Innovative Solid Slippery Coating: Uniting Mechanical Durability, Optical Transparency, Anti-Icing, and Anti-Graffiti Traits
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Coating Fabrication
2.3. Characterization
2.4. Durability Tests
2.5. Ice Adhesion Test
2.6. Anti-Graffiti and Anti-Sticking Test
3. Results and Discussion
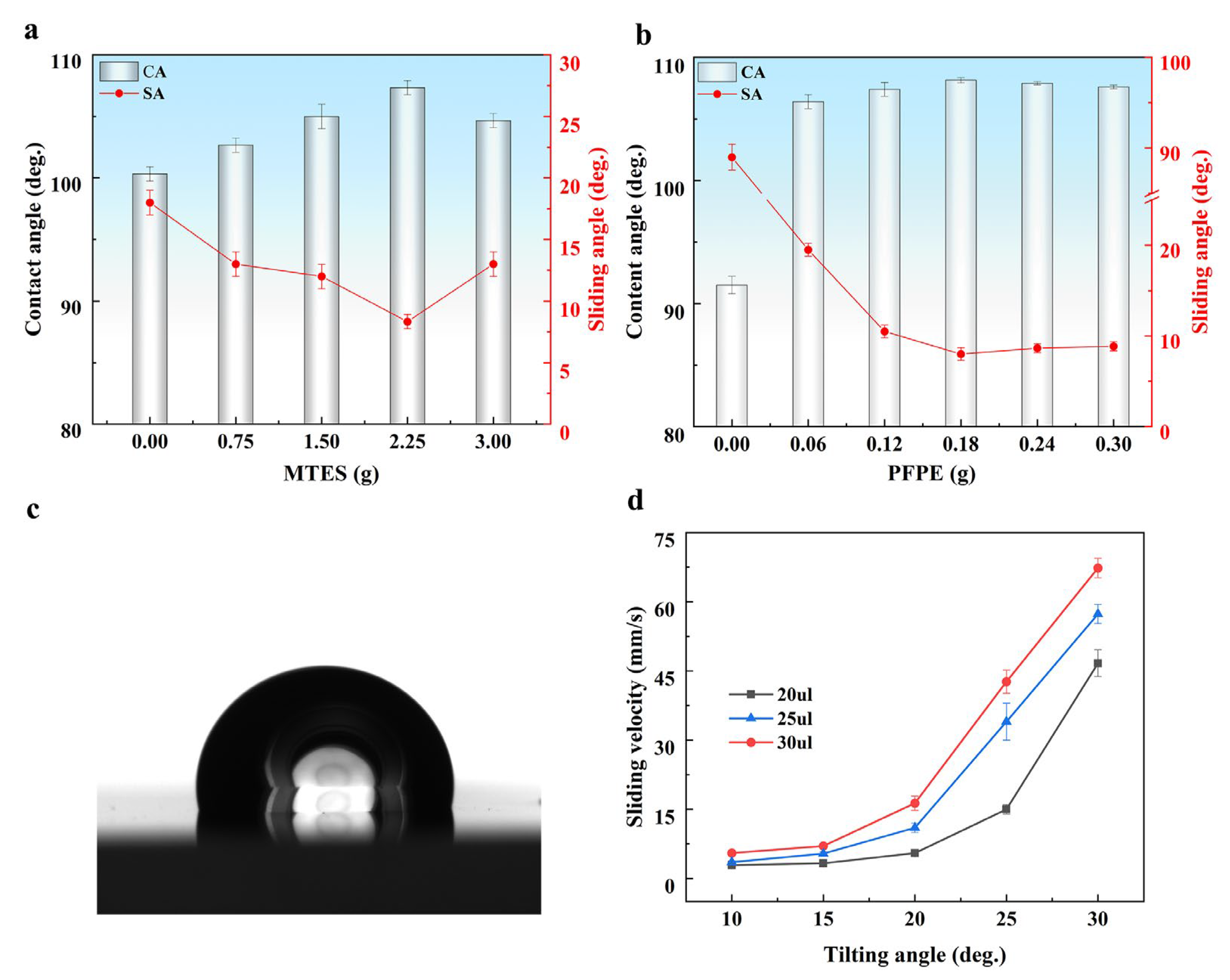
3.1. Component Optimization and Surface Characterization
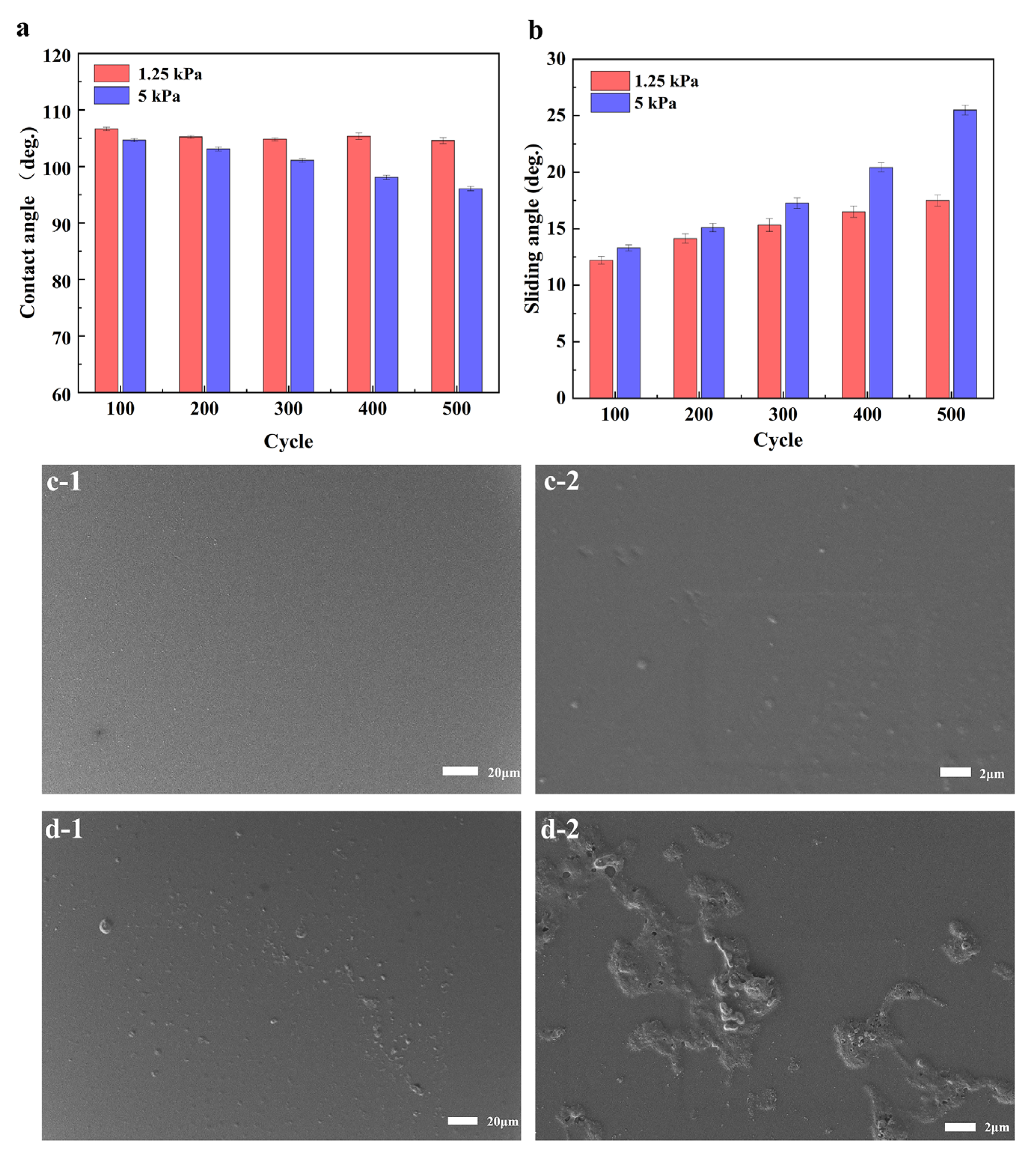
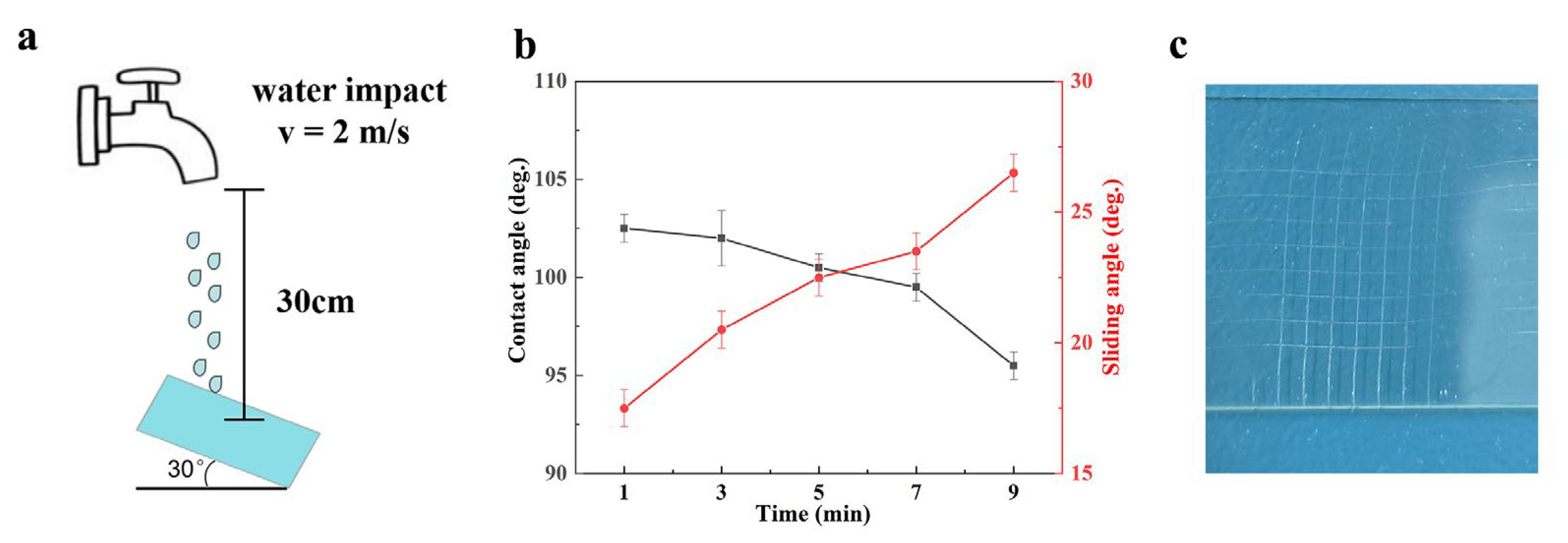
3.2. Durability and Protection Performance
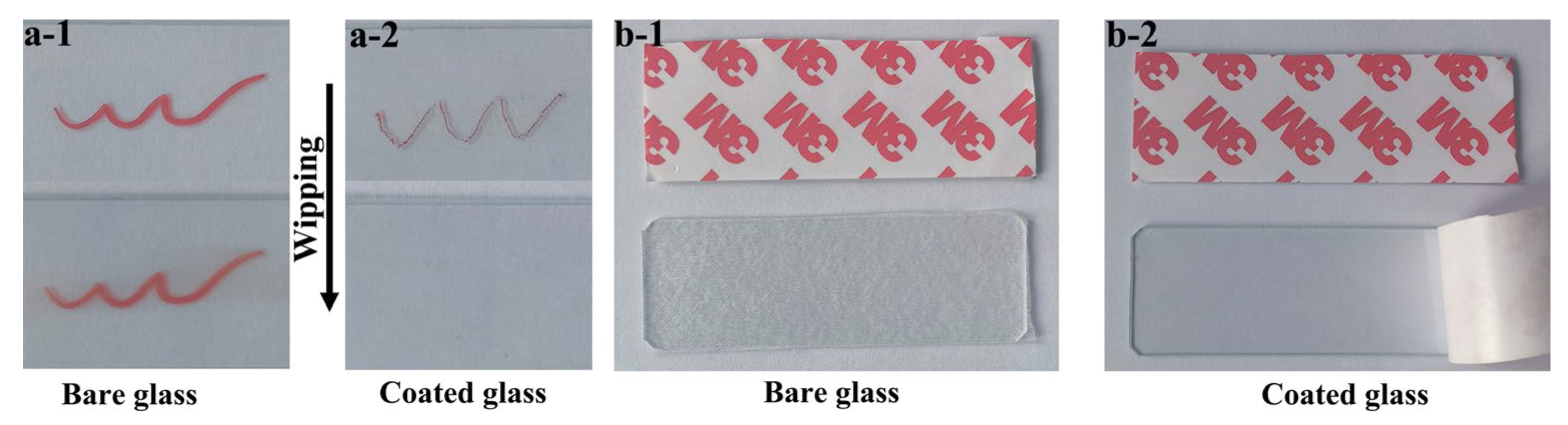
3.3. Applications: Anti-Icing, Anti-Graffiti and Anti-Sticking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, N.; Rasheed, S.; Ahmed, K.; Musharraf, S.G.; Najam-ul-Haq, M.; Hussain, D. Facile two-step functionalization of multifunctional superhydrophobic cotton fabric for UV-blocking, self cleaning, antibacterial, and oil-water separation. Sep. Purif. Technol. 2023, 306, 122626. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Han, Z.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, H.; Ji, H.; Chen, B. Slippery lubricant-infused textured aluminum surfaces. J. Adhes. Sci. Technol. 2014, 28, 1949–1957. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Sun, L.; Jiang, B.; Pan, F. Recent developments in slippery liquid-infused porous surface. Prog. Org. Coat. 2022, 166, 106806. [Google Scholar] [CrossRef]
- Tripathi, D.; Ray, P.; Singh, A.V.; Kishore, V.; Singh, S.L. Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances. Coatings 2023, 13, 1095. [Google Scholar] [CrossRef]
- Samaha, M.A.; Gad-el-Hak, M. Polymeric Slippery Coatings: Nature and Applications. Polymers 2014, 6, 1266–1311. [Google Scholar] [CrossRef]
