Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation
Abstract
:1. Introduction
2. Materials and Methods
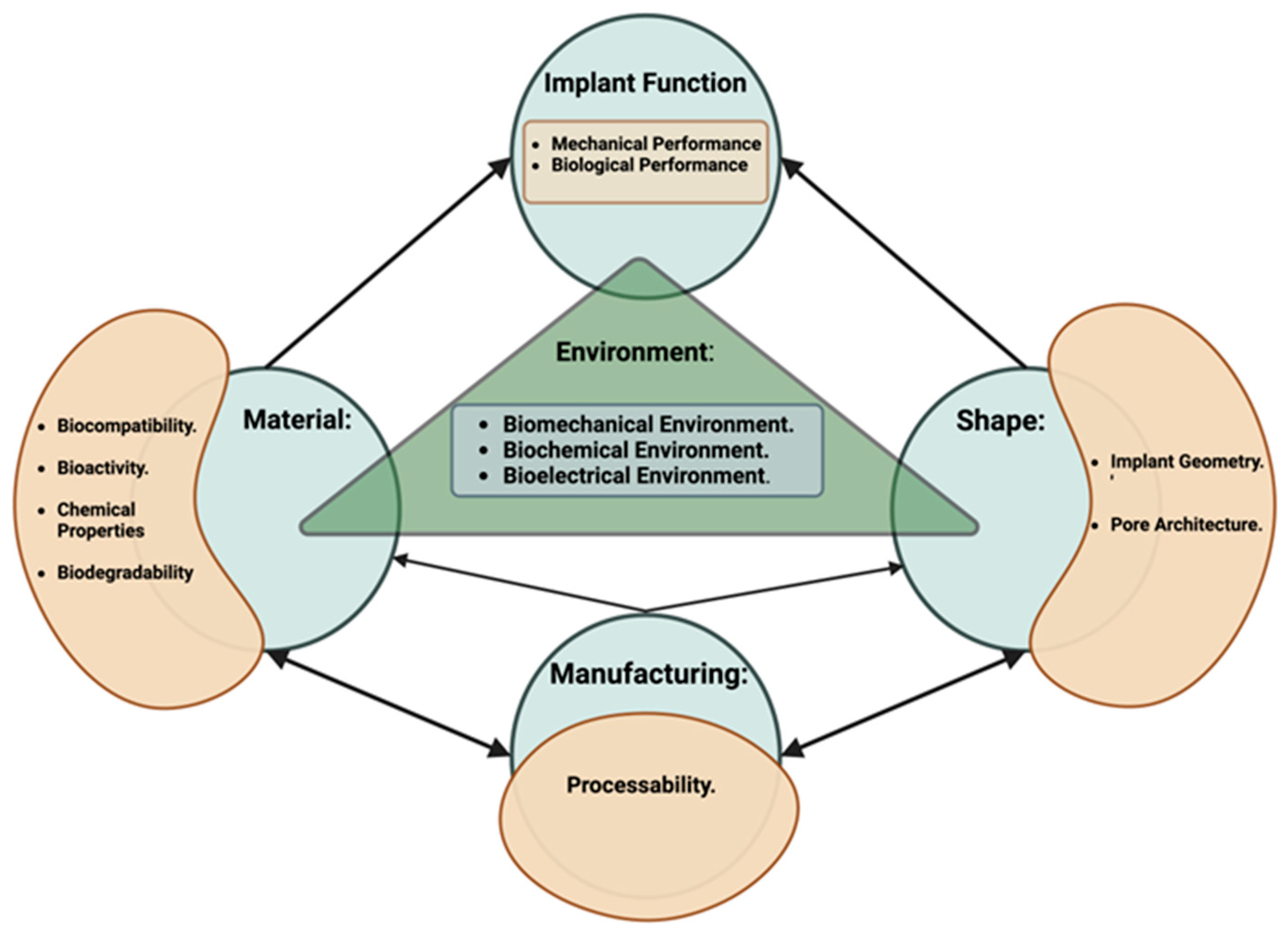
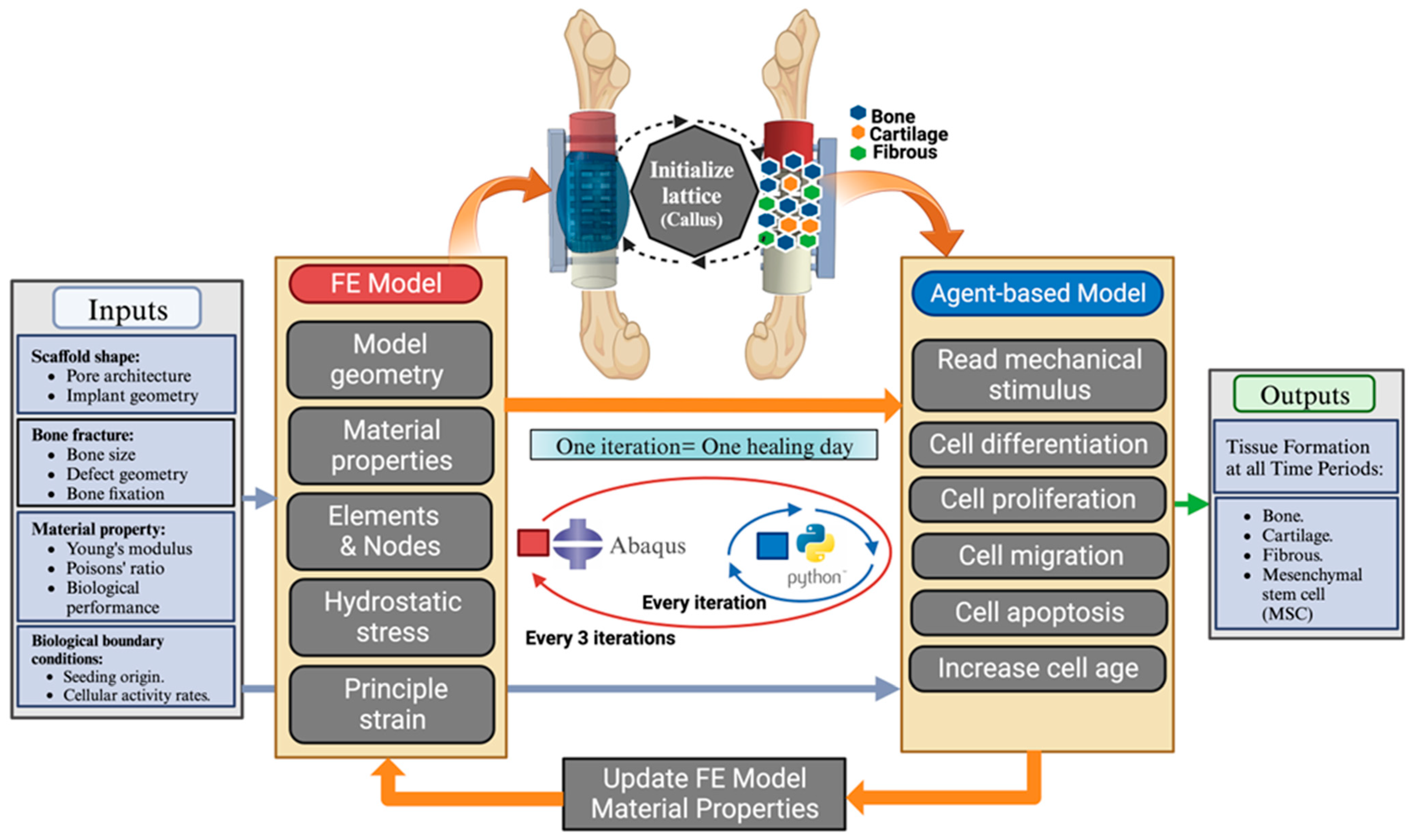
2.1. Virtual Design Framework
2.1.1. FE Model
2.1.2. Agent-Based Model (Python Model)
| Proliferation Rate per Day | Apoptosis Rate per Day | Differentiation Rate per Day | Migration Speed (µm/h) | |||
|---|---|---|---|---|---|---|
| Baseline | After Latency Period | Baseline | After Latency Period | |||
| Stem Cells | 0.12 | 0.06 | ||||
| Fibroblast | 0.11 | - | - | - | ||
| Chondrocyte | 0.1 | 0.04 | - | - | - | |
| Osteoblast | 0.15 | 0.06 | - | - | - | |
2.2. Model Validation
2.2.1. Experimental Data from the Literature
2.2.2. Virtual Design Framework Set Up
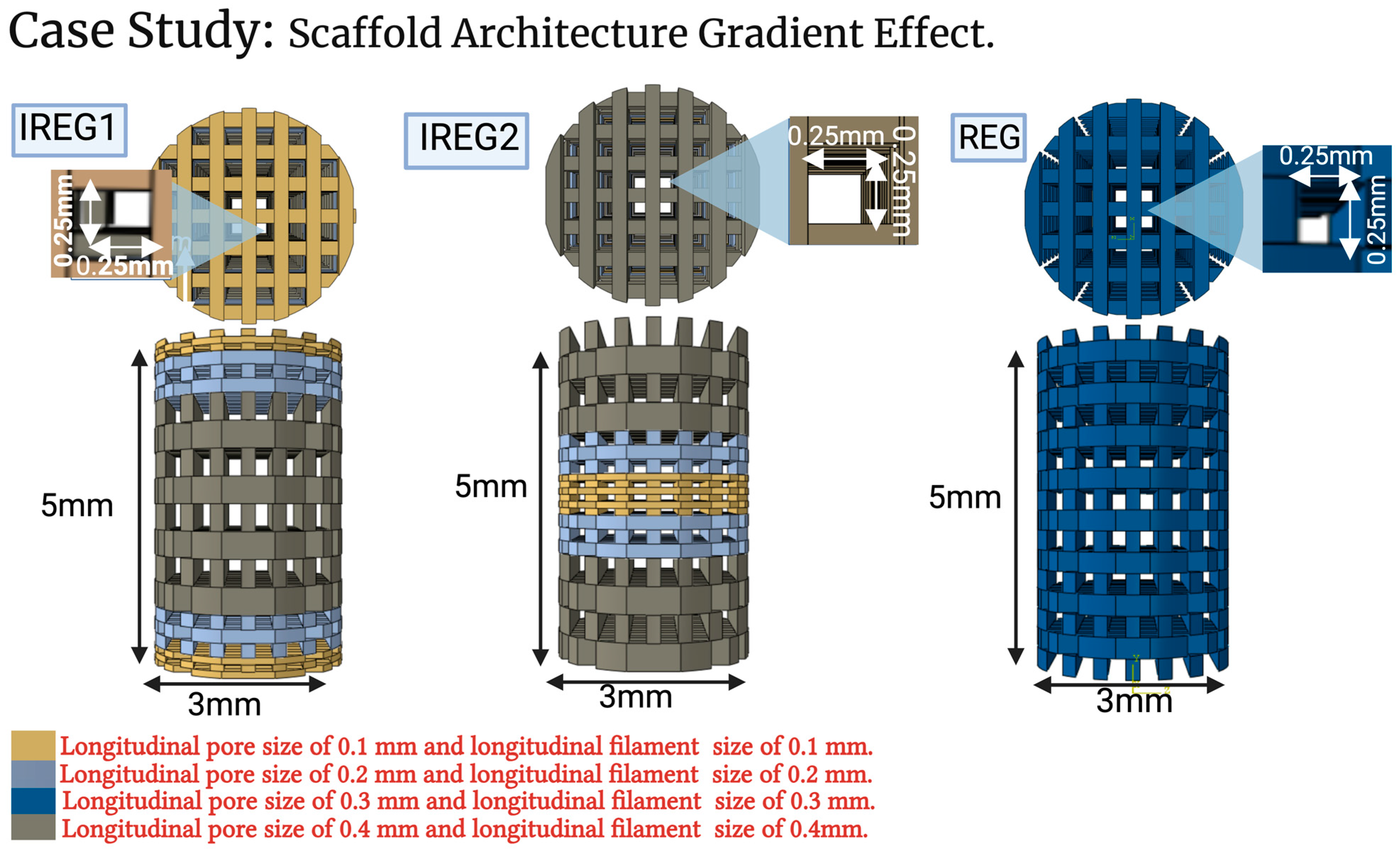
2.3. Case Study: Scaffold Architecture Gradient Effect
3. Results
3.1. Creation of Virtual Design Framework
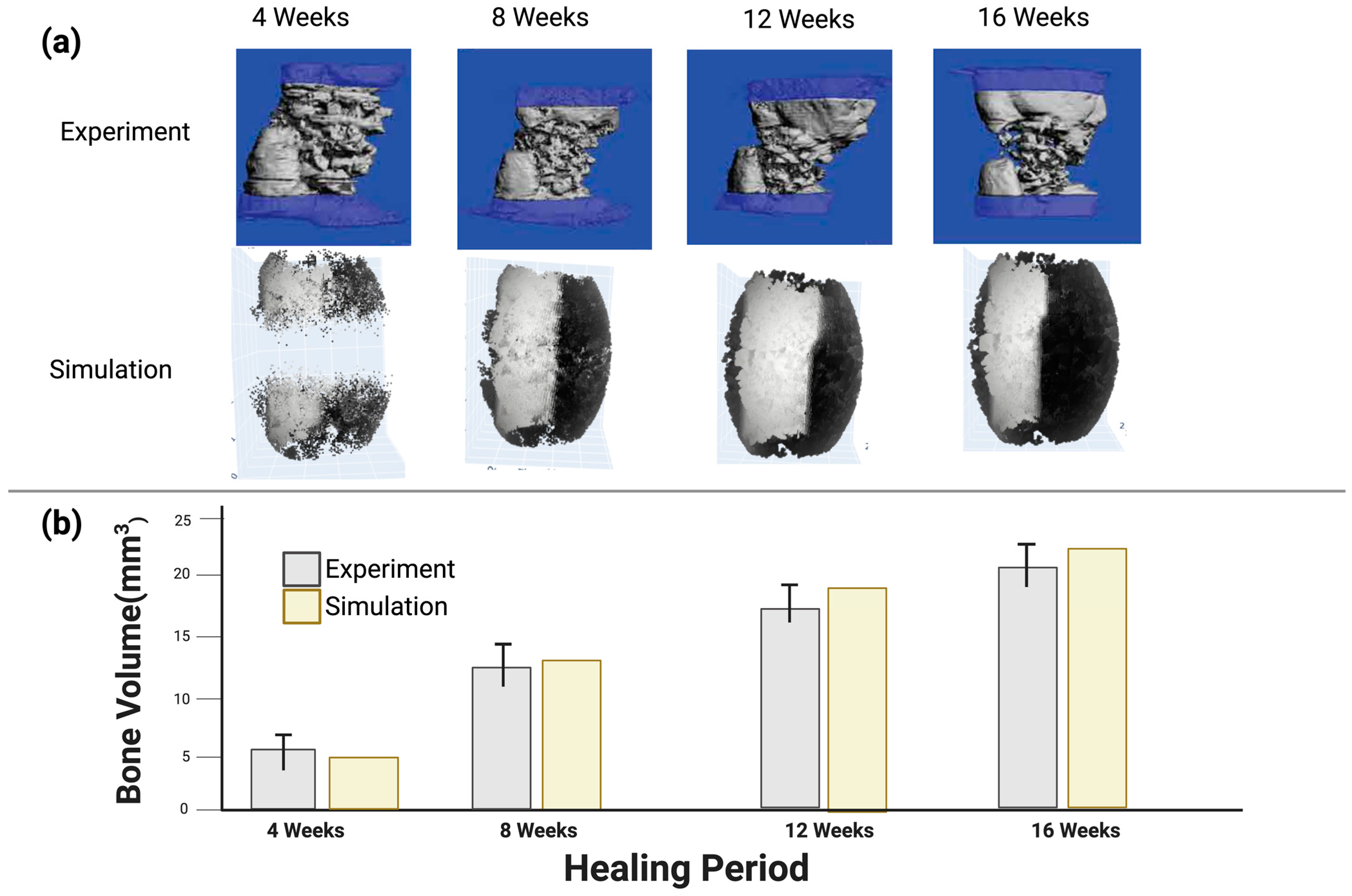
3.2. Model Validation Using In Vivo Experiment Results from the Literature
3.3. Case Study Results: The Influence of Longitudinal Pore Gradients
3.3.1. Bone Formation
3.3.2. Cartilage and Fibrous Formation
4. Discussion
- The predefined callus shape was created in silico and filled with granulation tissue, whereas no callus formation was observed in vivo. This is similar to many other mechanobiological models mentioned above [31,44,45,73], but one recent study