Possibilities of Influencing the Crystallization Process of Bisphenol A- and Bisphenol F-Based Epoxy Resins Used for Hydrophobic Coatings on Concrete
Abstract
:1. Introduction
2. Materials and Tested Formulations
2.1. Materials
2.1.1. Unmodified Epoxy Resins Based on Bisphenol A (DGEBA) and Bisphenol F (DGEBF)
2.1.2. Thixotropic Additive–Pyrogenic Silica (PS)
2.1.3. Fillers
- Baryte;
- Waste windshield glass;
- Silica flour Dorsilit.
2.1.4. Solvents
2.1.5. Nucleating Agents
2.1.6. Tested Formulations
3. Methods
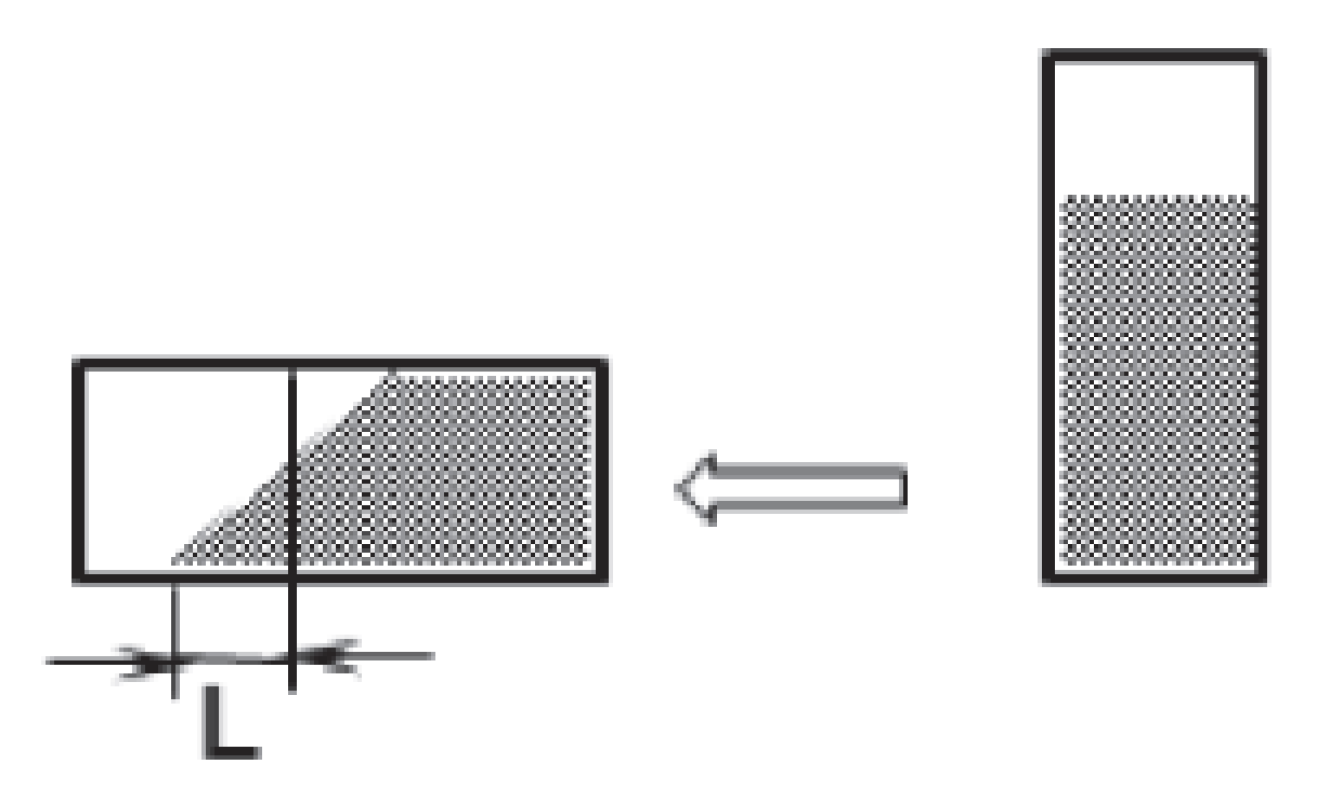
3.1. Determination of Tendency to Crystallize
- L ≥ 10 mm—the crystallization phase of the sample is evaluated by the letter “a”;
- L < 10 mm—the crystallization phase of the sample is evaluated by the letter “b”;
- L = 0 mm—the crystallization phase of the sample is evaluated by the letter “c”.