- Khammas, R.; Koivuluoto, H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability 2022, 14, 8422. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Chen, J.; Gu, H.; Liu, J.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of bioinspired slippery liquid-infused porous surfaces with anti-icing performance via delayed phase inversion process. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124384. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Jin, J. Omniphobic, ice-repellent, anti-bacterial, slippery liquid-infused porous surface (SLIPS) using sprayable chitin nanofiber coating. Macromol. Res. 2023, 31, 65–74. [Google Scholar] [CrossRef]
- Michael, W.; Anne-Marie, K.; François, L.-L. Icephobic Behavior of UV-Cured Polymer Networks Incorporated into Slippery Lubricant-Infused Porous Surfaces: Improving SLIPS Durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Zhao, Z.; Yan, Y.; Zhang, D. Slippery liquid-infused porous electric heating coating for anti-icing and de-icing applications. Surf. Coat. Technol. 2019, 374, 889–896. [Google Scholar] [CrossRef]
- Tas, M.; Memon, H.; Xu, F.; Ahmed, I.; Hou, X. Electrospun anofiber membrane based transparent slippery liquid-infused porous surfaces with icephobic properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124177. [Google Scholar] [CrossRef]
- Deng, R.; Shen, T.; Chen, H.; Lu, J.; Yang, H.-C.; Li, W. Slippery liquid-infused porous surfaces (SLIPSs): A perfect solution to both marine fouling and corrosion? J. Mater. Chem. A 2020, 8, 7536–7547. [Google Scholar] [CrossRef]
- Vicente, A.; Rivero, P.J.; García, P.; Mora, J.; Carreño, F.; Palacio, J.F.; Rodríguez, R. Icephobic and Anticorrosion Coatings Deposited by Electrospinning on Aluminum Alloys for Aerospace Applications. Polymers 2021, 13, 4164. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, C.; Song, J.; Huang, L.; Sun, Y.; Liu, Z.; Zhao, C.; Li, Y. Multi-functional application of oil-infused slippery Al surface: From anti-icing to corrosion resistance. J. Mater. Sci. 2018, 53, 16099–16109. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, J.; Gao, J.; Ma, Y. TiO2-based slippery liquid-infused porous surfaces with excellent ice-phobic performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129994. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Y.; Liu, X.; Xi, S.; Yan, Z.; Liu, Z.; Shi, T.; Liao, G. Employing micro pyramidal holes and porous nanostructures for enhancing the durability of lubricant-infused surfaces in anti-icing. Surf. Coat. Technol. 2021, 405, 126568. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, C.; Su, C.; Zhong, L.; Zhou, L.; Hang, T.; Lin, H.; Chen, W.; Li, L.; Xie, X. Slippery Liquid-Attached Surface for Robust Biofouling Resistance. ACS Biomater. Sci. Eng. 2020, 6, 358–366. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; Wang, L.; Heng, L.; Jiang, L. A stable solid slippery surface with thermally assisted self-healing ability. J. Mater. Chem. A 2018, 6, 16355–16360. [Google Scholar] [CrossRef]
- Seraji, M.M.; Sameri, G.; Davarpanah, J.; Bahramian, A.R. The effect of high temperature sol-gel polymerization parameters on the microstructure and properties of hydrophobic phenol-formaldehyde/silica hybrid aerogels. J. Colloid Interface Sci. 2017, 493, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Ma, J.; Xu, W.; Sun, J.; Liu, X.; Song, J. Solid-Like Slippery Coating with Highly Comprehensive Performance. Adv. Funct. Mater. 2023, 33, 2302311. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zheng, H.; Chen, R.; Ma, C.; Zhang, G. Multifunctional Hard Yet Flexible Coatings Fabricated Using a Universal Step-by-Step Strategy. Adv. Sci. 2022, 9, 2200268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Peart, M.R.; Jim, C.Y.; La, J. Alkaline rains on the Tibetan Plateau and their implication for the original pH of natural rainfall. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Hou, Y.; Fu, S.; Zhang, L.; Li, M.; Wang, D. Facile fabrication of biomimetic slippery lubricant-infused transparent and multifunctional omniphobic surfaces. J. Mater. Sci. 2020, 55, 4225–4237. [Google Scholar] [CrossRef]