modeled the callus behavior [74]. Nevertheless, the callus part was subjected to a biomechanically stressed environment, as determined by the mechanobiological model proposed by Claes et al. [39]. This mechanobiological formula makes it possible to predict the formation of various tissue types in callus areas, which may not be detectable in vivo with current measurement methods.
- No revascularization process of the defect was included in this simulation, despite the fact that other studies have found this to be a concerning issue in large bone defects [75,76,77,78]. In this simulation, this was mitigated by forming bone only in suitable biological and mechanical environments. This may have a slight impact on the results of the case studies focused on the effect of the longitudinal pore gradients.
- No scaffold degradation was included in the model. However, in this study Young’s modulus and the Poisson ratio for the three scaffold cases were 1000 MPa and 0.3, respectively, which are within the range of polymer−ceramics composites used in bone regeneration applications [61,79], which commonly use polycaprolactone (PCL), poly(l-lactide) (LPLA) or poly(lactic acid) (PLA) as the base polymers of the bone tissue scaffold implant. Lam et al. [80] reported that PCL scaffolds have no significant degradation and little effect on bone regeneration for periods of six months or less. Similar low degradation is reported for LPLA and PLA [81,82].
- Fixed relationships for cell behavior was a limitation of this model. Cell fates, migration, and multiplication are based on fixed relationships with the stress environment without any statistical variation. In an in vitro or in vivo experiment, statistical changes would be present. However, if these statistical changes were included the simulation would become more computationally expensive.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Software for Mechanobiological Model
- Create folder for the case requiring to be implemented.
- Create FE model with all the case set up, including geometry, material properties, and boundary conditions (Abaqus/CAE 2022 (Simulia, Johnston, RI, USA) license would be needed).
- Save the output files from Abaqus in the same folder (ex. job-1.inp, and job-1.dat).
- Create batch file in the same folder with the name of bone.run_abaqus.bat with these commands ‘abaqus job=bone ask_delete=OFF standard_parallel=all cups=2 interactive’.
- Open Python code; there are two main items to change:
- a.
- Change the index numbers to fit callus part in job.inp file, this can be changed in (1.Read Job File) in python code, see Figure A1 below.
- b.
- Change boundary conditions for your case in (3.create FE Model Job File (Abaqus), and 3.1 Update FE Model Job File With New Bone Formation) in Python code as in Figure A2 below.