3.2. Determination of Crystallinity
3.3. DSC Analysis
3.4. Determination of Viscosity
3.5. Determination of Hardness Shore D
3.6. Abrasion Resistance
3.7. Glass Transition Temperature (Tg) Determined by DMA
3.8. Adhesion on Concrete
3.9. Tensile Properties
3.10. Determination of Chemical Resistance
4. Results and Discussion
4.1. Determination of Tendency to Crystallize
4.2. Determination of Crystallinity
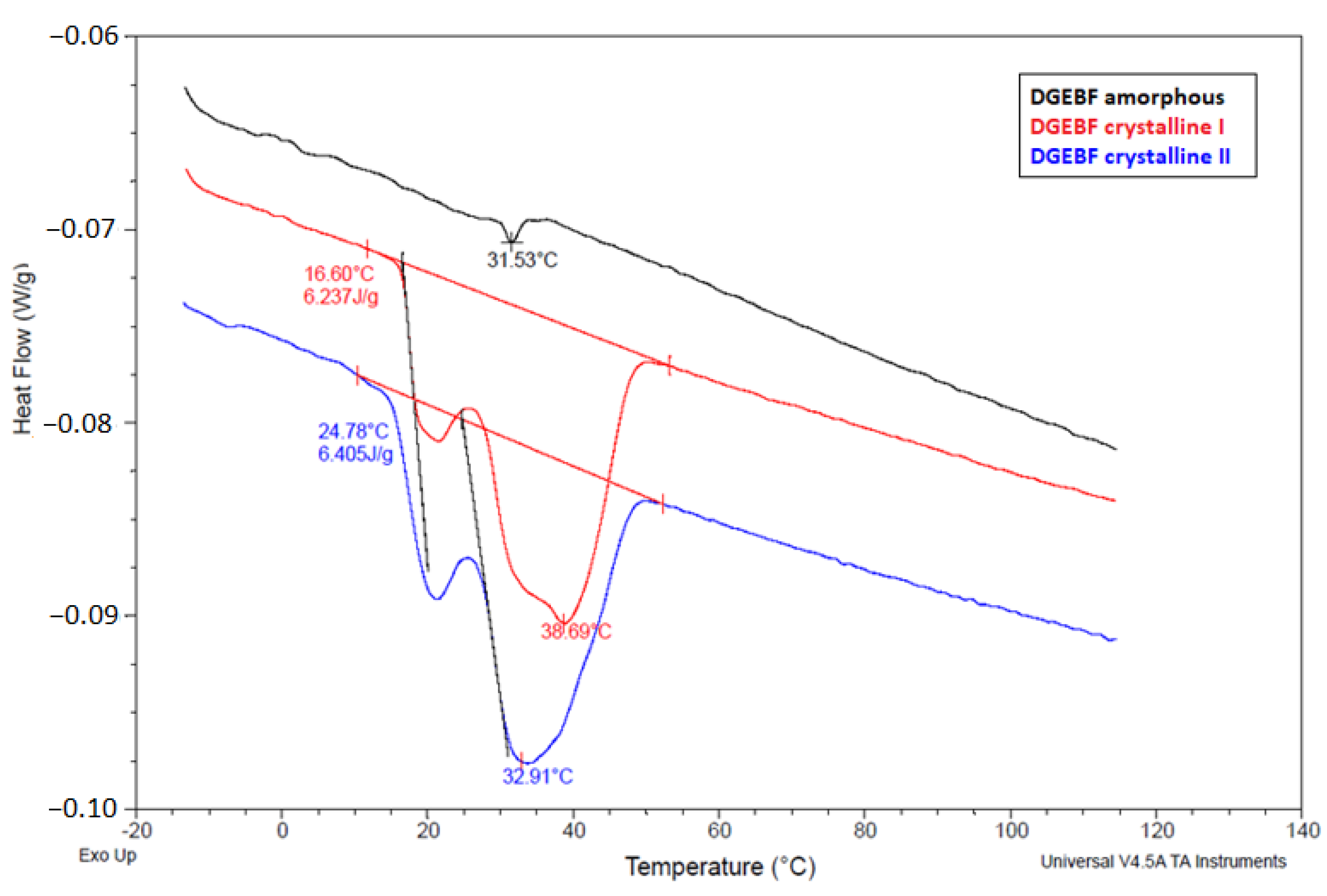
4.3. Determination of Crystallization Temperature by DSC Method
4.4. Determination of Viscosity
4.5. Determination of Hardness Shore D
4.6. Abrasion Resistance
4.7. Glass Transition Temperature Determined by DMA
4.8. Adhesion on Concrete
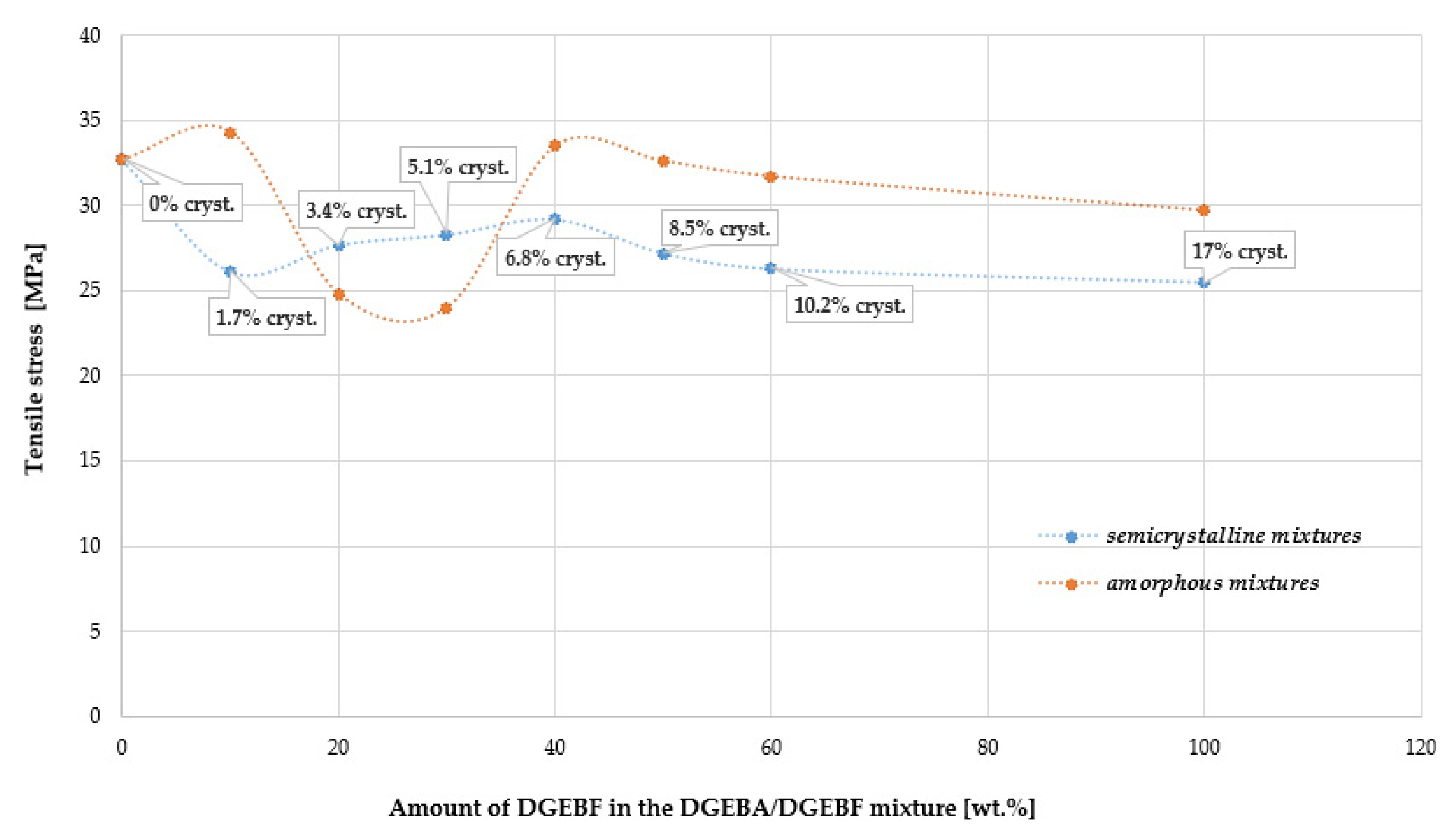
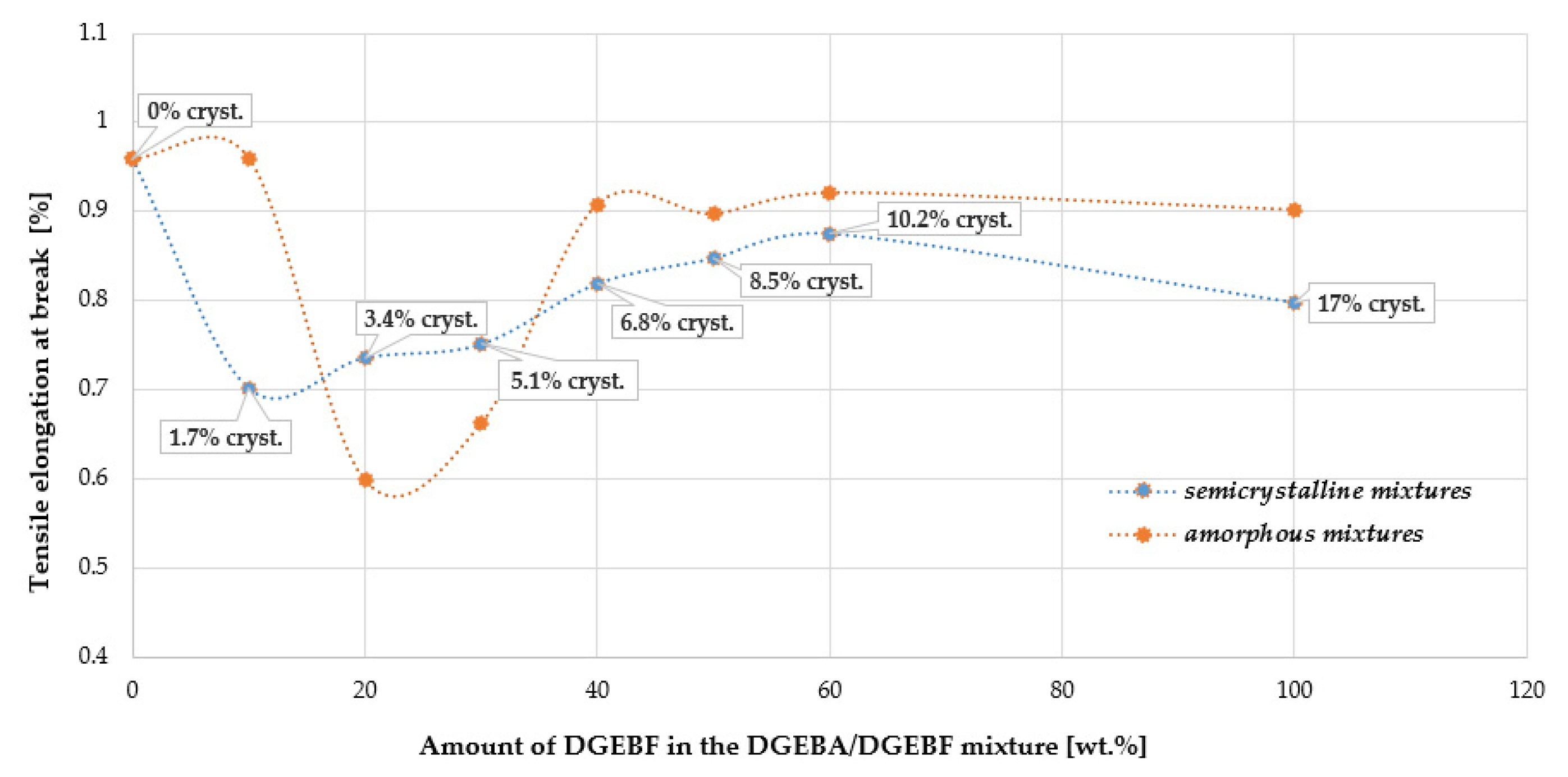
4.9. Tensile Properties
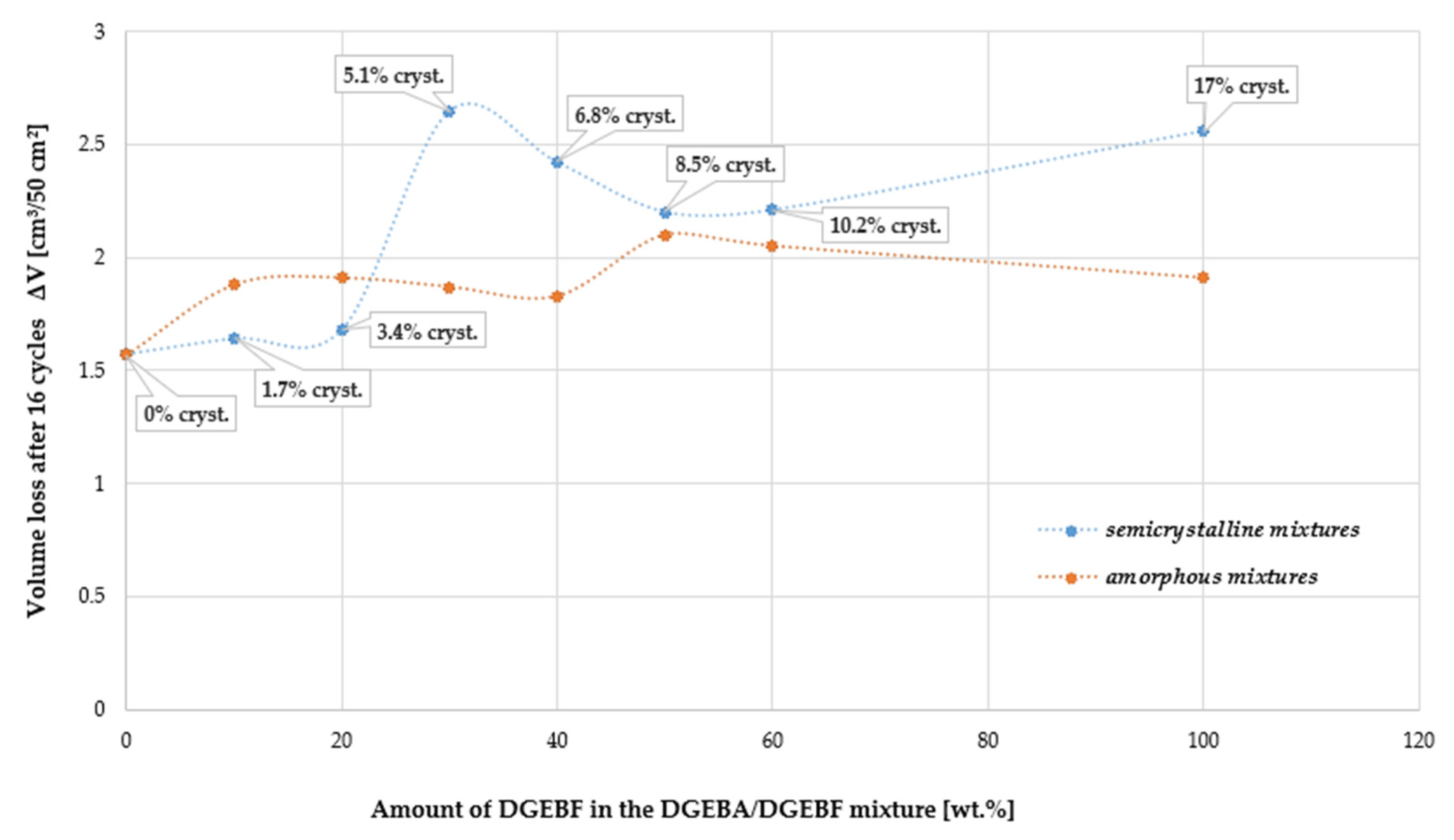
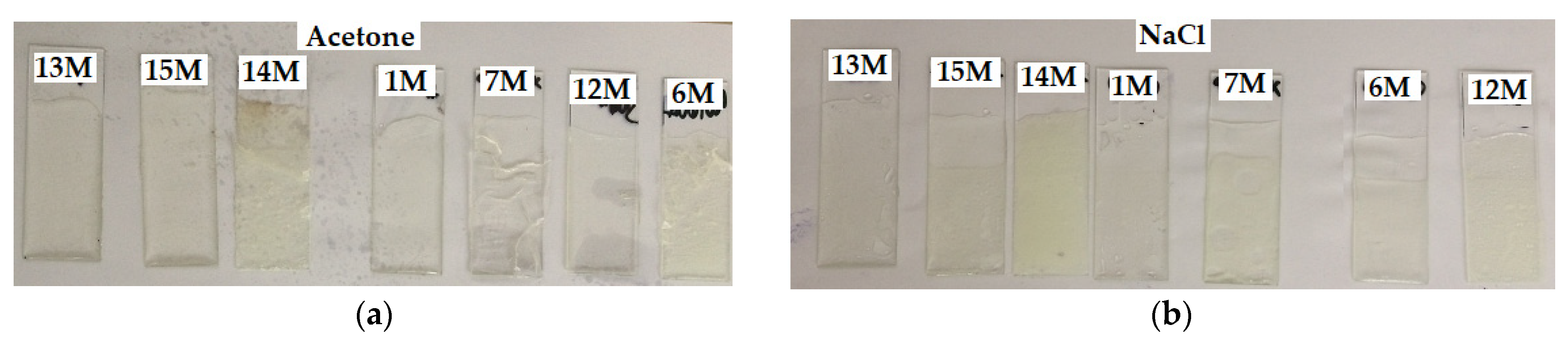
4.10. Chemical Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.C.; Guo, B.-H.; Xu, J. A Review on polymer crystallisation theories. Crystals 2017, 7, 4. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Silvestre, C. Non-isothermal crystallisation of polymers. Prog. Polym. Sci. 1999, 24, 919–950. [Google Scholar] [CrossRef]
- Ziabicki, A. Crystallisation of polymers in variable external conditions. Colloid. Polym. Sci. 1996, 274, 705–716. [Google Scholar] [CrossRef]
- Lotz, B.; Miyoshi, T.; Cheng, S.Z.D. 50th anniversary perspective: Polymer crystals and crystallisation: Personal journeys in a challenging Research field. Macromolecules 2017, 50, 5995–6025. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Prediction of PEKK properties related to crystallisation by molecular dynamics simulations with a united-atom model. Polymer 2019, 174, 25–32. [Google Scholar] [CrossRef]
