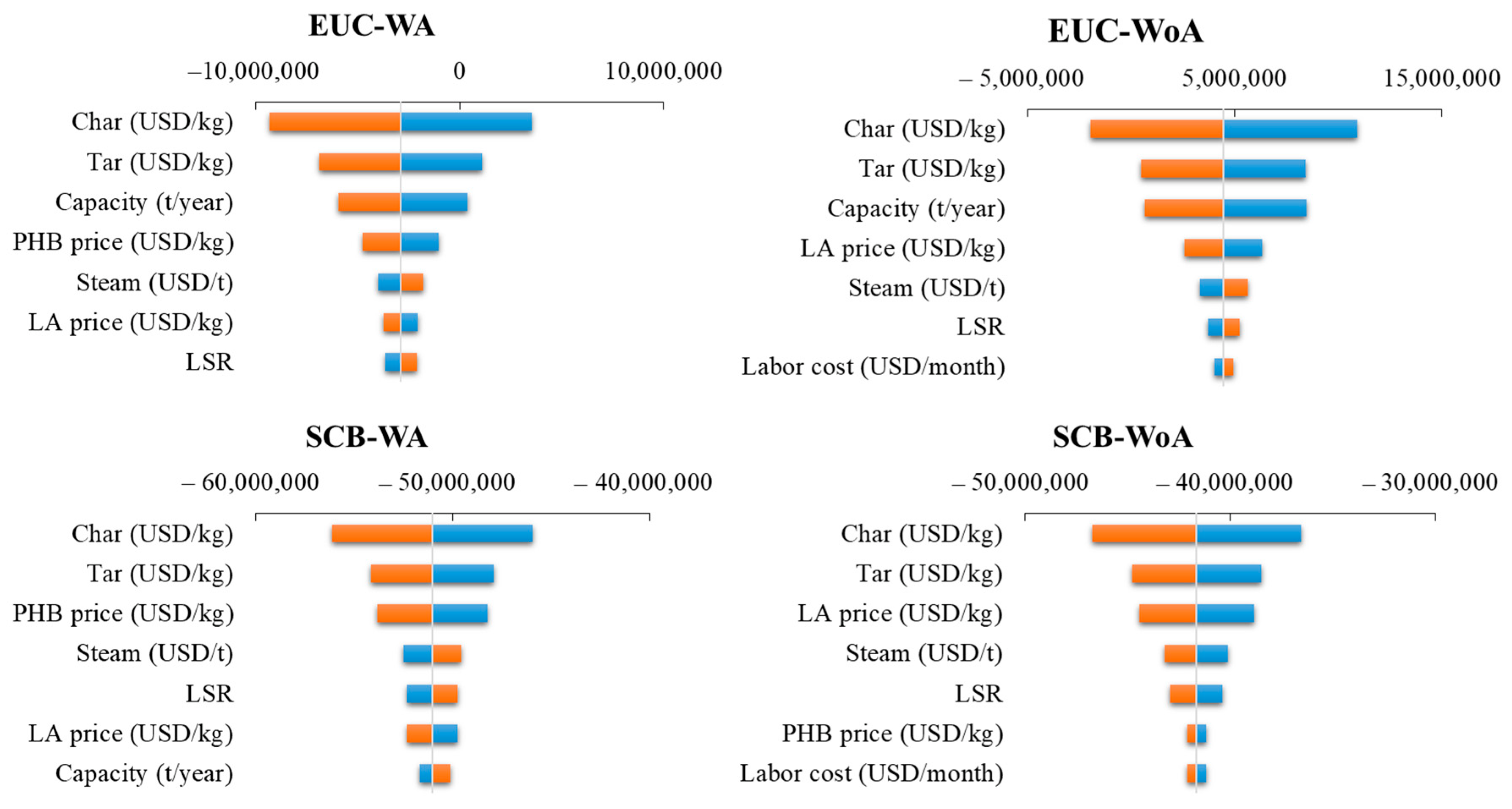
2.9. Sensitivity Assessment
3. Results
3.1. Technical Assessment
3.2. Economic Assessment
3.3. Sensitivity Assessment
3.4. Case Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida, L.P.; Silva, C.R.; Martins, T.B.; Pereira, R.D.; Esperança, M.N.; Cruz, A.J.; Badino, A.C. Heat transfer evaluation for conventional and extractive ethanol fermentations: Saving cooling water. J. Clean. Prod. 2021, 304, 127063. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. A framework for the design and analysis of integrated multi-product biorefineries from agricultural and forestry wastes. Renew. Sustain. Energy Rev. 2021, 139, 110687. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.; Bast, F.; Mehariya, S.; Bhatia, S.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Techno-economic assessment of carboxylic acids, furfural, and pellet production in a pine sawdust biorefinery. Biofuels Bioprod. Biorefin. 2018, 12, 997–1012. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E.; Gutiérrez, S. An energy-saving re-use strategy for the treatment of lignocellulosic biomass applied to the production of levulinc acid. J. Clean. Prod. 2020, 257, 120549. [Google Scholar] [CrossRef]
- Zanghellini, A.L. Fermentation Route for the Production of Levulinic Acid, Levulinate Esters and Valerolactone and Derivatives Thereof. U.S. Patent No. 9,523, 105, 20 December 2016. [Google Scholar]
- Pinto-Ibieta, F.; Cea, M.; Cabrera, F.; Abanto, M.; Felissia, F.; Area, M.; Ciudad, G. Strategy for biological co-production of levulinic acid and polyhydroxyalkanoates by using mixed microbial cultures fed with synthetic hemicellulose hydrolysate. Bioresour. Technol. 2020, 309, 123323. [Google Scholar] [CrossRef]
- Covinich, L.G.; Area, M.C. Biological Pathways for the Production of Levulinic Acid from Lignocellulosic Resources. In Sustainable Microbial Technologies for Valorization of Agro-Industrial Wastes; Saini, J.K., Singh, S., Nain, L., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 269–292. [Google Scholar] [CrossRef]