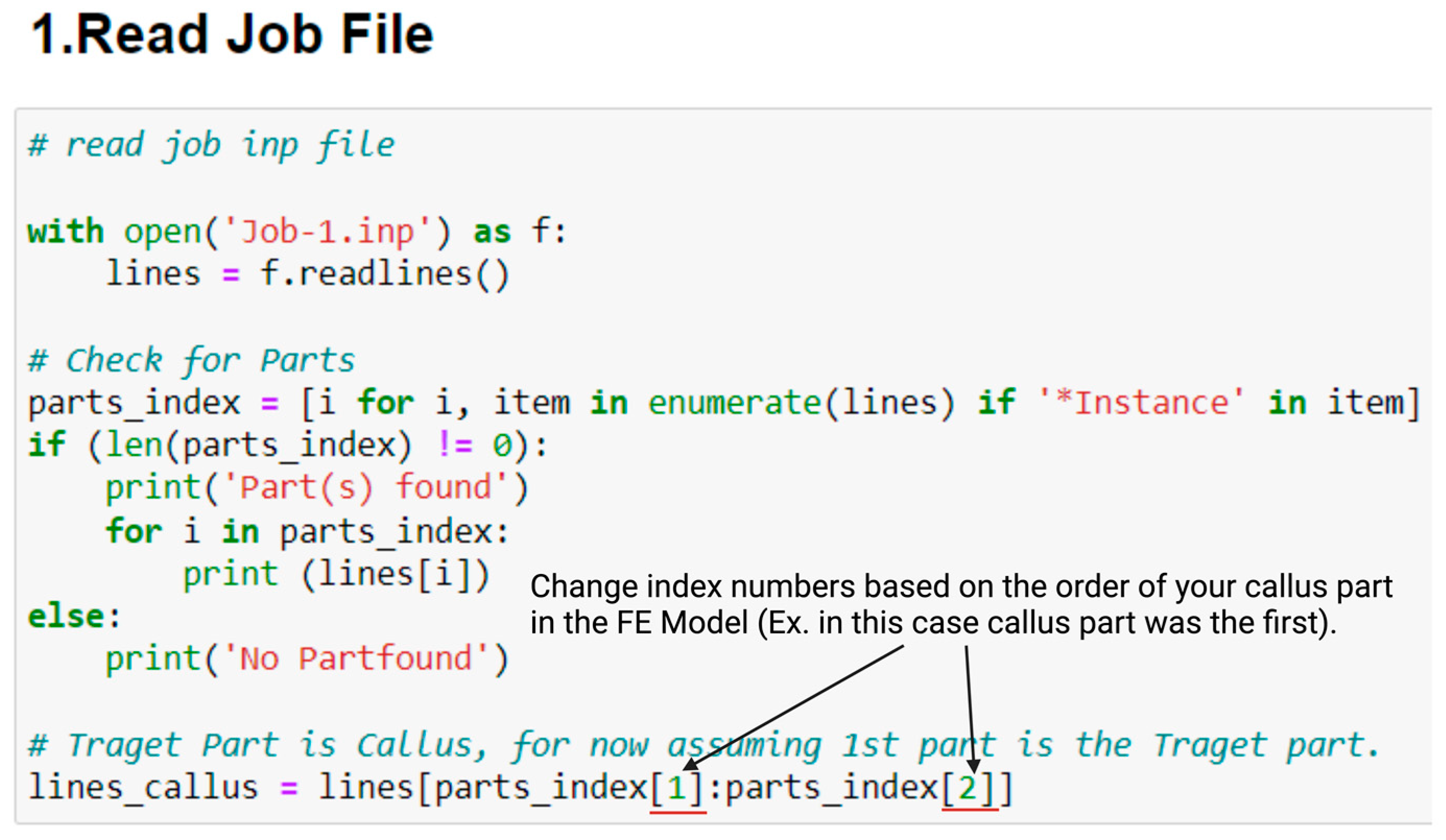
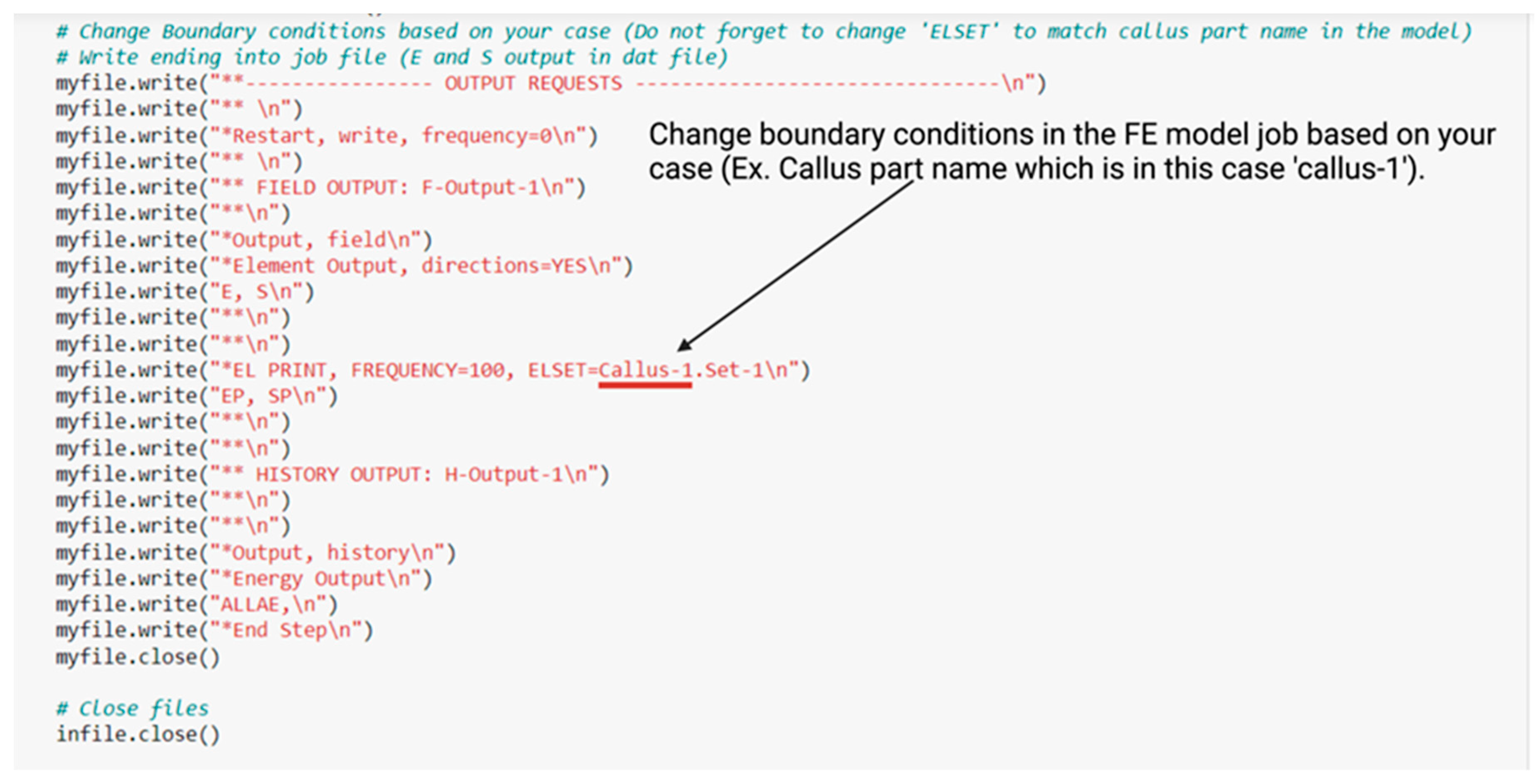
References
- Bone Fracture Facts|Osteo-Pharma. Available online: https://www.osteo-pharma.com/bone-fracture-facts/ (accessed on 29 November 2022).
