Anti-Obesity Effects of Chitosan and Its Derivatives
Abstract
1. Introduction
2. Effects of Chitooligosaccharides and Chitosan against Obesity
2.1. Chitooligosaccharides (COS)
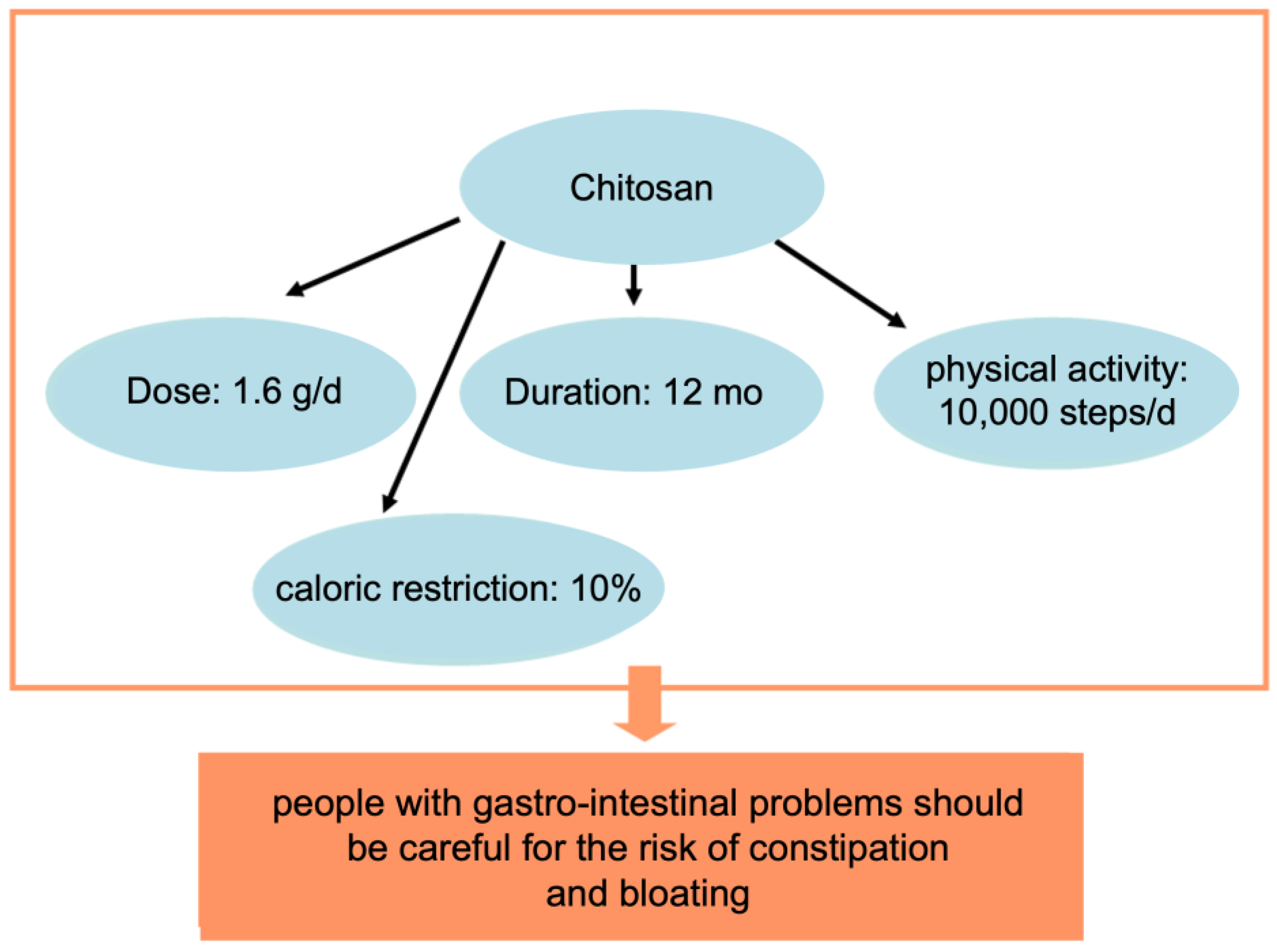
2.2. Chitosan
3. Effect of Chitosan Derivatives on Obesity
4. Regulation of Gut Flora by Chitosan and COS
5. Treatment of Obesity with Chitosan-Based Delivery Systems
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Review paper: Chitosan derivatives as promising materials for controlled drug delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023, 240, 124398. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, ISBN 9780081002575. [Google Scholar]
- Yildizbakan, L.; Iqbal, N.; Ganguly, P.; Kumi-Barimah, E.; Do, T.; Jones, E.; Giannoudis, P.V.; Jha, A. Fabrication and Characterisation of the Cytotoxic and Antibacterial Properties of Chitosan-Cerium Oxide Porous Scaffolds. Antibiotics 2023, 12, 1004. [Google Scholar] [CrossRef]
- World Health Organization World Obesity Day 2022—Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 25 May 2023).