- Vila-Santa, A.; Islam, M.A.; Ferreira, F.C.; Prather, K.L.J.; Mira, N.P. Prospecting Biochemical Pathways to Implement Microbe-Based Production of the New-to-Nature Platform Chemical Levulinic Acid. ACS Synth. Biol. 2021, 10, 724–736. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C.; Ehman, N.V.; Tarrés, Q.; Mutjé, P. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohydr. Polym. 2016, 139, 99–105. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Chade, M.; Mereles, E.B.; Bengoechea, D.I.; Brizuela, J.G.; Felissia, F.E.; Area, M.C. Strategies of detoxification and fermentation for biotechnological production of xylitol from sugarcane bagasse. Ind. Crop. Prod. 2016, 91, 161–169. [Google Scholar] [CrossRef]
- Saska, M.; Ozer, E. Aqueous extraction of sugarcane bagasse hemicellulose and production of xylose syrup. Biotechnol. Bioeng. 1995, 45, 517–523. [Google Scholar] [CrossRef]
- Geankoplis, C.J. (Ed.) Procesos de Transporte y Operaciones Unitarias; Prentice-All International: Mexico City, Mexico, 1998. [Google Scholar]
- Fatehi, P.; Catalan, L.; Cave, G. Simulation analysis of producing xylitol from hemicelluloses of pre-hydrolysis liquor. Chem. Eng. Res. Des. 2014, 92, 1563–1570. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.-N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.-C.; Yang, B.; et al. Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates from Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 6240210. [Google Scholar] [CrossRef] [PubMed]
- Jakob, A.; Grilc, M.; Teržan, J.; Likozar, B. Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents. Processes 2021, 9, 924. [Google Scholar] [CrossRef]
- Nhien, L.C.; Long, N.V.D.; Lee, M. Design and optimization of the levulinic acid recovery process from lignocellulosic biomass. Chem. Eng. Res. Des. 2016, 107, 126–136. [Google Scholar] [CrossRef]
- TAPPI. Kraft Pulping Short Course. In TAPPI Course Notes; TAPPI Press: Peachtree Corners, GA, USA, 1997; Volume 466. [Google Scholar]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Boer, F.D.; Valette, J.; Commandré, J.M.; Fournier, M.; Thévenon, M.F. Slow pyrolysis of sugarcane bagasse for the production of char and the potential of its by-product for wood protection. J. Renew. Mater. 2021, 9, 97. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O. Investigating the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef]
- Haydary, J. Chemical Process Design and Simulation: Aspen Plus and Aspen HYSYS Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hackett, B.W. The Essentials of Continuous Evaporation. Available online: https://www.aiche.org/resources/publications/cep/2018/may/essentials-continuous-evaporation (accessed on 1 September 2018).
- Kumar, S.; Dheeran, P.; Singh, S.P.; Mishra, I.M.; Adhikari, D.K. Cooling System Economy in Ethanol Production Using Thermotolerant Yeast Kluyveromyces Sp. IIPE453. Am. J. Microbiol. Res. 2013, 1, 39–44. [Google Scholar] [CrossRef]
- Stuart, P.R.; El-Halwagi, M.M. (Eds.) Integrated Biorefineries, Design, Analysis and Optimization; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Yang, H.; Kudo, S.; Kuo, H.-P.; Norinaga, K.; Mori, A.; Mašek, O.; Hayashi, J.-I. Estimation of Enthalpy of Bio-Oil Vapor and Heat Required for Pyrolysis of Biomass. Energy Fuels 2013, 27, 2675–2686. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Ng, K.S.; Martinez, E. Biorefineries and Chemical Processes: Design, Integration and Sustainability Analysis; Sadhukhan, J., Ng, K.S., Martinez, E., Eds.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Batsy, D.R.; Solvason, C.C.; Sammons, N.E.; Chambost, V.; Bilhartz, D.L.; Eden, M.R.; El-halwagi, M.M.; Stuart, P.R. Product Portfolio Selection and Process Design for the Forest Biorefinery. In Integrated Biorefineries, Design, Analysis and Optimization; El-Halwagi, M.M., Stuart, P.R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 4–33. [Google Scholar]
- Seider, W.D.; Seader, J.D.; Lewin, D.R. (Eds.) Product and Process Design Principles-Synthesis, Analysis, and Evaluation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Harmsen, J. Industrial Process Scale-Up: A Practical Innovation Guide from Idea to Commercial Implementation; Harmsen, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Da Silva, S.S.; Chandel, A.K. (Eds.) Biofuels in Brazil, Fundamental Aspects, Recent Developments, and Future Perspectives; Springer: Sao Paulo, Brazil, 2014. [Google Scholar] [CrossRef]
- National Agricultural Technology Institute (INTA), Argentina. Available online: http://inta.gob.ar (accessed on 13 August 2021).
- Geng, W.; Venditti, R.A.; Pawlak, J.J.; De Assis, T.; Gonzalez, R.W.; Phillips, R.B.; Chang, H. Techno-economic analysis of hemicellulose extraction from different types of lignocellulosic feedstocks and strategies for cost optimization. Biofuels Bioprod. Biorefin. 2019, 14, 225–241. [Google Scholar] [CrossRef]
- Iglesias, S.; Miyazaki, M.; Mariano, A.; Franco, T. Techno-economic assessment of bio-oil produced from Eucalyptus forestry residues. Ind. Crop. Prod. 2021, 171, 113936. [Google Scholar] [CrossRef]
- De Assis, C.A.; Gonzalez, R.; Kelley, S.; Jameel, H.; Bilek, T.; Daystar, J.; Handfield, R.; Golden, J.; Prestemon, J.; Singh, D. Risk management consideration in the bioeconomy. Biofuels Bioprod. Biorefin. 2017, 11, 549–566. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Design of nano and micro fibrillated cellulose production processes from forest industrial wastes in a multiproduct biorefinery. Chem. Eng. Res. Des. 2020, 167, 1–14. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Alternatives of Small-Scale Biorefineries for the Integrated Production of Xylitol from Sugarcane Bagasse. J. Renew. Mater. 2018, 6, 139–151. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Small-sized biorefineries as strategy to add value to sugarcane bagasse. Chem. Eng. Res. Des. 2016, 107, 137–146. [Google Scholar] [CrossRef]
- Pérez-López, P.; Montazeri, M.; Feijoo, G.; Moreira, M.T.; Eckelman, M.J. Integrating uncertainties to the combined environmental and economic assessment of algal biorefineries: A Monte Carlo approach. Sci. Total. Environ. 2018, 626, 762–775. [Google Scholar] [CrossRef]
- Shah, A. Techno-Economic Analysis and Life Cycle Assessment of The Corn Stover Biomass Feedstock Supply Chain System for a Midwest-Based First-Generation Cellulosic Biorefinery. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Cheali, P.; Quaglia, A.; Gernaey, K.V.; Sin, G. Effect of Market Price Uncertainties on the Design of Optimal Biorefinery Systems—A Systematic Approach. Ind. Eng. Chem. Res. 2014, 53, 6021–6032. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, M.S.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Sensitivity assessment of value-added products and pellet production in alternatives for softwood sawdust valorization. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Zambon, M.D.; Area, M.C.; Curvelo, A.A.D.S. Low liquid–solid ratio (LSR) hot water pretreatment of sugarcane bagasse. Green Chem. 2012, 14, 1982–1989. [Google Scholar] [CrossRef]
- Piris, V. A las Industrias les Cuesta Cada vez más Conseguir Chips de Madera. Available online: https://www.elterritorio.com.ar/ (accessed on 1 September 2021).
- Lopez-Arenas, T.; González-Contreras, M.; Anaya-Reza, O.; Sales-Cruz, M. Analysis of the fermentation strategy and its impact on the economics of the production process of PHB (polyhydroxybutyrate). Comput. Chem. Eng. 2017, 107, 140–150. [Google Scholar] [CrossRef]
- Kumar, L.R.; Kaur, R.; Tyagi, R.; Drogui, P. Identifying economical route for crude glycerol valorization: Biodiesel versus polyhydroxy-butyrate (PHB). Bioresour. Technol. 2020, 323, 124565. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Techno-economic assessment of a sustainable and cost-effective bioprocess for large scale production of polyhydroxybutyrate. Chemosphere 2021, 284, 131371. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Kleerebezem, R.; Cuellar, M.C.; Ramirez, A. Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: Techno-economic analysis and ex-ante environmental assessment. Bioresour. Technol. 2015, 185, 368–377. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P. Techno-economic analysis of cyanobacterial PHB bioplastic production. J. Environ. Chem. Eng. 2022, 10, 107502. [Google Scholar] [CrossRef]
- Nieder-Heitmann, M.; Haigh, K.; Görgens, J.F. Process design and economic evaluation of integrated, multi-product biorefineries for the co-production of bio-energy, succinic acid, and polyhydroxybutyrate (PHB) from sugarcane bagasse and trash lignocelluloses. Biofuels Bioprod. Biorefin. 2018, 13, 599–617. [Google Scholar] [CrossRef]



| Component | SCB | EUC |
|---|---|---|
| g/L | g/L | |
| Cellobiose | 3.40 | 0.00 |
| Glucose | 12.2 | 15.6 |
| Xylose | 147 | 153 |
| Arabinose | 9.10 | 3.70 |
| Formic acid | 1.70 | 3.10 |
| Acetic acid | 20.9 | 12.6 |
| HMF | 0.50 | 0.20 |
| Furfural | 2.80 | 10.0 |
| Step | Operational Conditions | Aim | Ref. |
|---|---|---|---|
| Autohydrolysis | EUC: 180 °C, LSR of 6, for 20 min. | Extract hemicelluloses | [13] |
| SCB: 180 °C, LSR of 10, for 20 min. | Extract hemicelluloses | [14] | |
| Washing of the solid fraction | 3.5 tons of water per ton of dry pulp | Recover all hemicellulosic sugars. | [22] |
| Evaporation 1 | With a steam economy of 2.6 | To reach 200 g/L of sugars | [15] |
| Post-hydrolysis | 120 °C for 60 min and 1% H2SO4 | To convert oligomers into monomers | |
| Conversion | Mixed microbial cultures and nutrients were added to the microbial culture at 60 rpm and maintained at 25 °C for 40 days. * | To convert sugars in PHB and LA | [9] |
| Filtration | After conversion to recover the cellular mass | To recover cellular mass | |
| PHB extraction | Using solvent CHCl3 at 60 °C. | To recover PHB | [19] |
| LA extraction | Using furfural as solvent. | To recover LA | [21] |
| Unit Prices at the Mill Gate | ||
| Sawdust (t/year) | 50,000 (Dry basis) | Assumption |
| Sawdust (USD/t) a | 7.0 | |
| Water (USD/m) b | 0.7 | |
| Electricity (USD/MWh) c | 85 | |
| Labor (USD/h) d | 9–21 | |
| Steam (USD/t) | 10 | Assumption |
| Maintenance and taxes | 2% (of TCI) | Assumption |
| Tax rate | 35% | Assumption |
| Chemicals for production | ||
| H2SO4 (USD/kg) e | 0.09 | |
| Nutrients (USD/kg) | 10 | Assumption |
| Waste treatment (USD/m3) f | 1 | |
| Products (assumptions) | ||
| Char (USD/t) g | 1.5 | |
| Tar (USD/t) g | 1.3 | |
| PHB (USD/kg) g | 6 | |
| LA (USD/kg) g | 3.5 | |
| Product | Raw Material | |||
|---|---|---|---|---|
| EUC | SCB | |||
| Without acetate | With acetate | Without acetate | With acetate | |
| LA (t/year) | 1807 | 816 | 2685 | 1213 |
| PHB (t/year) | 175 | 1049 | 260 | 1559 |
| Tar (t/year) | 10,275 | 10,275 | 8125 | 8125 |
| Char (t/year) | 14,385 | 14,385 | 11,375 | 11,375 |
| EUC | SCB | |||
|---|---|---|---|---|
| Without Acetate | With Acetate | Without Acetate | With Acetate | |
| IRR | 16.0% | 14.4% | 7.4% | 5.5% |
| NPV (MMUSD) | 4.5 | −2.9 | NV | NV |
| Investment (MMUSD) | 113 | 113 | 146 | 146 |
| Without Acetate | With Acetate | ||
|---|---|---|---|
| Increase 20% | Raw material (t/year) | 17,610,627 | 8,654,484 |
| Reduce 20% | LSR | 8,604,932 | 1,141,480 |
| Reduce 20% | Washing water | 7,294,834 | −168,618 |
| Reduce 20% | Steam | 6,793,459 | −669,993 |
| Base case (USD) | 4,462,196 | −2,886,175 | |
| Increase 20% | Steam | 2,130,932 | −5,102,358 |
| Increase 20% | Washing water | 1,458,368 | −5,774,923 |
| Increase 20% | LSR | −327,790 | −7,561,081 |
| Reduce 20% | Raw material (t/year) | −6,722,415 | −12,509,047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Process Design for Value-Added Products in a Biorefinery Platform from Agro and Forest Industrial Byproducts. Polymers 2023, 15, 274. https://doi.org/10.3390/polym15020274
Clauser NM, Felissia FE, Area MC, Vallejos ME. Process Design for Value-Added Products in a Biorefinery Platform from Agro and Forest Industrial Byproducts. Polymers. 2023; 15(2):274. https://doi.org/10.3390/polym15020274
Chicago/Turabian StyleClauser, Nicolás M., Fernando E. Felissia, María C. Area, and María E. Vallejos. 2023. "Process Design for Value-Added Products in a Biorefinery Platform from Agro and Forest Industrial Byproducts" Polymers 15, no. 2: 274. https://doi.org/10.3390/polym15020274
APA StyleClauser, N. M., Felissia, F. E., Area, M. C., & Vallejos, M. E. (2023). Process Design for Value-Added Products in a Biorefinery Platform from Agro and Forest Industrial Byproducts. Polymers, 15(2), 274. https://doi.org/10.3390/polym15020274