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2017, 28, 351–362. [Google Scholar] [CrossRef]
- Cobos, J.A.; Lindsey, R.W.; Gugala, Z. The Cylindrical Titanium Mesh Cage for Treatment of a Long Bone Segmental Defect: Description of a New Technique and Report of Two Cases. J. Orthop. Trauma 2000, 14, 54–59. [Google Scholar] [CrossRef]
- Crovace, A.M.; Lacitignola, L.; Forleo, D.M.; Staffieri, F.; Francioso, E.; Di Meo, A.; Becerra, J.; Crovace, A.; Santos-Ruiz, L. 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals 2020, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Lopa, S.; Recordati, C.; Talò, G.; Turrisi, C.; Bottagisio, M.; Losa, M.; Scanziani, E.; Moretti, M. In Vivo Bone Formation within Engineered Hydroxyapatite Scaffolds in a Sheep Model. Calcif. Tissue Int. 2016, 99, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Cebon, D. Materials Selection in Mechanical Design. J. Phys. IV 1993, 3, C7-1. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A Review of the Mechanical Behavior of CaP and CaP/Polymer Composites for Applications in Bone Replacement and Repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Rodrigues, I.C.P.; Perez, A.G.M.; Souto, E.M.B.; Gabriel, L.P.; Webster, T. Scaffolds for Tissue Engineering: A State-of-the-Art Review Concerning Types, Properties, Materials, Processing, and Characterization. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer: Cham, Switzerland, 2020; pp. 647–676. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lv, X.; Zhang, M.; Huang, X.; Shi, Y.; Lu, C.; Song, J.; Yang, H. Bioinspired Mineral–Organic Bone Adhesives for Stable Fracture Fixation and Accelerated Bone Regeneration. Adv. Funct. Mater. 2020, 30, 1908381. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Carter, A.; Popowski, K.; Cheng, K.; Greenbaum, A.; Ligler, F.S.; Moatti, A. Enhancement of Bone Regeneration through the Converse Piezoelectric Effect, A Novel Approach for Applying Mechanical Stimulation. Bioelectricity 2021, 3, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Bisht, B.; Hope, A.; Mukherjee, A.; Paul, M.K. Advances in the Fabrication of Scaffold and 3D Printing of Biomimetic Bone Graft. Ann. Biomed. Eng. 2021, 49, 1128–1150. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Y.; Francis, L.F. Porous Polymer/Bioactive Glass Composites for Soft-to-Hard Tissue Interfaces. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 61, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Walinska, K.; Iwan, A.; Gorna, K.; Gogolewski, S. The Use of Long-Chain Plant Polyprenols as a Means to Modify the Biological Properties of New Biodegradable Polyurethane Scaffolds for Tissue Engineering. A Pilot Study. J. Mater. Sci. Mater. Med. 2008, 19, 129–135. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Wu, H.; Lù, Z.; Deng, C.; Hong, Z.; Jing, X.; Chen, X. RGD-Conjugated Copolymer Incorporated into Composite of Poly(Lactide-Co-Glycotide) and Poly(l-Lactide)-Grafted Nanohydroxyapatite for Bone Tissue Engineering. Biomacromolecules 2011, 12, 2667–2680. [Google Scholar] [CrossRef] [PubMed]
- Beşkardeş, I.G.; Aydın, G.; Bektaş, Ş.; Cengiz, A.; Gümüşderelioğlu, M. A Systematic Study for Optimal Cell Seeding and Culture Conditions in a Perfusion Mode Bone-Tissue Bioreactor. Biochem. Eng. J. 2018, 132, 100–111. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S. Cell Culture-Based Models for Intestinal Permeability: A Critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Li, Q. Microstructure Design of Biodegradable Scaffold and Its Effect on Tissue Regeneration. Biomaterials 2011, 32, 5003–5014. [Google Scholar] [CrossRef]
- Lu, H.Z.; Ma, H.W.; Luo, X.; Wang, Y.; Wang, J.; Lupoi, R.; Yin, S.; Yang, C. Microstructure, Shape Memory Properties, and in Vitro Biocompatibility of Porous NiTi Scaffolds Fabricated via Selective Laser Melting. J. Mater. Res. Technol. 2021, 15, 6797–6812. [Google Scholar] [CrossRef]
- Neurohr, A.J.; Dunand, D.C. Shape-Memory NiTi with Two-Dimensional Networks of Micro-Channels. Acta Biomater. 2011, 7, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Maliha, S.G.; Lopez, C.D.; Coelho, P.G.; Witek, L.; Cox, M.; Meskin, A.; Rusi, S.; Torroni, A.; Cronstein, B.N.; Flores, R.L. Bone Tissue Engineering in the Growing Calvaria Using Dipyridamole-Coated, Three-Dimensionally-Printed Bioceramic Scaffolds: Construct Optimization and Effects on Cranial Suture Patency. Plast. Reconstr. Surg. 2020, 145, 337e–347e. [Google Scholar] [CrossRef] [PubMed]