- Xing, K.; Li, Z.; Wang, Z.; Qian, S.; Feng, J.; Gu, C.; Tu, J. Slippery coatings with mechanical robustness and self-replenishing properties as potential application on magnesium alloys. Chem. Eng. J. 2021, 418, 129079. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, X.; Lin, D.; Xu, F.; Li, Y.; Wang, H. A novel slippery surface with enhanced stability and corrosion resistance. Prog. Org. Coat. 2020, 142, 105563. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Y.; Yang, D.-Q.; Sacher, E. A facile method to prepare mechanically durable super slippery polytetrafluoroethylene coatings. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 99–105. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z. An ionic liquid-infused slippery surface for temperature stability, shear resistance and corrosion resistance. J. Mater. Chem. A 2020, 8, 24075–24085. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, H.; Yang, L.; Sheng, J.; Fu, H. Robust Hyperbranched Polyester-Based Anti-Smudge Coatings for Self-Cleaning, Anti-Graffiti, and Chemical Shielding. ACS Appl. Mater. Interfaces 2019, 11, 14305–14312. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Sheng, J.; Fu, H. A novel UV/sunlight-curable anti-smudge coating system for various substrates. Chem. Eng. J. 2018, 345, 659–668. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-H.; Wu, S.-L.; Liu, C.; Yang, H.-C.; Darling, S.B.; Xu, Z.-K. When SLIPS meets TIPS: An endogenous lubricant-infused surface by taking the diluent as the lubricant. Chem. Eng. J. 2021, 425, 130600. [Google Scholar] [CrossRef]