- Guo, Q.; Groeninckx, G. Crystallisation kinetics of poly(ε-caprolactone) in miscible thermosetting polymer blends of epoxy resin and poly(ε-caprolactone). Polymer 2001, 42, 8647–8655. [Google Scholar] [CrossRef]
- Kulshreshtha, B.; Ghosh, A.K.; Misra, A. Crystallisation kinetics and morphological behavior of reactively processed PBT/epoxy blends. Polymer 2003, 44, 4723–4734. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, S.; Guo, Q. Thermosetting polymer blends of unsaturated polyester resin and poly(ethylene oxide). I. Miscibility and thermal properties. J. Polym. Sci. A Polym Chem. 1997, 35, 3161–3168. [Google Scholar] [CrossRef]
- Zhong, Z.; Guo, Q. The miscibility and morphology of hexamine cross-linked novolac/poly(epsilon-caprolactone) blends. Polymer 1997, 38, 279–286. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, H.; Guo, Q. Epoxy resin/poly(ϵ-caprolactone) blends cured with 2,2-bis[4-(4-aminophenoxy)phenyl]propane. I. Miscibility and crystallisation kinetics. J. Polym. Sci. B Polym. Phys. 2003, 41, 1085–1098. [Google Scholar] [CrossRef]
- Resinlab, An Ellsworth adhesives company. Available online: https://www.resinlab.com/resources/resin-crystallisation/ (accessed on 22 June 2023).
- Martuscelli, E. Influence of composition, crystallisation conditions and melt phase structure on solid morphology, kinetics of crystallisation and thermal behavior of binary polymer/polymer blends. Polym. Eng. Sci. 1984, 24, 563–586. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Influence of processing conditions on polymer crystallisation measured by fast scanning DSC. J. Therm. Anal. Calorim. 2013, 116, 1165–1173. [Google Scholar] [CrossRef]