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular genetics of human obesity: A comprehensive review. Comptes Rendus Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Kim, O.T. Epigenetics of obesity. Cardiovasc. Ther. Prev. 2020, 19, 94–100. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Alfredo Martínez, J. Precision Nutrition and Metabolic Syndrome Management. Nutrients 2019, 11, 2411. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Pérusse, L.; Rao, D.C.; Bouchard, C. Familial risk of overweight and obesity in the Canadian population using the WHO/NIH criteria. Obes. Res. 2000, 8, 194–197. [Google Scholar] [CrossRef]
- Alonso, R.; Farías, M.; Alvarez, V.; Cuevas, A. The Genetics of Obesity. Transl. Cardiometab. Genom. Med. 2016, 161–177. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Girardet, C.; Butler, A.A. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim. Biophys. Acta 2014, 1842, 482–494. [Google Scholar] [CrossRef]
- Montan, P.D.; Sourlas, A.; Olivero, J.; Silverio, D.; Guzman, E.; Kosmas, C.E. Pharmacologic therapy of obesity: Mechanisms of action and cardiometabolic effects. Ann. Transl. Med. 2019, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Deska, K.; Bąk, B.; Różycka-Kosmalska, M.; Pietras, T. Pharmacological Support for the Treatment of Obesity—Present and Future. Healthcare 2023, 11, 433. [Google Scholar] [CrossRef]
- Chao, A.M.; Tronieri, J.S.; Amaro, A.; Wadden, T.A. Semaglutide for the treatment of obesity. Trends Cardiovasc. Med. 2023, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Egorov, A.D.; Penkov, D.N.; Tkachuk, V.A. Molecular and cellular mechanisms of adipogenesis. Diabetes Mellit. 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed. Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Bahar, B.; O’Doherty, J.V.; O’Doherty, A.M.; Sweeney, T. Chito-Oligosaccharide Inhibits the De-Methylation of a “CpG” Island within the Leptin (LEP) Promoter during Adipogenesis of 3T3-L1 Cells. PLoS ONE 2013, 8, e60011. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Rahman, A.; Kim, S.W.; Baek, Y.M.; Hwang, H.J.; Oh, J.Y.; Hwang, H.S.; Lee, S.H.; Yun, J.W. Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2008, 18, 80–87. [Google Scholar] [PubMed]
- Kong, C.S.; Kim, J.A.; Eom, T.K.; Kim, S.K. Phosphorylated glucosamine inhibits adipogenesis in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Karagozlu, M.Z.; Pyun, S.Y.; Kim, S.K. Sulfation of chitosan oligomers enhances their anti-adipogenic effect in 3T3-L1 adipocytes. Carbohydr. Polym. 2011, 86, 666–671. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rahman, A.M.; Lee, S.H.; Hwang, H.S.; Kim, H.A.; Yun, J.W. Plasma proteome analysis for anti-obesity and anti-diabetic potentials of chitosan oligosaccharides in ob/ob mice. Proteomics 2009, 9, 2149–2162. [Google Scholar] [CrossRef]
- Liao, A.H.; Ma, W.C.; Wu, M.F. Evaluation of Ultrasound Combined with Chitosan for the Control of Weight and Local Fat in Mice. Ultrasound Med. Biol. 2013, 39, 1794–1803. [Google Scholar] [CrossRef]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Sweeney, T. A potential role of IL-6 in the chito-oligosaccharide-mediated inhibition of adipogenesis. Br. J. Nutr. 2011, 106, 1142–1153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; He, W.; Yang, D.; Cao, H.; Bai, Y.; Guo, J. Beneficial Metabolic Effects of Chitosan and Chitosan Oligosaccharide on Epididymal WAT Browning and Thermogenesis in Obese Rats. Molecules 2019, 24, 4455. [Google Scholar] [CrossRef]
- Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Su, Z. Anti-obesity effect of chitosan oligosaccharide capsules (COSCs) in obese rats by ameliorating leptin resistance and adipogenesis. Mar. Drugs 2018, 16, 198. [Google Scholar] [CrossRef]
- Melzner, I.; Scott, V.; Dorsch, K.; Fischer, P.; Wabitsch, M.; Brüderlein, S.; Hasel, C.; Möller, P. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. J. Biol. Chem. 2002, 277, 45420–45427. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents*. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Yen, T.E.; Liu, S.H.; Chiang, M.T. Comparative effects and mechanisms of chitosan and its derivatives on hypercholesterolemia in high-fat diet-fed rats. Int. J. Mol. Sci. 2020, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, R.Y.; Chiang, M.T. Effects of chitosan oligosaccharide on plasma and hepatic lipid metabolism and liver histomorphology in normal sprague-dawley rats. Mar. Drugs 2020, 18, 408. [Google Scholar] [CrossRef]
- Bonetti, G.; Herbst, K.L.; Donato, K.; Dhuli, K.; Kiani, A.K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Dietary supplements for obesity. J. Prev. Med. Hyg. 2022, 63, 160–168. [Google Scholar]
- Batsis, J.A.; Apolzan, J.W.; Bagley, P.J.; Blunt, H.B.; Divan, V.; Gill, S.; Golden, A.; Gundumraj, S.; Heymsfield, S.B.; Kahan, S.; et al. A Systematic Review of Dietary Supplements and Alternative Therapies for Weight Loss. Obesity 2021, 29, 1102–1113. [Google Scholar] [CrossRef]
- Wawrzyniak, N.; Skrypnik, K.; Suliburska, J. Dietary supplements in therapy to support weight reduction in obese patients. Acta Sci. Pol. Technol. Aliment. 2022, 21, 67–80. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2010, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Feng, S.A.; Liu, S.H.; Chiang, M.T. Functional comparison for lipid metabolism and intestinal and fecal microflora enzyme activities between low molecular weight chitosan and chitosan oligosaccharide in high-fat-diet-fed rats. Mar. Drugs 2017, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Multi-Functional Roles of Chitosan as a Potential Protective Agent against Obesity. PLoS ONE 2013, 8, e53828. [Google Scholar] [CrossRef]
- Di Santo, M.C.; Alaimo, A.; Acebedo, S.L.; Spagnuolo, C.; Pozner, R.; Pérez, O.E. Biological responses induced by high molecular weight chitosan administrated jointly with Platelet-derived Growth Factors in different mammalian cell lines. Int. J. Biol. Macromol. 2020, 158, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Rathore, V.K.; Parikh, J.K. Chitosan: Derivatives, Properties and Applications. In Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2022; pp. 759–770. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Song, T.; Zeng, A.; Wang, Q.; Sun, Z.; Shen, J. Therapeutic effect of carboxymethylated and quanternized chitosan on insulin resistance in high-fat-diet-induced rats and 3T3-L1 adipocytes. J. Biomater. Sci. Polym. Ed. 2012, 23, 1271–1284. [Google Scholar] [CrossRef]