- Wieding, J.; Fritsche, A.; Heinl, P.; Körner, C.; Cornelsen, M.; Seitz, H.; Mittelmeier, W.; Bader, R. Biomechanical Behavior of Bone Scaffolds Made of Additive Manufactured Tricalciumphosphate and Titanium Alloy under Different Loading Conditions. J. Appl. Biomater. Funct. Mater. 2013, 11, 159–166. [Google Scholar] [CrossRef]
- Wieding, J.; Lindner, T.; Bergschmidt, P.; Bader, R. Biomechanical Stability of Novel Mechanically Adapted Open-Porous Titanium Scaffolds in Metatarsal Bone Defects of Sheep. Biomaterials 2015, 46, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, P.; Yilmaz, G.; Onal, M.B.; Solmaz, I.; Gundogdu, S.; Keskil, S.; Sousa, R.A.; Reis, R.L.; Hasirci, N.; Hasirci, V. An in Vivo Study on the Effect of Scaffold Geometry and Growth Factor Release on the Healing of Bone Defects. J. Tissue Eng. Regen. Med. 2012, 7, 687–696. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Yoon, D.M.; Henslee, A.M.; Nair, M.B.; Smid, C.; Kasper, F.K.; Tasciotti, E.; Mikos, A.G.; Decuzzi, P.; Ferrari, M. Shaping the Micromechanical Behavior of Multi-Phase Composites for Bone Tissue Engineering. Acta Biomater. 2010, 6, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, J.; Ma, X.; Ma, Y.; Kan, C.; Ma, H.; Li, Y.; Yuan, Y.; Liu, C. β-Tricalcium Phosphate/Poly(Glycerol Sebacate) Scaffolds with Robust Mechanical Property for Bone Tissue Engineering. Mater. Sci. Eng. C 2015, 56, 37–47. [Google Scholar] [CrossRef]
- Reichert, J.C.; Wullschleger, M.E.; Cipitria, A.; Lienau, J.; Cheng, T.K.; Schütz, M.A.; Duda, G.N.; Nöth, U.; Eulert, J.; Hutmacher, D.W. Custom-Made Composite Scaffolds for Segmental Defect Repair in Long Bones. Int. Orthop. 2011, 35, 1229–1236. [Google Scholar] [CrossRef]
- Dutta Roy, T.; Simon, J.L.; Ricci, J.L.; Dianne Rekow, E.; Thompson, V.P.; Russell Parsons, J. Performance of Hydroxyapatite Bone Repair Scaffolds Created via Three-Dimensional Fabrication Techniques. J. Biomed. Mater. Res. Part A 2003, 67, 1228–1237. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Duda, G.N.; Checa, S. Initial Mechanical Conditions within an Optimized Bone Scaffold Do Not Ensure Bone Regeneration—An in Silico Analysis. Biomech. Model. Mechanobiol. 2021, 20, 1723–1731. [Google Scholar] [CrossRef]
- De Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Kruse Gujer, A.; Grätz, K.W.; Weber, F.E. Bone Regeneration by the Osteoconductivity of Porous Titanium Implants Manufactured by Selective Laser Melting: A Histological and Micro Computed Tomography Study in the Rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical Properties of Bioactive Glass (13-93) Scaffolds Fabricated by Robotic Deposition for Structural Bone Repair. Acta Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef]
- Li, C.; Yan, T.; Lou, Z.; Jiang, Z.; Shi, Z.; Chen, Q.; Gong, Z.; Wang, B. Characterization and in Vitro Assessment of Three-Dimensional Extrusion Mg-Sr Codoped SiO2-Complexed Porous Microhydroxyapatite Whisker Scaffolds for Biomedical Engineering. Biomed. Eng. Online 2021, 20, 116. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Simon, C.G. Self-Hardening Calcium Phosphate Composite Scaffold for Bone Tissue Engineering. J. Orthop. Res. 2004, 22, 543. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Zhu, Y.; Friis, T.; Xiao, Y. Structure-Property Relationships of Silk-Modified Mesoporous Bioglass Scaffolds. Biomaterials 2010, 31, 3429–3438. [Google Scholar] [CrossRef]
- Killeen, D.; Frydrych, M.; Chen, B. Porous Poly(Vinyl Alcohol)/Sepiolite Bone Scaffolds: Preparation, Structure and Mechanical Properties. Mater. Sci. Eng. C 2012, 32, 749–757. [Google Scholar] [CrossRef]
- Penk, A.; Förster, Y.; Scheidt, H.A.; Nimptsch, A.; Hacker, M.C.; Schulz-Siegmund, M.; Ahnert, P.; Schiller, J.; Rammelt, S.; Huster, D. The Pore Size of PLGA Bone Implants Determines the de Novo Formation of Bone Tissue in Tibial Head Defects in Rats. Magn. Reson. Med. 2013, 70, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.E.; Heigele, C.A. Magnitudes of Local Stress and Strain along Bony Surfaces Predict the Course and Type of Fracture Healing. J. Biomech. 1999, 32, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P.J.; Huiskes, R.; Søballe, K. Biophysical Stimuli on Cells during Tissue Differentiation at Implant Interfaces. J. Biomech. 1997, 30, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Checa, S.; Prendergast, P.J. A Mechanobiological Model for Tissue Differentiation That Includes Angiogenesis: A Lattice-Based Modeling Approach. Ann. Biomed. Eng. 2009, 37, 129–145. [Google Scholar] [CrossRef]
- Isaksson, H.; van Donkellar, C.C.; Huiskes, R.; Ito, K. Corroboration of Mechanoregulatory Algorithms for Tissue Differentiation during Fracture Healing: Comparison with in Vivo Results. J. Orthop. Res. 2006, 24, 898–907. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. A Mathematical Model for Bone Tissue Regeneration inside a Specific Type of Scaffold. Biomech. Model. Mechanobiol. 2007, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Perier-Metz, C.; Cipitria, A.; Hutmacher, D.W.; Duda, G.N.; Checa, S. An in Silico Model Predicts the Impact of Scaffold Design in Large Bone Defect Regeneration. Acta Biomater. 2022, 145, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Poh, P.S.P.; Duda, G.N.; Checa, S. PCL Strut-like Scaffolds Appear Superior to Gyroid in Terms of Bone Regeneration within a Long Bone Large Defect: An in Silico Study. Front. Bioeng. Biotechnol. 2022, 10, 995266. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fang, J.; Entezari, A.; Sun, G.; Swain, M.V.; Xu, Y.; Steven, G.P.; Li, Q. A Time-Dependent Mechanobiology-Based Topology Optimization to Enhance Bone Growth in Tissue Scaffolds. J. Biomech. 2021, 117, 110233. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.; Shanjani, Y.; Vlasea, M.; Toyserkani, E. On the Bio-Mechanical Properties of a Dual-Porous Osteochondral Scaffold. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Boston, MA, USA, 31 October–6 November 2008. [Google Scholar]
- Boccaccio, A.; Ballini, A.; Pappalettere, C.; Tullo, D.; Cantore, S.; Desiate, A.; Boccaccio, A.; Ballini, A. Finite Element Method (FEM), Mechanobiology and Biomimetic Scaffolds in Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 112–132. [Google Scholar] [CrossRef]
- Zhang, B.; Skelly, J.D.; Maalouf, J.R.; Ayers, D.C.; Song, J. Multifunctional Scaffolds for Facile Implantation, Spontaneous Fixation, and Accelerated Long Bone Regeneration in Rodents. Sci. Transl. Med. 2019, 11, 7411. [Google Scholar] [CrossRef] [PubMed]
- Checa, S.; Prendergast, P.J.; Duda, G.N. Inter-Species Investigation of the Mechano-Regulation of Bone Healing: Comparison of Secondary Bone Healing in Sheep and Rat. J. Biomech. 2011, 44, 1237–1245. [Google Scholar] [CrossRef]
- Barengolts, E.I.; Curry, D.J.; Bapna, M.S.; Kukreja, S.C. Calcified Tissue International Effects of Two Non-Endurance Exercise Protocols on Established Bone Loss in Ovariectomized Adult Rats. Calcif. Tissue Int. 1993, 52, 239–243. [Google Scholar] [CrossRef]
- Jimenez-Palomar, I.; Shipov, A.; Shahar, R.; Barber, A.H.; Bosia, F.; Genin, G.M. Mechanical Behavior of Osteoporotic Bone at Sub-Lamellar Length Scales. Front. Mater. 2015, 2, 9. [Google Scholar] [CrossRef]
- Zhang, B.; Filion, T.M.; Kutikov, A.B.; Song, J. Facile Stem Cell Delivery to Bone Grafts Enabled by Smart Shape Recovery and Stiffening of Degradable Synthetic Periosteal Membranes. Adv. Funct. Mater. 2017, 27, 1604784. [Google Scholar] [CrossRef]
- Jin, W.; Chu, P.K. Orthopedic Implants Introduction 425 Classification of Orthopedic Implants 425 Permanent Orthopedic Implants 425 Temporary Orthopedic Implants 426 Consideration of Orthopedic Implant Materials. Biomed. Sci. 2019, 425–439. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, N.; Kango, S.; Sharma, S. Developments of PEEK (Polyetheretherketone) as a Biomedical Material: A Focused Review. Eur. Polym. J. 2021, 147, 110295. [Google Scholar] [CrossRef]
- Iwata, H.; Sakano, S.; Itoh, T.; Bauer, T.W. Demineralized Bone Matrix and Native Bone Morphogenetic Protein in Orthopaedic Surgery. Clin. Orthop. Relat. Res. 2002, 395, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Fan, W.; Crawford, R.; Xiao, Y. Structural and Cellular Differences between Metaphyseal and Diaphyseal Periosteum in Different Aged Rats. Bone 2007, 42, 81–89. [Google Scholar] [CrossRef]
- Postigo, S.; Schmidt, H.; Rohlmann, A.; Putzier, M.; Simón, A.; Duda, G.; Checa, S. Investigation of Different Cage Designs and Mechano-Regulation Algorithms in the Lumbar Interbody Fusion Process—A Finite Element Analysis. J. Biomech. 2014, 47, 1514–1519. [Google Scholar] [CrossRef]
- Skes, R.H.; Van Driel, W.D.; Prendergast, P.J.; Søballe, K. A Biomechanical Regulatory Model for Periprosthetic Fibrous-Tissue Differentiation. J. Mater. Sci. Mater. Med. 1997, 8, 785–788. [Google Scholar]
- Appeddu, P.A.; Shur, B.D. Molecular Analysis of Cell Surface Beta-1,4-Galactosyltransferase Function during Cell Migration. Proc. Natl. Acad. Sci. USA 1994, 91, 2095–2099. [Google Scholar] [CrossRef]
- Isaksson, H.; Van Donkelaar, C.; Huiskes, R.; Ito, K. A Mechano-Regulatory Bone-Healing Model Incorporating Cell-Phenotype Specific Activity. J. Theor. Biol. 2008, 252, 230–246. [Google Scholar] [CrossRef]
- Wehner, T.; Wolfram, U.; Henzler, T.; Niemeyer, F.; Claes, L.; Simon, U. Internal Forces and Moments in the Femur of the Rat during Gait. J. Biomech. 2010, 43, 2473–2479. [Google Scholar] [CrossRef]
- Mckinney, W. Pandas: A Foundational Python Library for Data Analysis and Statistics. Python High Perform. Sci. Comput. 2011, 14, 1–9. [Google Scholar]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D Printed Porous Ti6Al4V Implants with Different Pore Sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2015, 68, 355–369. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-Pore-Size Ti6Al4V Scaffolds with Different Pore Structures for Vascularized Bone Regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Asadi-Eydivand, M.; Abolfathi, N.; Chehrehsaz, Y.; Solati-Hashjin, M.; Negar, Z. The Effect of Pore Size and Layout on Mechanical and Biological Properties of 3D-Printed Bone Scaffolds with Gradient Porosity. Polym. Compos. 2022, 44, 1343–1359. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in Pore Size Enhance the Osteogenic Differentiation of Human Mesenchymal Stromal Cells in Three-Dimensional Scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef]
- Shi, X.; Nommeots-Nomm, A.; Todd, N.M.; Devlin-Mullin, A.; Geng, H.; Lee, P.D.; Mitchell, C.A.; Jones, J.R. Bioactive Glass Scaffold Architectures Regulate Patterning of Bone Regeneration in Vivo. Appl. Mater. Today 2020, 20, 100770. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Chmidt-Bleek, K.; Windolf, M.; Tatai, A.A.; Roth, C.P.; Schaser, K.D.; et al. Mechanobiologically Optimized 3D Titanium-Mesh Scaffolds Enhance Bone Regeneration in Critical Segmental Defects in Sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Prendergast, P.J. Three-Dimensional Simulation of Fracture Repair in the Human Tibia. Comput. Methods Biomech. Biomed. Eng. 2002, 5, 369–376. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Duda, G.N.; Checa, S. Mechano-Biological Computer Model of Scaffold-Supported Bone Regeneration: Effect of Bone Graft and Scaffold Structure on Large Bone Defect Tissue Patterning. Front. Bioeng. Biotechnol. 2020, 8, 1245. [Google Scholar] [CrossRef] [PubMed]
- Naveiro, J.M.; Puértolas, S.; Rosell, J.; Hidalgo, A.; Ibarz, E.; Albareda, J.; Gracia, L. A New Approach for Initial Callus Growth during Fracture Healing in Long Bones. Comput. Methods Programs Biomed. 2021, 208, 106262. [Google Scholar] [CrossRef]
- Laschke, M.W.; Harder, Y.; Amon, M.; Martin, I.; Farhadi, J.; Ring, A.; Torio-Padron, N.; Schramm, R.; Rücker, M.; Junker, D.; et al. Angiogenesis in Tissue Engineering: Breathing Life into Constructed Tissue Substitutes. Tissue Eng. 2006, 12, 2093–2104. [Google Scholar] [CrossRef]
- Bienert, M. Angiogenesis in Bone Tissue Engineering. J. Stem Cell Res. Med. 2019, 4, 1–2. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef]
- Gan, A.W.T.; Puhaindran, M.E.; Pho, R.W.H. The Reconstruction of Large Bone Defects in the Upper Limb. Injury 2013, 44, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A Tissue Engineering Solution for Segmental Defect Regeneration in Load-Bearing Long Bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in Vitro Polymer Degradation of Polycaprolactone-Based Scaffolds: Accelerated versus Simulated Physiological Conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Modification of Poly(Lactic Acid) Films: Enhanced Wettability from Surface-Confined Photografting and Increased Degradation Rate due to an Artifact of the Photografting Process. Macromolecules 2004, 37, 9151–9159. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
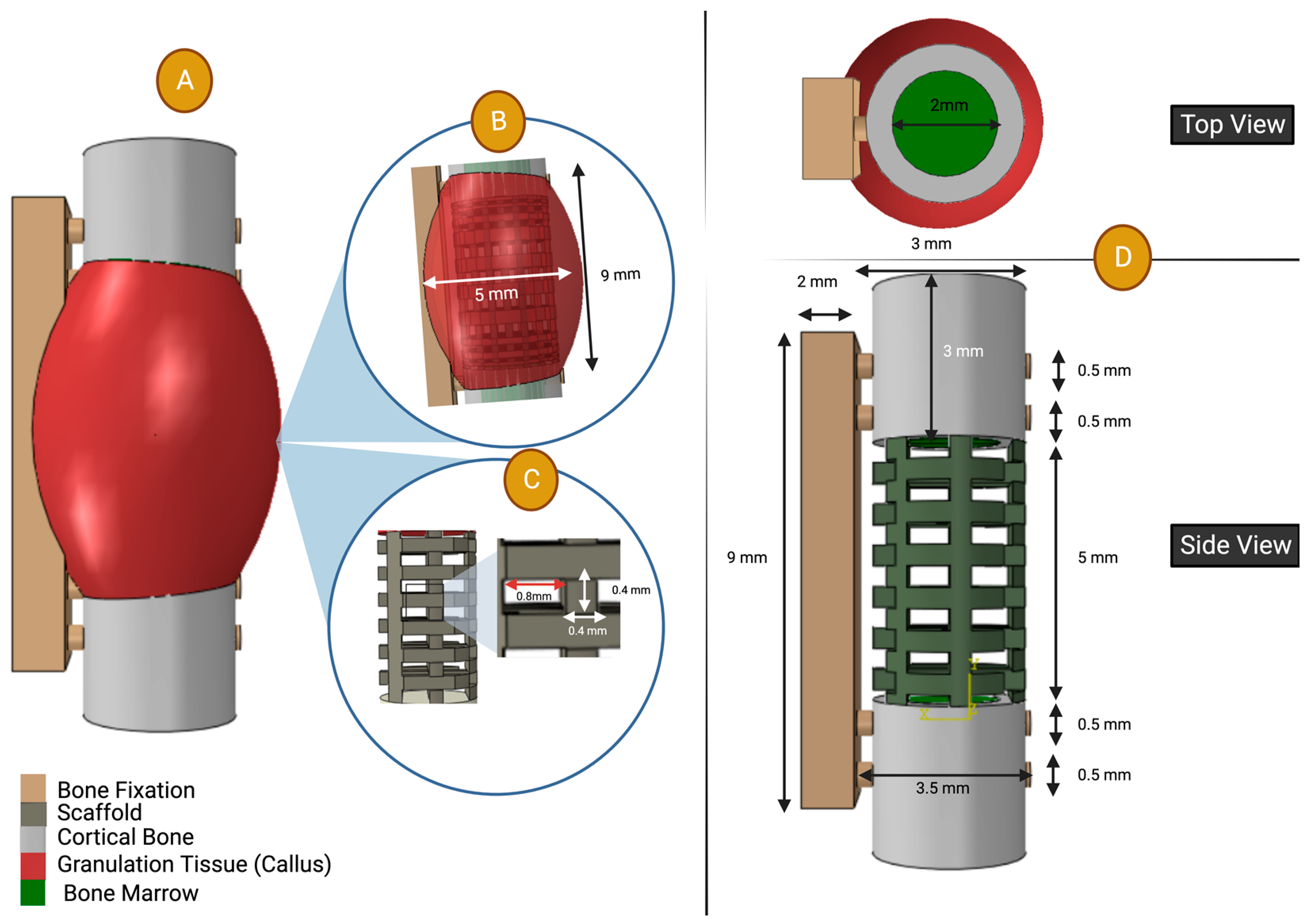


| Material | Young’s Modulus (MPa) | Poisson’s Ratio |
|---|---|---|
| Granulation tissue | 0.2 | 0.167 |
| Fibrous tissue | 2 | 0.167 |
| Cartilage | 10 | 0.3 |
| Cortical bone | 8000 | 0.3 |
| Bone marrow | 2 | 0.167 |
| HA-PELGA scaffold | 350 | 0.3 |
| Polyether-ether-ketone (PEEK) fixation | 3800 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, A.; Alabdah, F.; Wang, W.; Cooper, G. Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation. Polymers 2023, 15, 3918. https://doi.org/10.3390/polym15193918
Alshammari A, Alabdah F, Wang W, Cooper G. Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation. Polymers. 2023; 15(19):3918. https://doi.org/10.3390/polym15193918
Chicago/Turabian StyleAlshammari, Adel, Fahad Alabdah, Weiguang Wang, and Glen Cooper. 2023. "Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation" Polymers 15, no. 19: 3918. https://doi.org/10.3390/polym15193918
APA StyleAlshammari, A., Alabdah, F., Wang, W., & Cooper, G. (2023). Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation. Polymers, 15(19), 3918. https://doi.org/10.3390/polym15193918