- Menczel, J.; Varga, J. Influence of nucleating agents on crystallisation of polypropylene I. Talc as a nucleating agent. J. Therm. Anal. Calorim. 2006, 28, 161–174. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G.; Nagarajan, S. Dendritic polymer spherulites: Birefringence correlating with lamellae assembly and origins of superimposed ring bands. J. Polym. Res. 2019, 27, 7. [Google Scholar] [CrossRef]
- Norton, D.R.; Keller, A. The spherulitic and lamellar morphology of melt-crystallised isotactic polypropylene. Polymer 1985, 27, 704–716. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Y.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete—Part II: Characteristics and performance. Cem. Conr. Compos. 2012, 34, 313–321. [Google Scholar] [CrossRef]
- Schueremans, L.; Van Gemert, D.; Giessler, S. Chloride penetration in RC-structures in marine environment—Long term assessment of a preventive hydrophobic treatment. Constr. Build. Mater. 2007, 21, 1238–1249. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Sprenger, S. Epoxy resin composites with surface-modified silicon dioxide nanoparticles: A review. J. Appl. Polym. Sci. 2013, 130, 1421–1428. [Google Scholar] [CrossRef]
- Barbosa, A.Q.; da Silva, L.F.M.; Abenojar, J.; Figueiredo, M.; Öchsner, A. Toughness of a brittle epoxy resin reinforced with micro cork particles: Effect of size, amount and surface treatment. Compos. Part B Eng. 2017, 114, 299–310. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Desai, S. Nanofiller-reinforced polymer nanocomposites. Polym. Adv. Technol. 2010, 19, 947–959. [Google Scholar] [CrossRef]
- PalmerHolland, Technical Data Sheet of DER 331 Liquid Epoxy Resin. Available online: https://www.palmerholland.com/All-Products/DER-331 (accessed on 22 June 2023).