- Chen, T.C.; Ho, Y.Y.; Tang, R.C.; Ke, Y.C.; Lin, J.N.; Yang, I.H.; Lin, F.H. Thiolated chitosan as an intestinal absorption carrier with hesperidin encapsulation for obesity treatment. Nutrients 2021, 13, 4405. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Antibacterial and wound healing activities of micro/nanocarriers based on carboxymethyl and quaternized chitosan derivatives. In Biopolymer-Based Nano Films: Applications in Food Packaging and Wound Healing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–201. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Zhang, J.; Gu, G.; Guo, Z. Significantly enhanced antioxidant activity of chitosan through chemical modification with coumarins. Polym. Chem. 2019, 10, 1480–1488. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Y.; Kang, W.; Zhou, J.; Dong, R.; Feng, Q. Chitosan attenuates obesity by modifying the intestinal microbiota and increasing serum leptin levels in mice. J. Funct. Foods 2020, 64, 103659. [Google Scholar] [CrossRef]
- Elebeedy, D.; Ghanem, A.; Saleh, A.; Ibrahim, M.H.; Kamaly, O.A.; Abourehab, M.A.S.; Ali, M.A.; Abd El Maksoud, A.I.; El Hassab, M.A.; Eldehna, W.M. In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation. Int. J. Mol. Sci. 2022, 23, 12200. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Kristensen, M.; Foged, C.; Berthelsen, J.; Mørck Nielsen, H. Peptide-enhanced oral delivery of therapeutic peptides and proteins. J. Drug Deliv. Sci. Technol. 2013, 23, 365–373. [Google Scholar] [CrossRef]
- Rostami, E. Progresses in targeted drug delivery systems using chitosan nanoparticles in cancer therapy: A mini-review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Heikal, L.A.; El-Kamel, A.H.; Mehanna, R.A.; Khalifa, H.M.; Hassaan, P.S. Improved oral nutraceutical-based intervention for the management of obesity: Pterostilbene-loaded chitosan nanoparticles. Nanomedicine 2022, 17, 1055–1075. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef]
- Xian, J.; Zhong, X.; Huang, Q.; Gu, H.; Feng, Y.; Sun, J.; Wang, D.; Li, J.; Zhang, C.; Wu, Y.; et al. N-Trimethylated chitosan coating white adipose tissue vascular-targeting oral nano-system for the enhanced anti-obesity effects of celastrol. Int. J. Biol. Macromol. 2023, 236, 124023. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Li, H.; Liu, M.; Wen, J. N-trimethyl chitosan coated nano-complexes enhance the oral bioavailability and chemotherapeutic effects of gemcitabine. Carbohydr. Polym. 2021, 273, 118592. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Zou, Y.; Chi, H. The effects of chitosan supplementation on body weight and body composition: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Bessell, E.; Maunder, A.; Lauche, R.; Adams, J.; Sainsbury, A.; Fuller, N.R. Efficacy of dietary supplements containing isolated organic compounds for weight loss: A systematic review and meta-analysis of randomised placebo-controlled trials. Int. J. Obes. 2021, 45, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, S.; Sayyari, A.A.; Salehi, M.; Safa, M.; Sohouli, M.; Shidfar, F.; Santos, H.O. The effects of chitosan supplementation on anthropometric indicators of obesity, lipid and glycemic profiles, and appetite-regulated hormones in adolescents with overweight or obesity: A randomized, double-blind clinical trial. BMC Pediatr. 2022, 22, 527. [Google Scholar] [CrossRef]
- Valero-Pérez, M.; Bermejo, L.M.; López-Plaza, B.; García, M.A.; Palma-Milla, S.; Gómez-Candela, C. Regular consumption of LIPIGO® promotes the reduction of body weight and improves the rebound effect of obese people undergo a comprehensive weight loss program. Nutrients 2020, 12, 1960. [Google Scholar] [CrossRef]
- Pittler, M.H.; Abbot, N.C.; Harkness, E.F.; Ernst, E. Randomized, double-blind trial of chitosan for body weight reduction. Eur. J. Clin. Nutr. 1999, 53, 379–381. [Google Scholar] [CrossRef]