- Shi, J.; Cao, C.; Zhang, L.; Quan, Y.; Wang, Q.; Xie, H. Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface. Polymers 2021, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Men, X.; Tiwari, M.K. Transparent and Robust Amphiphobic Surfaces Exploiting Nanohierarchical Surface-grown Metal–Organic Frameworks. Nano Lett. 2021, 21, 3480–3486. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Zavahir, S.; Abusrafa, A.E.; Abdulkareem, A.; Sobolčiak, P.; Lehocky, M.; Vesela, D.; Humpolíček, P.; Popelka, A. Slippery Liquid-Infused Porous Polymeric Surfaces Based on Natural Oil with Antimicrobial Effect. Polymers 2021, 13, 206. [Google Scholar] [CrossRef]





| Test | Testing Standard or Details and the Results | Ref |
|---|---|---|
| Visible light transmittance | 88% | [9] |
| 85% | [10] | |
| 73.1% | [26] | |
| 89.7% | This work | |
| Abrasion resistance | SA increased to 25° after 20 cycles of abrasion. Abrasion distance of 10 cm each cycle with a load of 10 kPa against 1000-mesh sandpaper | [27] |
| SA increased to 8° after 10 cycles. Abrasion distance of 20 cm each cycle with a load of 1 kPa against 1500-mesh sandpaper | [28] | |
| SA increased to 12° after 11 cycles. Abrasion distance of 20 cm each cycle with a load of 100 g against 1000-mesh sandpaper | [29] | |
| CA decreased to 95° and SA increased to 25° after 500 cycles. Abrasion distance of 10 cm each cycle with a load of 5 kPa against medical gauze | This work | |
| Water jetting | Sample was tilted at a 30° and jetted with water at 2 m/s for 1 min. After the test, water droplets slid off easily | [23] |
| Sample was tilted at a 45° and jetted with water at 1.34 m/s for 5 min. After the test, water droplets slid off easily | [26] | |
| Sample was tilted at a 45° and jetted with water at 1.5 m/s for 5 min. After the test, the sample lost its slippery performance | [29] | |
| Sample was tilted at a 30° and jetted with water at 2 m/s for 7 min. After the test, water droplets slid off easily | This work | |