- PalmerHolland, Technical Data Sheet of DER 354 Liquid Epoxy Resin. Available online: https://www.palmerholland.com/All-Products/DER-354 (accessed on 22 June 2023).
- Tang, J.; Bai, Y.; Chen, X. An investigation of inorganic fillers on rheological properties and tensile strength of epoxy repair coating using mixture design method. Constr. Build. Mater. 2021, 307, 124866. [Google Scholar] [CrossRef]
- EVONIK, Leading Beyond Chemistry, Product Overview of AEROSIL 200 Hydrophilic Fumed Silica. Available online: https://www.silica-specialist.com/en/product/PR_52000005?page=1&orderBy=Name%20asc&c[id]=en-us&c[name]=en_US (accessed on 22 June 2023).
- EN ISO 4895; Plastics—Plastics Liquid Epoxy Resins—Determination of Tendency to Crystallise. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC61/SC12: Geneva, Switzerland, 2014.
- Leicy, D.; Hogg, P.J. The Effect of crystallinity on the impact properties of advanced thermoplastic composites. In Developments in the Science and Technology of Composite Materials; Bunsell, A.R., Lamicq, P., Massiah, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Richards, R.B. Polyethylene-structure, crystallinity and properties. J. Appl. Chem. 1951, 1, 370–376. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Y.; Li, L.; Wang, P.; Yang, Q.; Li, G. Mechanical properties, rheology, and crystallisation of epoxy-resin-compatibilized polyamide 6/polycarbonate blends: Effect of mixing sequences. J. Macromol. Sci. B Phys. 2012, 51, 96–108. [Google Scholar] [CrossRef]
- EN ISO 2884-2; Paints and Varnishes—Determination of Viscosity Using Rotary Viscometers—Part 2: Disc or Ball Viscometer Operated at a Specified Speed. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC35/SC9: Geneva, Switzerland, 2003.
- EN ISO 868; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC61/SC2: Geneva, Switzerland, 2003.
- EN 13892-3; Methods of Test for Screed Materials—Part 3: Determination of Wear Resistance—Böhme. European Committee for Standardization (CEN): Brussels, Belgium, 2014.
- EN ISO 4624; Plastics—Paints and Varnishes—Pull-Off Test for Adhesion. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC35/SC9: Geneva, Switzerland, 2016.
- EN ISO 527-1; Plastics—Determination of Tensile Properties—Part 1: General Principles. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC61/SC2: Geneva, Switzerland, 2019.
- EN ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC61/SC2: Geneva, Switzerland, 2012.
- Zhang, J.; Xu, Y.C.; Huang, P. Effect of cure cycle on curing process and hardness for epoxy resin. Express Polym. Lett. 2009, 3, 534–541. [Google Scholar] [CrossRef]
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Startsev, O.V.; Vapirov, Y.M.; Lebedev, M.P.; Kychkin, A.K. Comparison of Glass-Transition Temperatures for Epoxy Polymers Obtained by Methods of Thermal Analysis. Mech. Compos. Mater. 2020, 56, 227–240. [Google Scholar] [CrossRef]
- Ratna, D. Modification of epoxy resins for improvement of adhesion: A critical review. J. Adhes. Sci. Technol. 2003, 17, 1655–1668. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Del Rosario, G.; Ureña, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- Punchaipetch, P.; Ambrogi, V.; Giamberini, M.; Brostow, W.; Carfagna, C.; D’Souza, N.A. Epoxy+liquid crystalline epoxy coreacted networks: II. Mechanical properties. Polymer. 2002, 43, 839–848. [Google Scholar] [CrossRef]
- Singhal, R.; Nagpal, A.K. Thermal and chemical resistance behavior of cured epoxy based on diglycidyl ether of bisphenol-A and thiol treated liquid polysulfide blends. Adv. Mater. Lett. 2010, 1, 238–245. [Google Scholar] [CrossRef]







| Parameter | DGEBA | DGEBF |
|---|---|---|
| Epoxide equivalent weight (EEW) (g/eq) | 182–192 | 167–174 |
| Viscosity (25 °C) (mPa·s) | 11,000–14,000 | 3400–4200 |
| Density (25 °C) (g/cm3) | 1.16 | 1.19 |
| Hydrolysable chloride (ppm) | <500 | <1000 |
| Type of Filler | Producer | Max. Particle Size (μm) | Particle Content > 63 μm (%) | Specific Gravity (kg/L) |
|---|---|---|---|---|
| Baryte | Koltex Color | 63 | – | 4.5 |
| Waste windshield glass | – | 63 | – | 2.5 |
| Silica flour Dorsilit 1600 | Dorfner | 160 | 36 | 2.6 |
| Silica flour Dorsilit 12100 | Dorfner | 63 | – | 2.6 |
| Type of Solvent | Solvent | Viscosity at +25 °C (mPa·s) | Boiling /Flash Point (°C) | Density at +25 °C (g/cm3) |
|---|---|---|---|---|
| Reactive | Epilox P 13–18 | 4–12 | >200/142 | 0.89 |
| Reactive | Araldite DY-E | 5–10 | >200/142 | 0.89 |
| Non-reactive | Benzyl alcohol | 6.6 | 205/101 | 1.045 |
| Non-reactive | Methylisobutylketon | 0.6 | 117/16 | 0.802 |
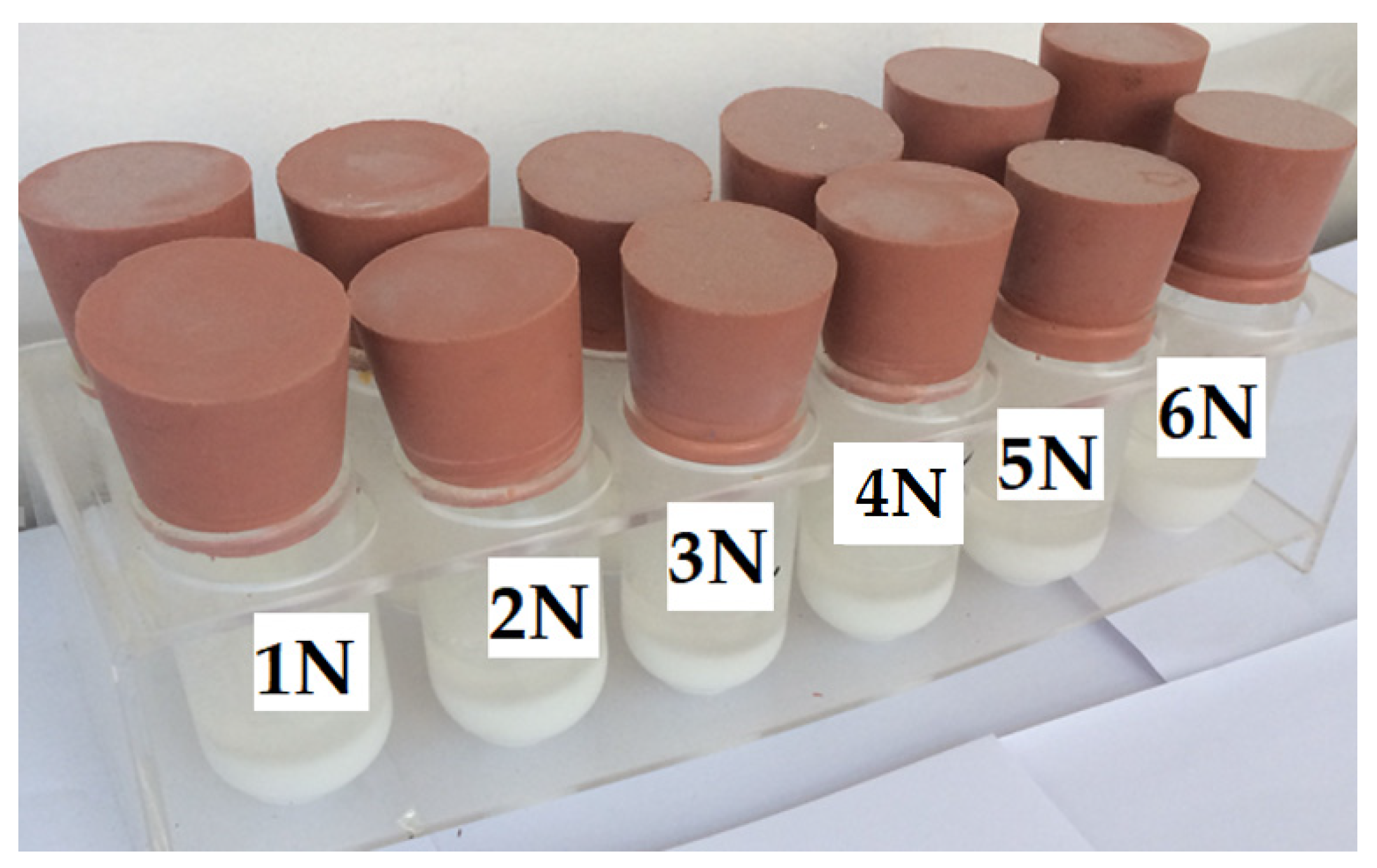
| Sample | Component | Amount (g) | Nucleating Agent | Amount (g) |
|---|---|---|---|---|
| 1 N | DGEBA | 20 | CaCO3 | 20 |
| Ethanol | 2 | |||
| 2 N | DGEBF | 20 | CaCO3 | 20 |
| Ethanol | 2 | |||
| 3 N | DGEBA | 14 | CaCO3 | 20 |
| Epilox P 13–18 | 6 | Ethanol | 2 | |
| 4 N | DGEBA | 14 | CaCO3 | 20 |
| Araldite DY-E | 6 | Ethanol | 2 | |
| 5 N | DGEBA | 14 | CaCO3 | 20 |
| benzyl alcohol | 6 | Ethanol | 2 | |
| 6 N | DGEBA | 14 | CaCO3 | 20 |
| methylisobutylketon | 6 | Ethanol | 2 | |
| 7 N | DGEBA | 19 | CaCO3 | 20 |
| PS 200 m2/g | 1 | Ethanol | 2 | |
| 8 N | DGEBA | 19 | CaCO3 | 20 |
| PS 380 m2/g | 1 | Ethanol | 2 | |
| 9 N | DGEBA | 10 | CaCO3 | 20 |
| windshield glass | 10 | Ethanol | 2 | |
| 10 N | DGEBA | 15 | CaCO3 | 20 |
| baryte | 5 | Ethanol | 2 | |
| 11 N | DGEBA | 10 | CaCO3 | 20 |
| silica flour 1600 | 10 | Ethanol | 2 | |
| 12 N | DGEBA | 15 | CaCO3 | 20 |
| silica flour 12,100 | 5 | Ethanol | 2 |
| Sample | Components | Amount (g) |
|---|---|---|
| 1 M | DGEBA amorphous | 180 |
| DGEBF amorphous | 20 | |
| Isophoronediamine-based hardener | 102 | |
| 2 M | DGEBA amorphous | 160 |
| DGEBF amorphous | 40 | |
| Isophoronediamine-based hardener | 103 | |
| 3 M | DGEBA amorphous | 140 |
| DGEBF amorphous | 60 | |
| Isophoronediamine-based hardener | 103 | |
| 4 M | DGEBA amorphous | 120 |
| DGEBF amorphous | 80 | |
| Isophoronediamine-based hardener | 104 | |
| 5 M | DGEBA amorphous | 100 |
| DGEBF amorphous | 100 | |
| Isophoronediamine-based hardener | 105 | |
| 6 M | DGEBA amorphous | 80 |
| DGEBF amorphous | 120 | |
| Isophoronediamine-based hardener | 106 | |
| 7 M | DGEBA amorphous | 180 |
| DGEBF in the crystallization phase “c” | 20 | |
| Isophoronediamine-based hardener | 102 | |
| 8 M | DGEBA amorphous | 160 |
| DGEBF in the crystallization phase “c” | 40 | |
| Isophoronediamine-based hardener | 103 | |
| 9 M | DGEBA amorphous | 140 |
| DGEBF in the crystallization phase “c” | 60 | |
| Isophoronediamine-based hardener | 103 | |
| 10 M | DGEBA amorphous | 120 |
| DGEBF in the crystallization phase “c” | 80 | |
| Isophoronediamine-based hardener | 104 | |
| 11 M | DGEBA amorphous | 100 |
| DGEBF in the crystallization phase “c” | 100 | |
| Isophoronediamine-based hardener | 105 | |
| 12 M | DGEBA amorphous | 80 |
| DGEBF in the crystallization phase “c” | 120 | |
| Isophoronediamine-based hardener | 106 | |
| 13 M | DGEBA amorphous | 200 |
| Isophoronediamine-based hardener | 101 | |
| 14 M | DGEBF in the crystallization phase “c” | 200 |
| Isophoronediamine-based hardener | 109 | |
| 15 M | DGEBF amorphous | 200 |
| Isophoronediamine-based hardener | 109 |
| Type of Chemical | Concentration (%) |
|---|---|
| Acetone | 100 |
| Technical petrol | 100 |
| Distilled water | 100 |
| Beer | 100 |
| Wine | 100 |
| Aqueous NaCl solution (saturated) | 27 |
| Aqueous NaOH solution | 50 |
| Aqueous HCl solution | 35 |
| Aqueous H2SO4 solution | 50 |
| Aqueous solution of ethanol | 50 |
| Sample | 1/M 1–4/A 2 | 5/M–13/A | 14/M–19/A | 20/M–A | 21/A–25/A | 26/M–28/M | 28/A |
|---|---|---|---|---|---|---|---|
| 1 N | a | a | b | b | b | b | b |
| 2 N | a | b | b | c | c | c | c |
| 3 N | a | a | a | a | a | a | a |
| 4 N | a | a | a | a | a | a | a |
| 5 N | a | a | a | a | a | a | a |
| 6 N | a | a | a | a | a | a | a |
| 7 N | a | a | a | a | a | b | b |
| 8 N | a | a | a | a | b | b | b |
| 9 N | a | a | a | a | a | a | b |
| 10 N | a | a | a | a | a | a | b |
| 11 N | a | a | a | a | a | a | a |
| 12 N | a | a | a | a | a | a | b |
| The Crystalline Phase of the Sample | Specific Gravity (kg/L) | Calculated Crystallinity (%) |
|---|---|---|
| DGEBF amorphous | 1.1952 | – |
| DGEBF in the crystallization phase “c” | 1.1960 | 17 |
| DGEBF maximally spontaneously crystallized | 1.1999 | – |
| Sample/ Crystallinity | Time of Exposure (Days) | Acetone | Petrol | Distilled Water | Beer | Wine | NaCl Solution | 50% NaOH | 35% HCl | 50% H2SO4 | 50% Ethanol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 M/0 | 1 | +0.03 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.01 | +0.00 | +0.00 |
| 7 | N/A * | +0.00 | +0.05 | +0.01 | +0.02 | +0.00 | −0.02 | +0.06 | +0.02 | +1.25 | |
| 28 | N/A | +0.01 | +0.95 | +0.21 | +0.24 | +0.01 | −0.03 | +0.68 | +0.21 | +5.01 | |
| 6 M/0 | 1 | +0.05 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.02 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.13 | +0.05 | +0.09 | +0.00 | −0.03 | +0.08 | +0.07 | +2.02 | |
| 28 | N/A | +0.03 | +1.07 | +0.32 | +0.36 | +0.03 | −0.04 | +0.71 | +0.28 | +6.23 | |
| 7 M/1.7 | 1 | +0.06 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.02 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.07 | +0.02 | +0.02 | +0.00 | −0.03 | +0.08 | +0.04 | +1.33 | |
| 28 | N/A | +0.02 | +0.99 | +0.19 | +0.20 | +0.01 | −0.03 | +0.70 | +0.23 | +5.21 | |
| 12 M/10.2 | 1 | +0.06 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.02 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.21 | +0.07 | +0.11 | +0.00 | −0.04 | +0.11 | +0.10 | +3.84 | |
| 28 | N/A | +0.03 | +1.34 | +0.45 | +0.50 | +0.04 | −0.05 | +0.76 | +0.31 | +8.97 | |
| 13 M/0 | 1 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.02 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.04 | +0.02 | +0.02 | +0.00 | −0.01 | +0.07 | +0.02 | +1.18 | |
| 28 | N/A | +0.00 | +0.84 | +0.05 | +0.07 | +0.00 | −0.02 | +0.67 | +0.19 | +4.96 | |
| 14 M/17 | 1 | +0.11 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.04 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.53 | +0.32 | +0.37 | +0.00 | −0.06 | +0.11 | +0.14 | +5.67 | |
| 28 | N/A | +0.02 | +2.41 | +0.98 | +1.03 | +0.03 | −0.07 | +0.78 | +0.43 | +11.83 | |
| 15 M/0 | 1 | +0.06 | +0.00 | +0.00 | +0.00 | +0.00 | +0.00 | −0.00 | +0.02 | +0.00 | +0.00 |
| 7 | N/A | +0.00 | +0.23 | +0.12 | +0.09 | +0.00 | −0.03 | +0.07 | +0.09 | +2.46 | |
| 28 | N/A | +0.00 | +1.31 | +0.45 | +0.39 | +0.00 | −0.04 | +0.68 | +0.32 | +7.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidlová, M.; Hodul, J.; Žižková, N.; Borg, R.P. Possibilities of Influencing the Crystallization Process of Bisphenol A- and Bisphenol F-Based Epoxy Resins Used for Hydrophobic Coatings on Concrete. Polymers 2023, 15, 3871. https://doi.org/10.3390/polym15193871
Seidlová M, Hodul J, Žižková N, Borg RP. Possibilities of Influencing the Crystallization Process of Bisphenol A- and Bisphenol F-Based Epoxy Resins Used for Hydrophobic Coatings on Concrete. Polymers. 2023; 15(19):3871. https://doi.org/10.3390/polym15193871
Chicago/Turabian StyleSeidlová, Michaela, Jakub Hodul, Nikol Žižková, and Ruben Paul Borg. 2023. "Possibilities of Influencing the Crystallization Process of Bisphenol A- and Bisphenol F-Based Epoxy Resins Used for Hydrophobic Coatings on Concrete" Polymers 15, no. 19: 3871. https://doi.org/10.3390/polym15193871
APA StyleSeidlová, M., Hodul, J., Žižková, N., & Borg, R. P. (2023). Possibilities of Influencing the Crystallization Process of Bisphenol A- and Bisphenol F-Based Epoxy Resins Used for Hydrophobic Coatings on Concrete. Polymers, 15(19), 3871. https://doi.org/10.3390/polym15193871









