An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications
Abstract
:1. Introduction
2. Antibacterial Polymer Composite Materials
2.1. Metals-Incorporated Polymer Composites
2.2. Metal Oxide-Incorporated Polymer Composites
2.3. Carbon Derivates-Incorporated Polymer Composites
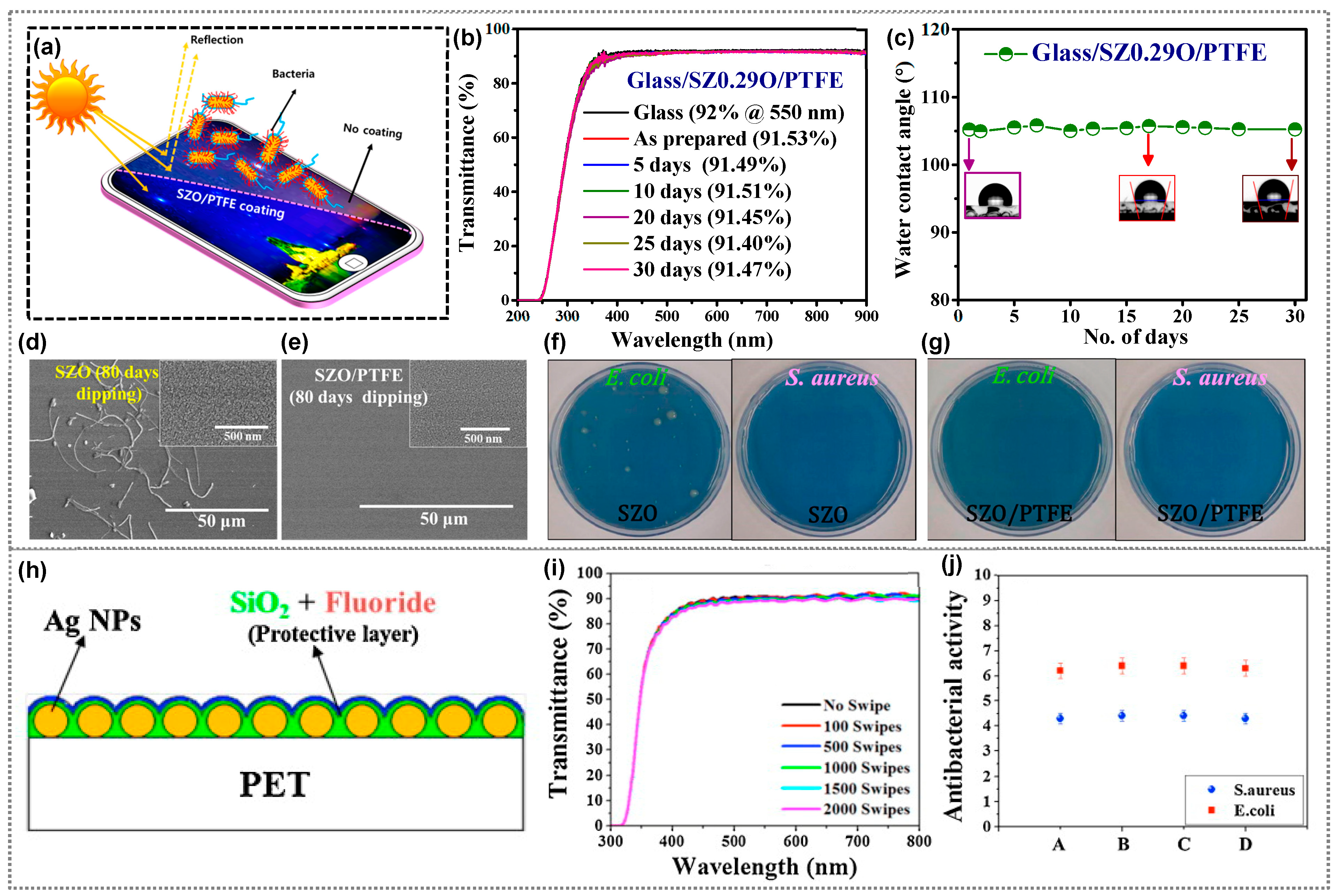
3. Antibacterial Polymer Composites for Display Coating Applications
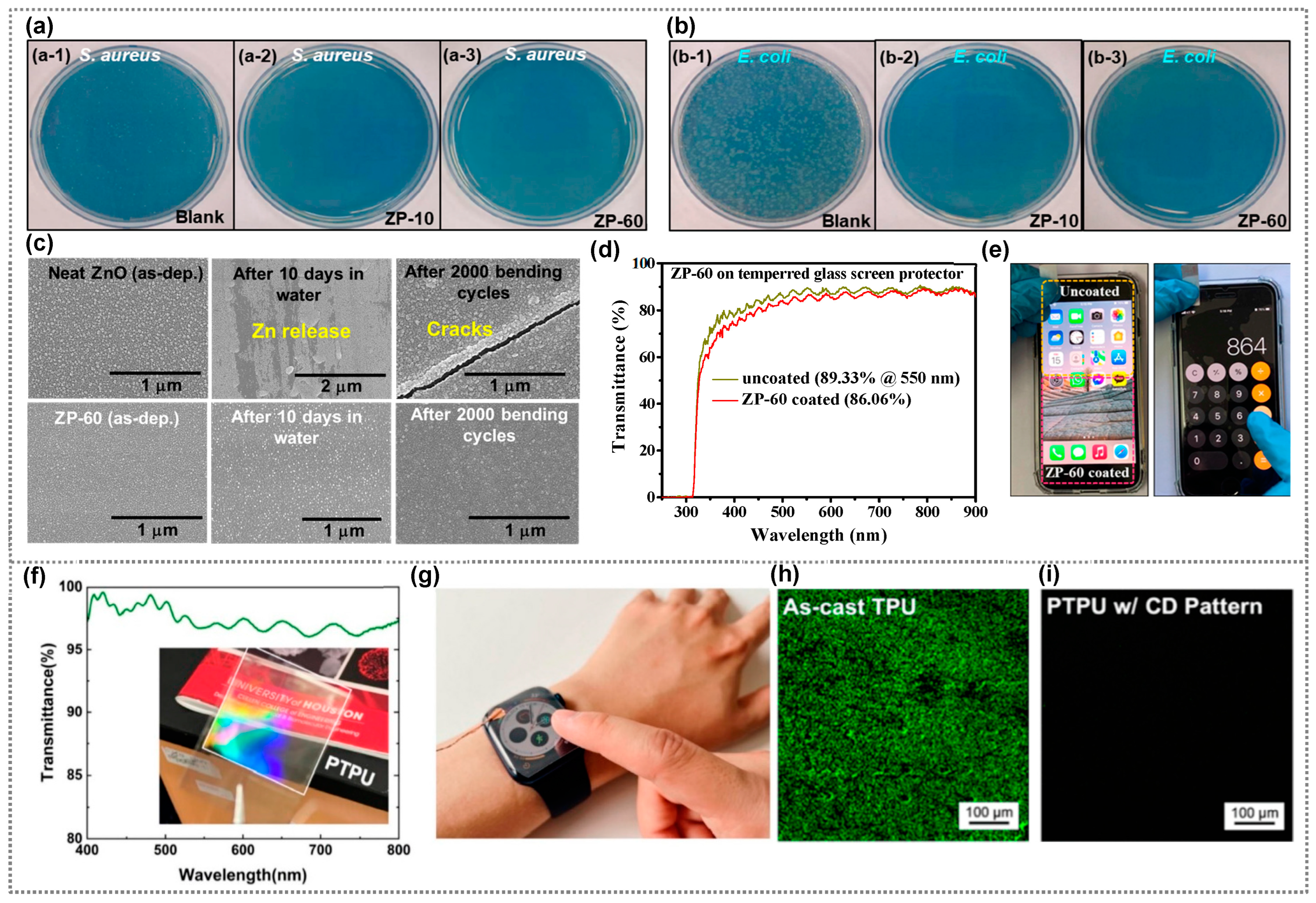
4. Antibacterial Polymer Composites for Sensor Applications
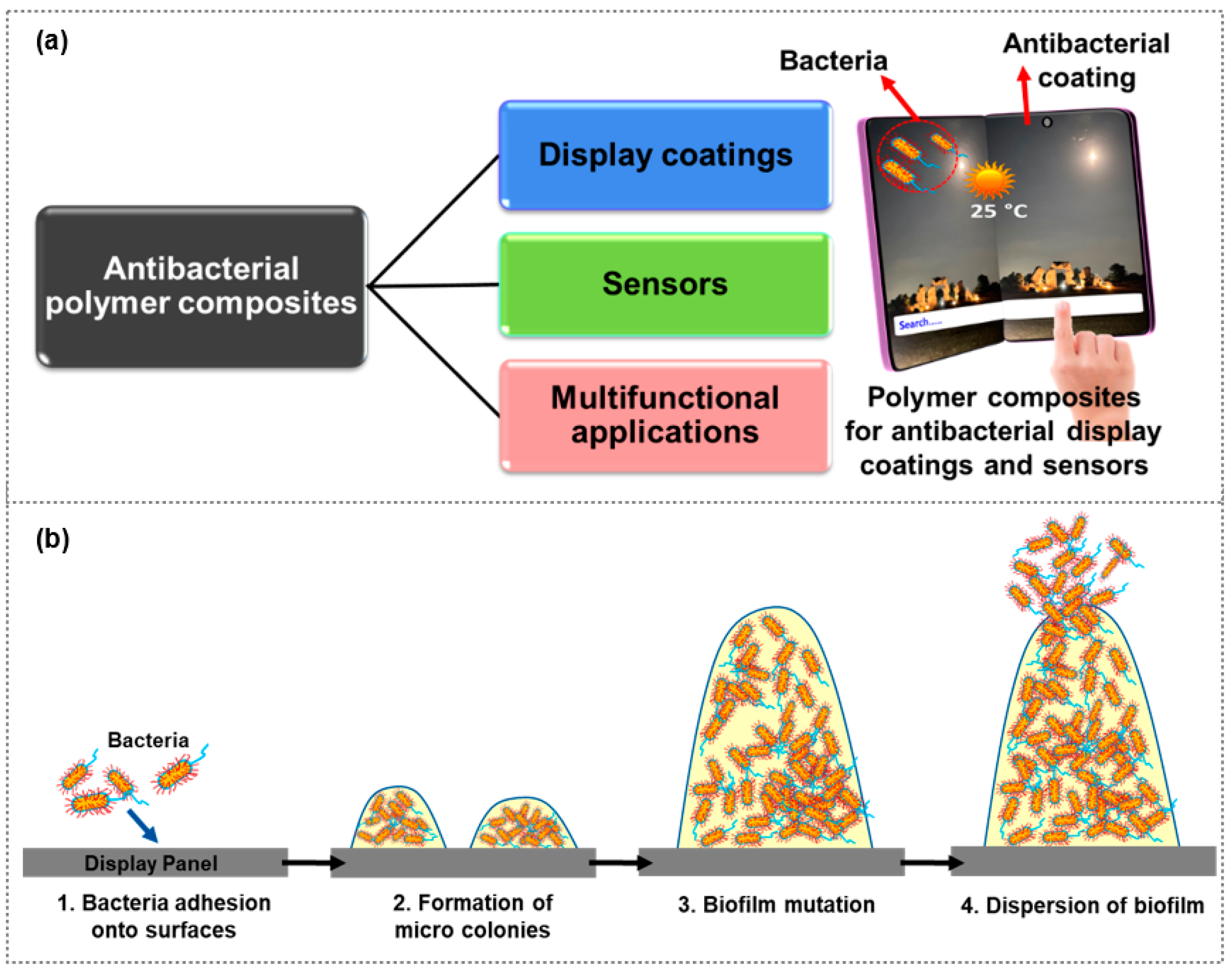
5. Antibacterial Polymer Composites for Multifunctional Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Janik, E.; Ceremuga, M.; Niemcewicz, M.; Bijak, M. Dangerous Pathogens as a Potential Problem for Public Health. Medicina 2020, 56, 591. [Google Scholar] [CrossRef]
- Oszutowska-Mazurek, D.; Fastowicz, J.; Mazurek, P. The Associations between Knowledge and Behaviours Related to Touch Screens and Microbiological Threats among IT Students’. Int. J. Env. Res. Public Health 2021, 18, 9269. [Google Scholar] [CrossRef]
- Alt, F.; Shirazi, A.S.; Kubitza, T.; Schmidt, A. Interaction Techniques for Creating and Exchanging Content with Public Displays. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Paris, France, 27 April–2 May 2013; ACM: New York, NY, USA, 2013; pp. 1709–1718. [Google Scholar]
- Jacucci, G.; Morrison, A.; Richard, G.T.; Kleimola, J.; Peltonen, P.; Parisi, L.; Laitinen, T. Worlds of Information. In Proceedings of the Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Atlanta, GA, USA, 10–15 April 2010; ACM: New York, NY, USA, 2010; pp. 2267–2276. [Google Scholar]
- Bhardwaj, N.; Khatri, M.; Bhardwaj, S.K.; Sonne, C.; Deep, A.; Kim, K.-H. A Review on Mobile Phones as Bacterial Reservoirs in Healthcare Environments and Potential Device Decontamination Approaches. Env. Res. 2020, 186, 109569. [Google Scholar] [CrossRef] [PubMed]
- Bhoonderowa, A.; Gookool, S.; Biranjia-Hurdoyal, S.D. The Importance of Mobile Phones in the Possible Transmission of Bacterial Infections in the Community. J. Community Health 2014, 39, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Wuollet, A.L.; Raisanen, P.; Lopez, G.U. Bacterial Contamination of Computer Touch Screens. Am. J. Infect. Control. 2016, 44, 358–360. [Google Scholar] [CrossRef]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Powers, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Env. Sci. Technol. Lett. 2021, 8, 168–175. [Google Scholar] [CrossRef]
- Muniz de Oliveira, R.; da Rosa Gioppo, N.M.; Oliveira de Carvalho, J.; Carvalho Oliveira, F.; Webster, T.J.; Marciano, F.R.; Oliveira Lobo, A. Decontamination of Mobile Phones and Electronic Devices for Health Care Professionals Using a Chlorhexidine/Carbomer 940® Gel. Front. Chem. Sci. Eng. 2019, 13, 192–198. [Google Scholar] [CrossRef]
- Kandel, C.E.; Simor, A.E.; Redelmeier, D.A. Elevator Buttons as Unrecognized Sources of Bacterial in Hospitals. Open Med. 2014, 8, e81. [Google Scholar]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of Wearable Healthcare Systems: Materials, Devices and System Integration. Mater. Sci. Eng. R Rep. 2020, 140, 100523. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing Prosthetic Electronic Skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- Yeom, C.; Chen, K.; Kiriya, D.; