Soybean-Based Polyol as a Substitute of Fossil-Based Polyol on the Synthesis of Thermoplastic Polyurethanes: The Effect of Its Content on Morphological and Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
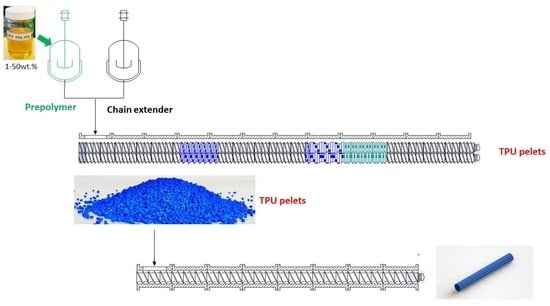
2.1. Reactive Extrusion and Injection Molding
2.2. Gel Content and Size Exclusion Chromatography (SEC)
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Differential Scanning Calorimetry (DSC)
2.5. Small Angle X-ray Scattering (SAXS)
2.6. Surface Energy
2.7. Dynamical Mechanical Analysis (DMA)
2.8. Physical–Mechanical Properties and Application in Tubing
3. Results and Discussion
3.1. Gel Content and Molecular Weight
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
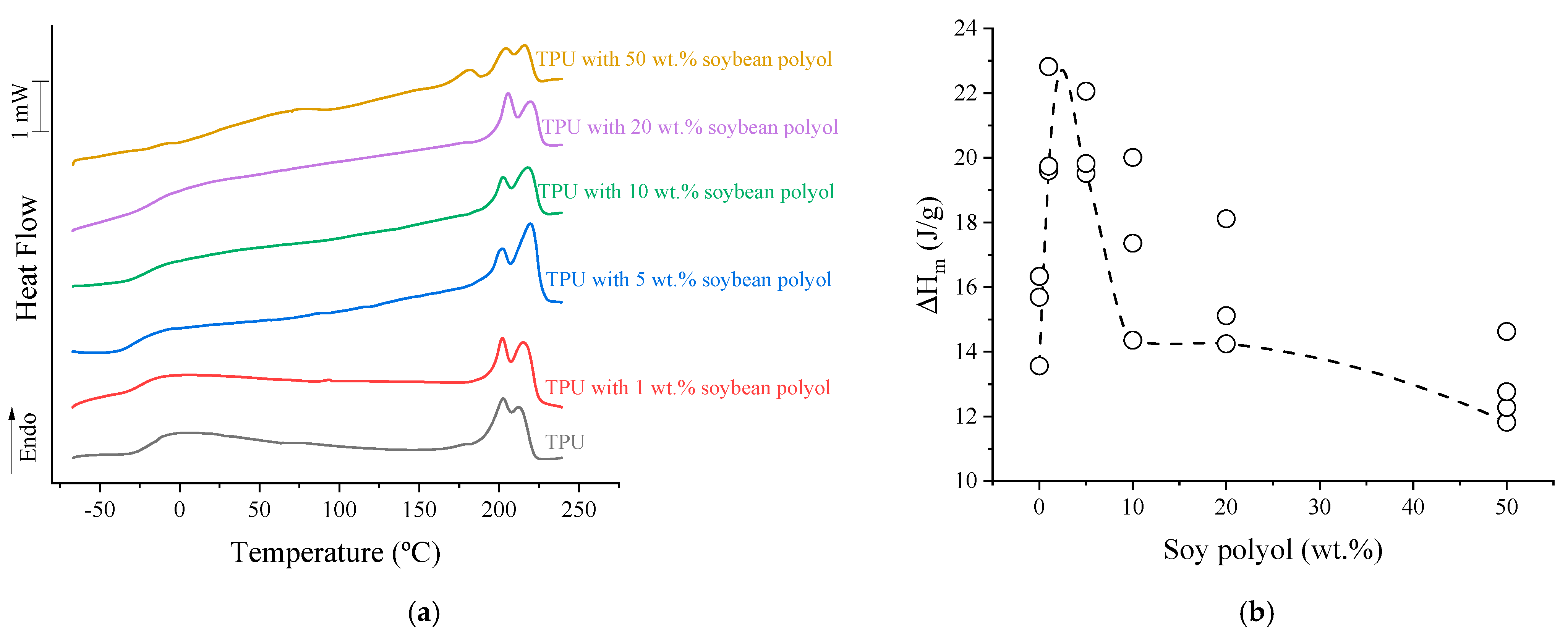
3.3. Differential Scanning Calorimetry (DSC)
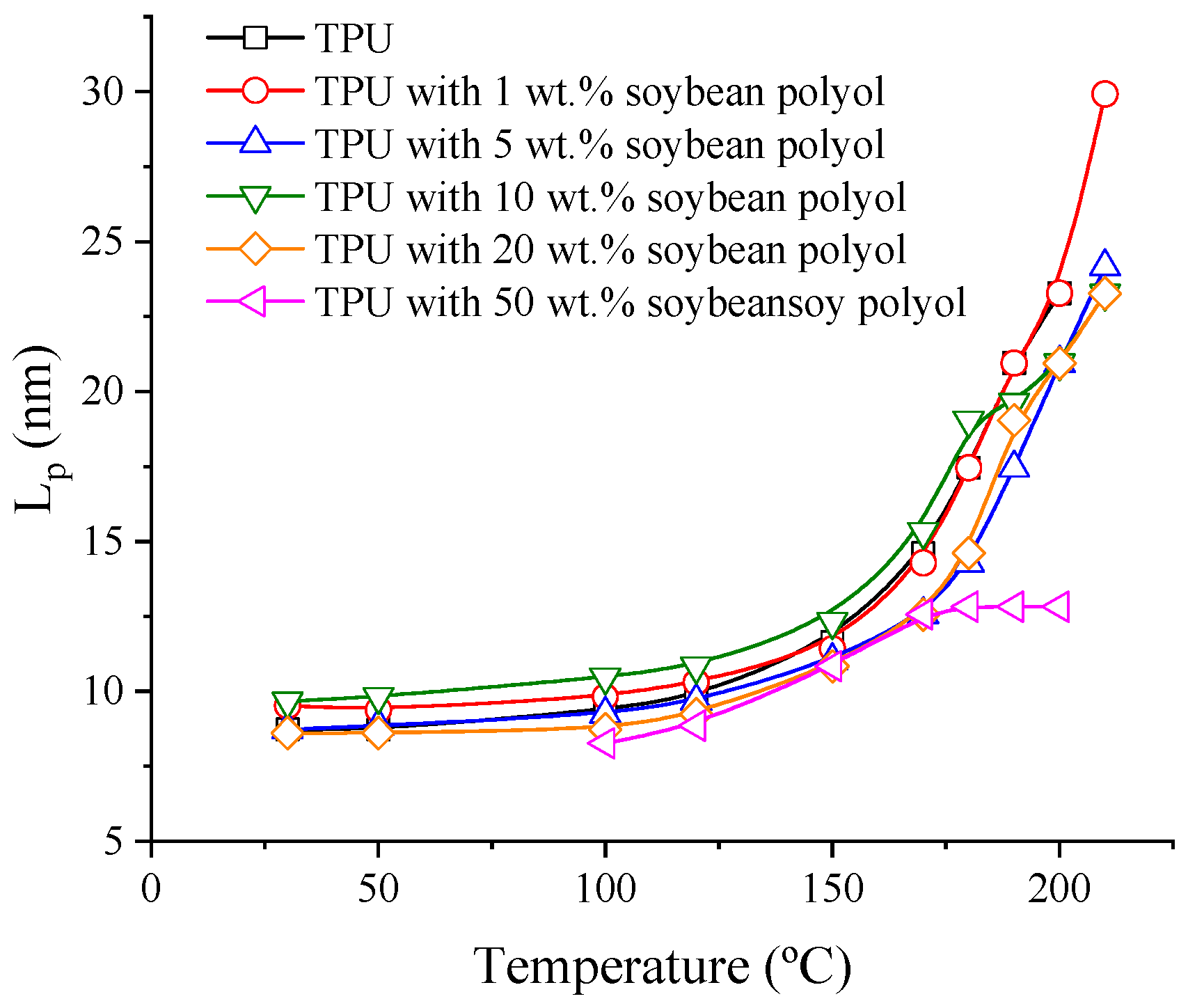
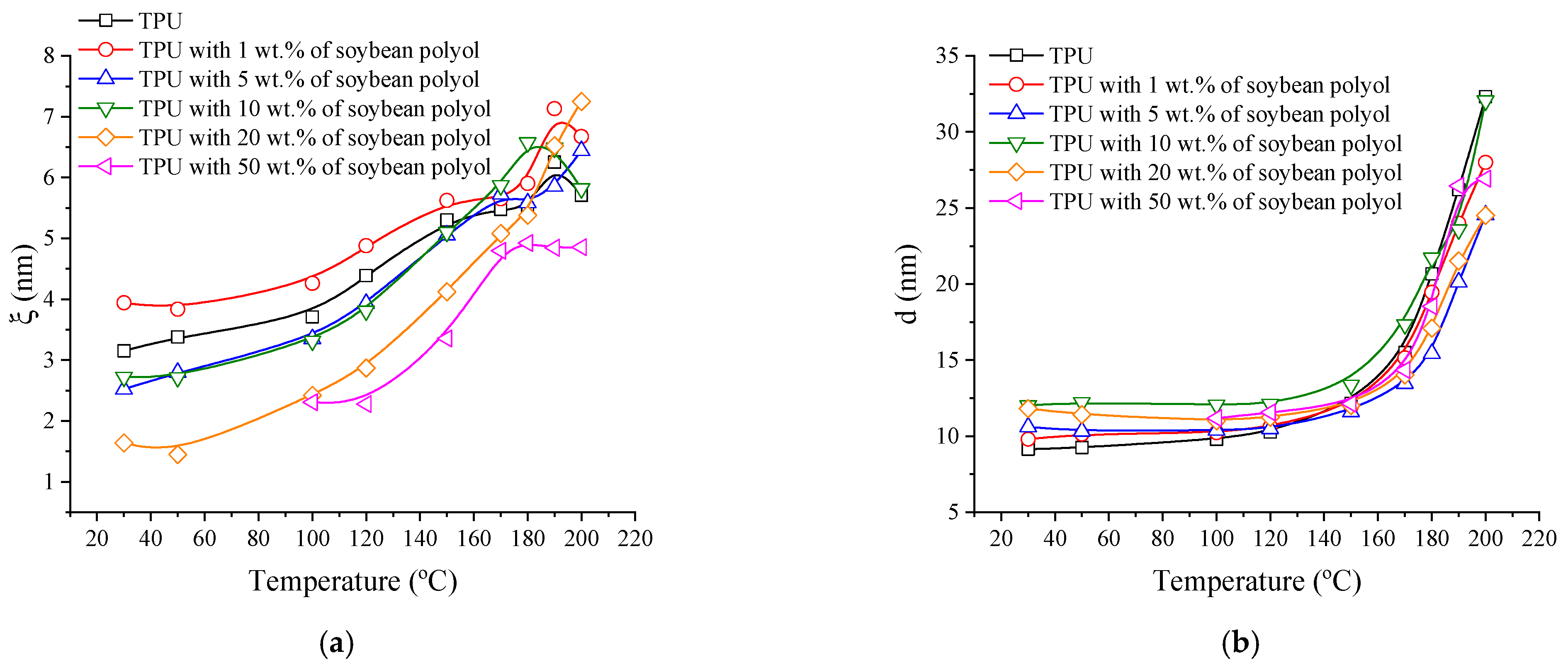
3.4. Small Angle X-ray Scattering (SAXS)
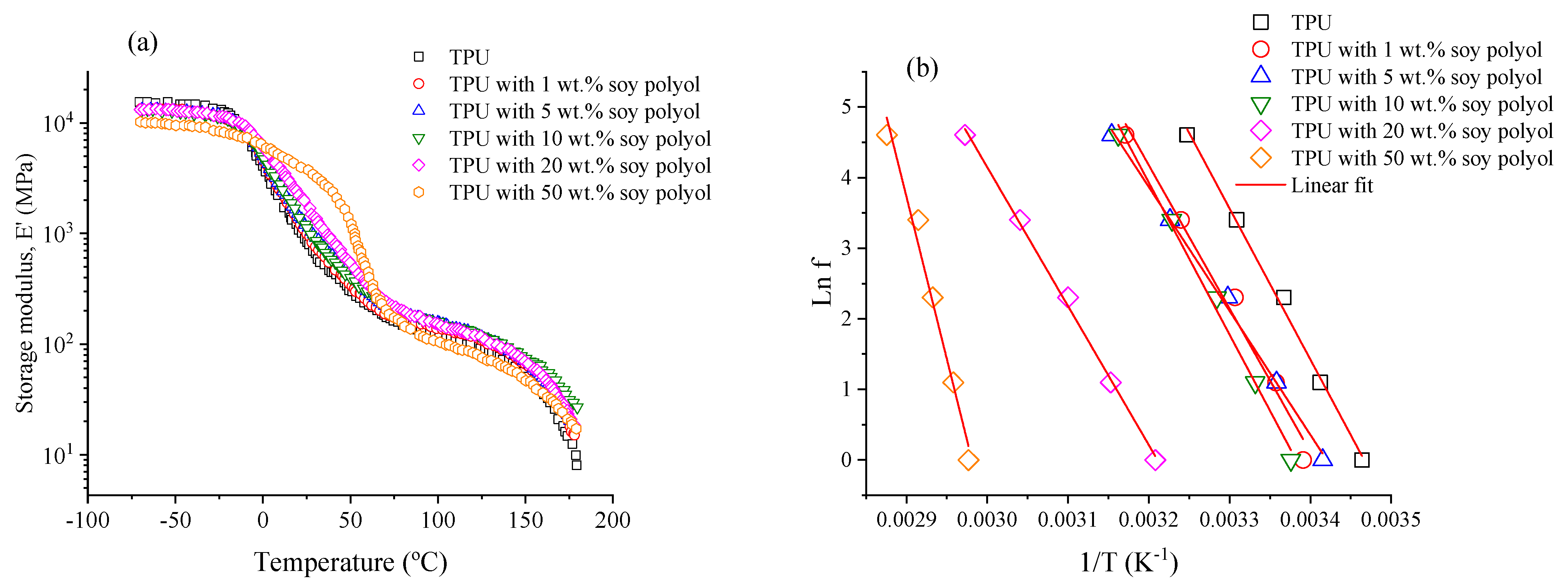
3.5. Dynamical Mechanical Analysis (DMA)
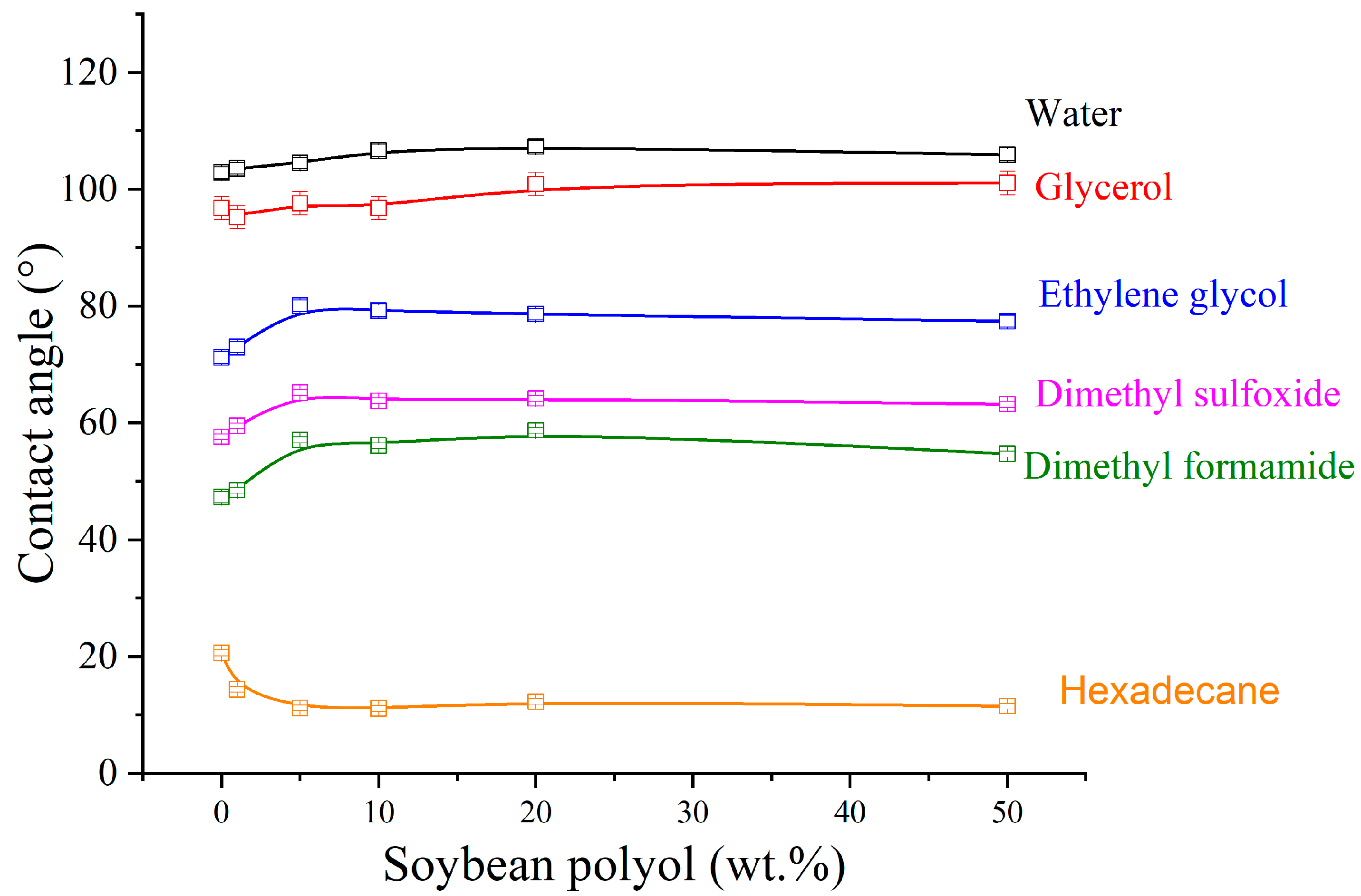
3.6. Contact Angle Measurements
3.7. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; iSmithers Rapra Publishing: Shewsbury, UK, 2005. [Google Scholar]
- Oertel, G.A. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties; Hanser Publishers: New York, NY, USA, 1985. [Google Scholar]
- Lee, D.K.; Tsai, H.B.; Tsai, R.S.; Chen, P.H. Preparation and properties of transparent thermoplastic segmented polyurethanes derived from different polyols. Polym. Eng. Sci. 2007, 47, 695–701. [Google Scholar] [CrossRef]
- Gomez, C.M.; Gutierrez, D.; Asensio, M.; Costa, V.; Nohales, A. Transparent thermoplastic polyurethanes based on aliphatic diisocyanates and polycarbonate diol. J. Elastomers Plast. 2017, 49, 77–95. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Rondan, N.; Wendt, B.L.; Cox, J.M. Synthesis of transparent thermoplastic polyurethane elastomers. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 271–278. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polymer Degradation and Stability 2002, 78, 323–333. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Nohales, A.; Asensio, M.; Gómez, C.M.; Bianchi, O. Effect of chain extenders on the thermal and thermodegradation behavior of carbonatodiol thermoplastic polyurethane. Polym. Bull. 2023. [Google Scholar] [CrossRef]
- Ourique, P.A.; Ornaghi, F.G.; Ornaghi, H.L.; Wanke, C.H.; Bianchi, O. Thermo-oxidative degradation kinetics of renewable hybrid polyurethane–urea obtained from air-oxidized soybean oil. J. Therm. Anal. Calorim. 2019, 137, 1969–1979. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2022, 15, 250–271. [Google Scholar] [CrossRef]
- Ourique, P.A.; Krindges, I.; Aguzzoli, C.; Figueroa, C.A.; Amalvy, J.; Wanke, C.H.; Bianchi, O. Synthesis, properties, and applications of hybrid polyurethane–urea obtained from air-oxidized soybean oil. Prog. Org. Coat. 2017, 108, 15–24. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Lee, S.; Park, J.; Shin, E.-J. One-Shot Synthesis of Thermoplastic Polyurethane Based on Bio-Polyol (Polytrimethylene Ether Glycol) and Characterization of Micro-Phase Separation. Polymers 2022, 14, 4269. [Google Scholar] [CrossRef]
- Ernzen, J.R.; Romoaldo, C.H.; Gommes, C.; Covas, J.A.; Marcos-Fernández, A.; Fiorio, R.; Bianchi, O. Tuning Thermal, Morphological, and Physicochemical Properties of Thermoplastic Polyurethanes (TPUs) by the 1,4-Butanediol (BDO)/Dipropylene Glycol (DPG) Ratio. Polymers 2022, 14, 3164. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- De Souza, F.M.; Kahol, P.K.; Gupta, R.K. Polyols from Sustainable Resources. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 25–49. [Google Scholar]
- Nguyen Dang, L.; Le Hoang, S.; Malin, M.; Weisser, J.; Walter, T.; Schnabelrauch, M.; Seppälä, J. Synthesis and characterization of castor oil-segmented thermoplastic polyurethane with controlled mechanical properties. Eur. Polym. J. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Cruz, J.A.; Amico, S.C.; Bianchi, O. Effect of the aramid pulp on the physicochemical, viscoelastic properties and rheokinetics of polyurethanes. J. Polym. Res. 2022, 30, 12. [Google Scholar] [CrossRef]
- Favero, D.; Marcon, V.; Figueroa, C.A.; Gómez, C.M.; Cros, A.; Garro, N.; Sanchis, M.J.; Carsí, M.; Bianchi, O. Effect of chain extender on the morphology, thermal, viscoelastic, and dielectric behavior of soybean polyurethane. J. Appl. Polym. Sci. 2021, 138, 50709. [Google Scholar] [CrossRef]
- Blache, H.; Méchin, F.; Rousseau, A.; Fleury, É.; Pascault, J.-P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New bio-based thermoplastic polyurethane elastomers from isosorbide and rapeseed oil derivatives. Ind. Crops Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Hogerat, R.; Ghosal, K.; Shaheen-Mualim, M.; Farah, S. Renewable polyol-based biodegradable polyesters as greener plastics for industrial applications. Chem. Eng. J. 2023, 459, 141211. [Google Scholar] [CrossRef]
- Rajput, B.S.; Hai, T.A.P.; Gunawan, N.R.; Tessman, M.; Neelakantan, N.; Scofield, G.B.; Brizuela, J.; Samoylov, A.A.; Modi, M.; Shepherd, J.; et al. Renewable low viscosity polyester-polyols for biodegradable thermoplastic polyurethanes. J. Appl. Polym. Sci. 2022, 139, e53062. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable Polyurethanes from Sustainable Biological Precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef]
- Favero, D.; Marcon, V.R.R.; Barcellos, T.; Gómez, C.M.; Sanchis, M.J.; Carsí, M.; Figueroa, C.A.; Bianchi, O. Renewable polyol obtained by microwave-assisted alcoholysis of epoxidized soybean oil: Preparation, thermal properties and relaxation process. J. Mol. Liq. 2019, 285, 136–145. [Google Scholar] [CrossRef]
- ASTM D2765-16; Standard Test Methods for Determination of Gel Content and Swell Ratio of Crosslinked Ethylene Plastics. ASTM International: West Conshohocken, PA, USA, 2016.
- Niemczyk, A.; Piegat, A.; Olalla, Á.S.; El Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Yilgör, I.; Yilgör, E.; Wilkes, G.L. Critical parameters in designing segmented polyurethanes and their effect on morphology and properties: A comprehensive review. Polymer 2015, 58, A1–A36. [Google Scholar] [CrossRef]
- Teubner, M.; Strey, R. Origin of the scattering peak in microemulsions. J. Chem. Phys. 1987, 87, 3195–3200. [Google Scholar] [CrossRef]
- Javni, I.; Bilić, O.; Bilić, N.; Petrović, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavský, J. Thermoplastic polyurethanes with controlled morphology based on methylenediphenyldiisocyanate/isosorbide/butanediol hard segments. Polym. Int. 2015, 64, 1607–1616. [Google Scholar] [CrossRef]
- Laity, P.R.; Taylor, J.E.; Wong, S.S.; Khunkamchoo, P.; Norris, K.; Cable, M.; Andrews, G.T.; Johnson, A.F.; Cameron, R.E. A review of small-angle scattering models for random segmented poly(ether-urethane) copolymers. Polymer 2004, 45, 7273–7291. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Schuster, J.M.; Schvezov, C.E.; Rosenberger, M.R. Analysis of the Results of Surface Free Energy Measurement of Ti6Al4V by Different Methods. Procedia Mater. Sci. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- ASTM D2240-15; Standard Test Method for Rubber Property–Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2010.
- ISO 527-2:2012; Plastics–Determination of Tensile Properties–Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: London, UK, 2012.
- Garrett, J.; Runt, J.; Lin, J. Microphase separation of segmented poly (urethane urea) block copolymers. Macromolecules 2000, 33, 6353–6359. [Google Scholar] [CrossRef]
- Ophir, Z.H.; Wilkes, G.L. Time dependence of mechanical properties and domain formation of linear and crosslinked segmented polyurethanes. In Multiphase Polymers; ACS Publications: Washington, DC, USA, 1979. [Google Scholar]
- Balko, J.; Fernández-d’Arlas, B.; Pöselt, E.; Dabbous, R.; Müller, A.J.; Thurn-Albrecht, T. Clarifying the Origin of Multiple Melting of Segmented Thermoplastic Polyurethanes by Fast Scanning Calorimetry. Macromolecules 2017, 50, 7672–7680. [Google Scholar] [CrossRef]
- Van Bogart, J.W.C.; Bluemke, D.A.; Cooper, S.L. Annealing-induced morphological changes in segmented elastomers. Polymer 1981, 22, 1428–1438. [Google Scholar] [CrossRef]
- Seymour, R.W.; Cooper, S.L. Thermal Analysis of Polyurethane Block Polymers. Macromolecules 1973, 6, 48–53. [Google Scholar] [CrossRef]
- Martin, D.J.; Meijs, G.F.; Renwick, G.M.; Mccarthy, S.J.; Gunatillake, P.A. The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers. I. Characterization of the series. J. Appl. Polym. Sci. 1996, 62, 1377–1386. [Google Scholar] [CrossRef]
- Martin, D.J.; Meijs, G.F.; Gunatillake, P.A.; McCarthy, S.J.; Renwick, G.M. The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers. II. SAXS-DSC annealing study. J. Appl. Polym. Sci. 1997, 64, 803–817. [Google Scholar] [CrossRef]
- Chen, T.K.; Shieh, T.S.; Chui, J.Y. Studies on the First DSC Endotherm of Polyurethane Hard Segment Based on 4,4‘-Diphenylmethane Diisocyanate and 1,4-Butanediol. Macromolecules 1998, 31, 1312–1320. [Google Scholar] [CrossRef]
- Camberlin, Y.; Pascault, J.P. Quantitative DSC evaluation of phase segregation rate in linear segmented polyurethanes and polyurethaneureas. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 415–423. [Google Scholar] [CrossRef]
- Xu, Y.; Petrovic, Z.; Das, S.; Wilkes, G.L. Morphology and properties of thermoplastic polyurethanes with dangling chains in ricinoleate-based soft segments. Polymer 2008, 49, 4248–4258. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Milić, J.; Zhang, F.; Ilavsky, J. Fast-responding bio-based shape memory thermoplastic polyurethanes. Polymer 2017, 121, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ryan, A.J.; Meier, I.K. Morphology Development via Reaction-Induced Phase Separation in Flexible Polyurethane Foam. Macromolecules 2002, 35, 5034–5042. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Osaka, N.; Murayama, S.; Saito, H. Thermal annealing behavior and structure development of crystalline hard segment domain in a melt-quenched thermoplastic polyurethane. Polymer 2013, 54, 2183–2189. [Google Scholar] [CrossRef]
- Prevost, S.; Lopian, T.; Pleines, M.; Diat, O.; Zemb, T. Small-angle scattering and morphologies of ultra-flexible microemulsions. J. Appl. Crystallogr. 2016, 49, 2063–2072. [Google Scholar] [CrossRef]
- Matsuoka, S. Chapter 3—Mechanical relaxation processes in polymers. In Handbook of Thermal Analysis and Calorimetry; Cheng, S.Z.D., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 3, pp. 111–146. [Google Scholar]
- Shen, J.; Lin, X.; Liu, J.; Li, X. Effects of Cross-Link Density and Distribution on Static and Dynamic Properties of Chemically Cross-Linked Polymers. Macromolecules 2019, 52, 121–134. [Google Scholar] [CrossRef]
- Reinitz, S.D.; Carlson, E.M.; Levine, R.A.C.; Franklin, K.J.; Van Citters, D.W. Dynamical mechanical analysis as an assay of cross-link density of orthopaedic ultra high molecular weight polyethylene. Polym. Test. 2015, 45, 174–178. [Google Scholar] [CrossRef]
- Bondan, F.; Ernzen, J.R.; Amalvy, J.; Machado, A.V.; Nardi Martins, J.D.; Bianchi, O. Influence of dynamic crosslinking on the morphology, crystallization, and dynamic mechanical properties of PA6,12/EVA blends. J. Appl. Polym. Sci. 2016, 133, 44206–44218. [Google Scholar] [CrossRef]

| Soybean Polyol Content (wt.%) | Mn (kDa) | Mw (kDa) | Đ |
|---|---|---|---|
| 0 | 70.4 | 123.1 | 1.74 |
| 1 | 115.1 | 202.9 | 1.76 |
| 5 | 136.2 | 366.5 | 2.68 |
| 10 * | 98.4 | 215.5 | 2.19 |
| 20 * | 98.9 | 269.2 | 2.72 |
| 50 * | 83.8 | 181.5 | 2.01 |
| Soybean Polyol Content (wt.%) | f | Xb | Wh | |||
|---|---|---|---|---|---|---|
| 0 | 0.462 | 0.528 | 0.288 | 0.133 | 0.671 | 0.329 |
| 1 | 0.462 | 0.523 | 0.286 | 0.132 | 0.669 | 0.331 |
| 5 | 0.462 | 0.520 | 0.289 | 0.134 | 0.671 | 0.329 |
| 10 | 0.462 | 0.515 | 0.288 | 0.133 | 0.671 | 0.329 |
| 20 | 0.462 | 0.433 | 0.315 | 0.145 | 0.682 | 0.318 |
| 50 | 0.462 | 0.444 | 0.292 | 0.135 | 0.672 | 0.328 |
| Soybean Polyol (wt.%) | TgSP (°C) | ΔCpS [J/(g.°C)] | (TgSP-TgSS) (°C) | Degree of Phase Separation (DPS) (%) |
|---|---|---|---|---|
| 0 | −18.7 | 0.423 | 5.3 | 79.9 |
| 1 | −22.6 | 0.403 | 1.9 | 76.3 |
| 5 | −24.5 | 0.383 | 2.3 | 73.1 |
| 10 | −17.2 | 0.377 | 12.4 | 72.8 |
| 20 | −10.3 | 0.297 | 24.9 | 58.8 |
| 50 | 82.6 | 0.170 | 134.7 | 43.1 |
| Soybean Polyol (wt.%) | Tg (DMA) (°C) * | Ee′0 (MPa) | νe (mol.cm−3) × 10−4 | Ea (kJ.mol−1) |
|---|---|---|---|---|
| 0 | 15.5 | 158.4 | 1.040 | 177.8 |
| 1 | 21.7 | 178.7 | 1.043 | 168.9 |
| 5 | 19.6 | 205.6 | 1.050 | 145.9 |
| 10 | 23.0 | 194.4 | 1.078 | 179.1 |
| 20 | 38.5 | 195.3 | 1.074 | 163.6 |
| 50 | 62.3 | 98.9 | 1.103 | 415.5 |
| Soybean Polyol (wt.%) | γL (mN.m) | γSD (mN.m) | γSP (mN.m) |
|---|---|---|---|
| 0 | 27.26 | 0.19 | 27.08 |
| 1 | 27.38 | 0.14 | 27.24 |
| 5 | 24.97 | 0.11 | 24.86 |
| 10 | 26.07 | 0.03 | 26.05 |
| 20 | 25.64 | 0.01 | 25.63 |
| 50 | 25.44 | 0.04 | 25.40 |
| Properties | Reference TPU (without Soybean Polyol) | TPU with 5 wt.% Soybean Polyol |
|---|---|---|
| Hardness (shore D) | 53 ± 2 | 52 ± 2 |
| Tensile strength at 100%, MPa | 11.6 ± 0.3 | 10.9 ± 0.2 |
| Tensile strength at 300%, MPa | 13.8 ± 0.3 | 15.3 ± 0.2 |
| Tensile strength, MPa | 27.8 ± 0.9 | 46.7 ± 3.1 |
| Burst pressure, bar (6 mm × 1 mm) | 33.7 ± 0.5 | 30.4 ± 0.9 |
| Burst pressure, bar (8 mm × 1.25 mm) | 30.9 ± 0.6 | 30.8 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernzen, J.R.; Covas, J.A.; Marcos-Fernández, A.; Fiorio, R.; Bianchi, O. Soybean-Based Polyol as a Substitute of Fossil-Based Polyol on the Synthesis of Thermoplastic Polyurethanes: The Effect of Its Content on Morphological and Physicochemical Properties. Polymers 2023, 15, 4010. https://doi.org/10.3390/polym15194010
Ernzen JR, Covas JA, Marcos-Fernández A, Fiorio R, Bianchi O. Soybean-Based Polyol as a Substitute of Fossil-Based Polyol on the Synthesis of Thermoplastic Polyurethanes: The Effect of Its Content on Morphological and Physicochemical Properties. Polymers. 2023; 15(19):4010. https://doi.org/10.3390/polym15194010
Chicago/Turabian StyleErnzen, Juliano R., José A. Covas, Angel Marcos-Fernández, Rudinei Fiorio, and Otávio Bianchi. 2023. "Soybean-Based Polyol as a Substitute of Fossil-Based Polyol on the Synthesis of Thermoplastic Polyurethanes: The Effect of Its Content on Morphological and Physicochemical Properties" Polymers 15, no. 19: 4010. https://doi.org/10.3390/polym15194010
APA StyleErnzen, J. R., Covas, J. A., Marcos-Fernández, A., Fiorio, R., & Bianchi, O. (2023). Soybean-Based Polyol as a Substitute of Fossil-Based Polyol on the Synthesis of Thermoplastic Polyurethanes: The Effect of Its Content on Morphological and Physicochemical Properties. Polymers, 15(19), 4010. https://doi.org/10.3390/polym15194010