- Jung, E.Y.; Jun, S.C.; Chang, U.J.; Suh, H.J. L-ascorbic acid addition to chitosan reduces body weight in overweight women. Int. J. Vitam. Nutr. Res. 2014, 84, 5–11. [Google Scholar] [CrossRef]
- Trivedi, V.; Satia, M.; Deschamps, A.; Maquet, V.; Shah, R.; Zinzuwadia, P.; Trivedi, J. Single-blind, placebo controlled randomised clinical study of chitosan for body weight reduction. Nutr. J. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Cornelli, U.; Belcaro, G.; Recchia, M.; D’Orazio, N. Long-Term Treatment of Overweight and Obesity with Polyglucosamine (PG L112): Randomized Study Compared with Placebo in Subjects after Caloric Restriction. Curr. Dev. Nutr. 2017, 1, e000919. [Google Scholar] [CrossRef] [PubMed]
- Qiang, F.; Di, T. Application of Chitosan in Preparation of Anti-Obesity Food. CN Patent 110477398, 22 November 2019. [Google Scholar]
- Luciano, M.; Andrea, C. Body Weight Control Preparation Based on Chitosan and Cellulose. EP Patent 3225239, 4 October 2017. [Google Scholar]
- Joo, S.H. Chitosan Mixture Containing Complex Additives for Antioxidation. KR Patent 1020110010018, 31 January 2011. [Google Scholar]
- Sun, H.H.; Won, Y.J. Anti-Obesity Composition Containing Chitosan Oligosaccharide and Deep Sea Water with Less Side Effects. KR Patent 1020090119319, 19 November 2009. [Google Scholar]
- Ju, C.H.; Sil, Y.I.; Jun, L.W. Functional Anti-Obesity Biohealth Material Containing Low Molecular Chitosan as Effective Ingredient. KR Patent 1020060082351, 18 July 2006. [Google Scholar]
- June, K.Y.; Hyeong, C.S. Dietary Agent Comprising Chitosan Microparticles Useful for Health-Aid Food for Treating Obesity. KR Patent 1020050055643, 13 June 2005. [Google Scholar]
- Suk, H.H.; Hyeong, K.S.; Tae, K.S.; Mun, P.G. Composition Containing Mycelium of Lentinus Edodes and Agaricus Blazei Murill and Water-Soluble Chitosan for Controlling Obesity. KR Patent 1020030056753, 4 July 2003. [Google Scholar]
- Kazuo, S.; Yoshimori, T.; Shiori, S. Anorectic Agent. JP Patent 2002104975, 10 April 2002. [Google Scholar]
| MW, kDa | Cells, Species | Route | Doses, Duration | Anti-Obesity Effects | Ref. |
|---|---|---|---|---|---|
| 1–10 | 3T3-L1 | - | 0.5–4 mg/mL | Decreased lipid accumulation, mRNA expression of C/EBP-α, PPAR-γ, leptin, adiponectin, and resistin levels | [26] |
| 1–10 | 3T3-L1 | - | 0.6–4.8 mg/mL | Decreased lipid accumulation, free glycerol release, expression of adipogenicity marker genes, and activation of IL-6 and PTGS2 genes | [33] |
| 5–10 | 3T3-L1 | - | 0.6–4.8 mg/mL | Suppression of lipid metabolism and lipid accumulation genes; inhibition of leptin expression | [25] |
| 1–3 | 3T3-L1 | - | 0.5–5 mg/mL | Reduced level of intracellular lipid droplets and TG; decreased level of extracellular glucose | [34] |
| Sprague–Dawley rats, HFD | Oral | 600 mg/kg·day, 8 weeks | Significant reduction in weight gain and in TG, TC, LDL-C, glucose, and FFA levels; significant increase in HDL-C levels; effective suppression of adipose tissue hypertrophy and hyperplasia; and increased expression of UCP1, PGC1α, PRMD16, and ATF2 in white adipose tissue and brown adipose tissue | ||
| ≤1 | Sprague–Dawley rats, HFD | Oral | 300–1200 mg/kg·day, 8 weeks | Reduced body weight; suppressed perirenal, epididymal, subcutaneous, and total fat accumulation; reduced TC, TG, and LDL-C; and improved HDL-C levels in blood serum | [32] |