| High temperature resistance | Almost no change in CA, while SA increased to 50° after 10 min at 240 °C | [23] |
| CA decreased to 65° and SA increased to 3.5° after 15 min at 220 °C | [30] | |
| CA decreased to 102° and SA increased to 19° after 1 h at 350 °C | This work | |
| Bending | No cracking observed after 20 cycle of bending the coating on aluminum | [24] |
| No cracking observed after 1 cycles of bending the coating on PET film | [31] | |
| No cracking observed after 50 cycles of bending the coating on PET film No cracking observed after 10 cycles of bending the coating on aluminum | This work | |
| Pencil Hardness | 3 H | [23] |
| 3 H | [32] | |
| 4H | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Ou, J.; Lei, S.; Hu, Y.; Wang, F.; Fang, X.; Li, C.; Li, W.; Amirfazli, A. Innovative Solid Slippery Coating: Uniting Mechanical Durability, Optical Transparency, Anti-Icing, and Anti-Graffiti Traits. Polymers 2023, 15, 3983. https://doi.org/10.3390/polym15193983
Shen J, Ou J, Lei S, Hu Y, Wang F, Fang X, Li C, Li W, Amirfazli A. Innovative Solid Slippery Coating: Uniting Mechanical Durability, Optical Transparency, Anti-Icing, and Anti-Graffiti Traits. Polymers. 2023; 15(19):3983. https://doi.org/10.3390/polym15193983
Chicago/Turabian StyleShen, Jiayi, Junfei Ou, Sheng Lei, Yating Hu, Fajun Wang, Xinzuo Fang, Changquan Li, Wen Li, and Alidad Amirfazli. 2023. "Innovative Solid Slippery Coating: Uniting Mechanical Durability, Optical Transparency, Anti-Icing, and Anti-Graffiti Traits" Polymers 15, no. 19: 3983. https://doi.org/10.3390/polym15193983
APA StyleShen, J., Ou, J., Lei, S., Hu, Y., Wang, F., Fang, X., Li, C., Li, W., & Amirfazli, A. (2023). Innovative Solid Slippery Coating: Uniting Mechanical Durability, Optical Transparency, Anti-Icing, and Anti-Graffiti Traits. Polymers, 15(19), 3983. https://doi.org/10.3390/polym15193983