| ≤1–3 | Sprague–Dawley rats, HFD | Oral | 150–600 mg/kg/day, 5 weeks | Mostly weight loss; significant decrease in serum total cholesterol and LDL-C levels; decrease in PPARγ and LXRα gene expression in white adipose tissue | [29] |
| ≤1 | Sprague–Dawley rats, HFD | Oral | 150–600 mg/kg·day, 8 weeks | Inhibition of body weight gain; reduction in adipocyte hypertrophy and fat accumulation; reduction in hepatic steatosis; significant reduction in leptin; increase in LepRb expression and JAK2-STAT3 phosphorylation levels; inhibition of lipid synthesis in the liver; regulation of SREBP-1c, FAS, ACC, HMGCR, and adiponectin gene expression | [35] |
| MW, kDa | Species | Route | Doses, Duration | Anti-Obesity Effects | Ref. |
|---|---|---|---|---|---|
| 80 | Sprague– Dawley rats, HFD | Food supplement | 5%, 10 weeks | Suppression of weight gain; improved balance of plasma, liver, and feces lipids and intestinal disaccharidase activity. Reduced mucinase and β-glucuronidase activities in fecal microflora. | [44] |
| 80, 740 | Sprague– Dawley rats, HFD | Food supplement | 5%, 8 weeks | Effective improvement of hypercholesterolemia and cholesterol homeostasis through activation and inhibition of hepatic AMPKα, PPARα, and intestinal ACAT2. | [38] |
| - | Sprague– Dawley rats, HFD | Oral | 600 mg/kg·day, 8 weeks | Significantly reduced weight gain, TG, TC, and LDL-C levels, and glucose and FFA levels. Promotion of energy release; increase in HDL-C levels; suppression of adipose tissue hypertrophy and hyperplasia; and increased UCP1, PGC1α, PRMD16, and ATF2 in white and brown adipose tissue. | [34] |
| 21, 46, 130 | C57BL/6J mice, HFD | Oral | 100 and 300 mg/kg·day, 10 weeks | Suppression of body weight and adipose tissue gain; induction of lipid-lowering effects; increase in fecal excretion of fat and/or bile acids; decreased absorption of triacylglycerol and cholesterol. | [43] |
| 300 | ICR mice | Oral | 60 mg/kg·day, 5 weeks | Body weight reduction; epididymal fat pad and intra-abdominal fat thickness reduction; decrease in TC, TG, and LDL-C plasma levels. | [31] |
| - | Pigs, basal diet | Food supplement | 300–1200 ppm, 1 week | Decreased crude fat digestibility, daily food intake, and final body weight; increased leptin concentration; and decreased serum C-reactive protein concentration. | [45] |
| Objects | MW, DD/DS | Cells, Species | Routes | Doses, Duration | Anti-Obesity Effects | Ref. |
|---|---|---|---|---|---|---|
| (N,O)-sulfatedchitosan | <1kDa | 3T3-L1 | - | 0.1–4 mg/mL | Reduced lipid and triglyceride accumulation; facilitated lipolysis and mRNA expression; and reduced PPAR-γ receptor and C/EBP-α protein levels | [28] |
| Phosphorylated glucosamine(PG1c) | - | 3T3-L1 | - | 0.2 mg/mL | Reduction in lipid accumulation; dose-dependent suppression of PPAR-γ receptor and C/EBP-α protein activity; induction of preadipocyte factor 1 mRNA activation; suppression of fatty acid synthase, lipoprotein lipase, and leptin | [27] |
| O-carboxymethyl chitosan (O-CMCs), N-[(2-hydroxy-3-N,N-dimethylhexadecyl ammonium)propyl] chitosan chloride (N-CQCs) | 50 kDa, DD 85%; O-CMCs DS 72% N-CQCs DS 21% | 3T3-L1 | - | 0.1 mg/mL | Suppression of leptin and resistin mRNA expression; increased adiponectin and PPAR-γ mRNA expression | [50] |
| Clean Wistar rats, HFD | Oral | 100 mg/kg·day, 6 weeks | Reduction in plasma leptin, glucose, insulin, and total cholesterol levels | |||
| Chitosan –thioglycolic acid | - | C57BL/6 mice, HFD | Oral | 250 mg/kg day, 8 weeks | Significant reduction in weight gain and fat distribution | [51] |
| Patent № | Patent Title | Description | Ref. |
|---|---|---|---|
| CN110477398 | Application of chitosan in preparation of anti-obesity food | This invention relates to the use of chitosan in the preparation of food products against obesity. In a food product with a high fat content, chitosan is added in an amount of 3–10% of the weight of the product. | [77] |
| EP3225239 | Body weight control preparation based on chitosan and cellulose | Compositions for oral administration are proposed that contain chitosan, capable of binding fatty acids and cellulose, and capable of forming a gel. These compositions are intended to combat obesity and overweight. | [78] |
| KR1020110010018 | Chitosan mixture containing complex additives for antioxidation | A chitosan mixture containing added vitamin C and organic acid is presented, designed to suppress fat accumulation and enhance the fight against obesity. | [79] |
| KR1020090119319 | Anti-obesity composition containing chitosan oligosaccharide and deep-sea water with fewer side effects | An anti-obesity composition is presented, containing chitosan oligosaccharides and deep-sea water with chlorine, sulfate, and magnesium. Designed to inhibit fat accumulation and for use as an additive in functional foods. | [80] |
| KR1020060082351 | Functional anti-obesity biohealth material containing low-molecular-weight chitosan as effective ingredient | Anti-obesity biomaterials consisting of low-molecular-weight chitosan are proposed, which improve physiological metabolism in obesity by reducing cell mass and abdominal fat size and improving serum lipid levels. | [81] |
| KR1020050055643 | Dietary agent comprising chitosan microparticles useful in health-promoting food for treating obesity | A dietary preparation containing chitosan microparticles is proposed, which can be used as a therapeutic food for the treatment of obesity without causing the so-called “yo-yo” phenomenon. | [82] |
| KR1020030056753 | Composition containing mycelium of Lentinus edodes and Agaricus blazei Murill and water-soluble chitosan for controlling obesity | A composition is presented that contains as the main components a mixture of Lentinus edodes mycelium, Agaricus blazei Murill mycelium, and water-soluble chitosan. This composition is used as a healthy food product to combat obesity and prevent cancer. | [83] |
| JP2002104975 | Anorectic agent | It is proposed to add an anorectic agent containing low-molecular-weight chitosan or its derivative to healthy foods and drinks to prevent obesity. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shagdarova, B.; Konovalova, M.; Varlamov, V.; Svirshchevskaya, E. Anti-Obesity Effects of Chitosan and Its Derivatives. Polymers 2023, 15, 3967. https://doi.org/10.3390/polym15193967
Shagdarova B, Konovalova M, Varlamov V, Svirshchevskaya E. Anti-Obesity Effects of Chitosan and Its Derivatives. Polymers. 2023; 15(19):3967. https://doi.org/10.3390/polym15193967
Chicago/Turabian StyleShagdarova, Balzhima, Mariya Konovalova, Valery Varlamov, and Elena Svirshchevskaya. 2023. "Anti-Obesity Effects of Chitosan and Its Derivatives" Polymers 15, no. 19: 3967. https://doi.org/10.3390/polym15193967
APA StyleShagdarova, B., Konovalova, M., Varlamov, V., & Svirshchevskaya, E. (2023). Anti-Obesity Effects of Chitosan and Its Derivatives. Polymers, 15(19), 3967. https://doi.org/10.3390/polym15193967






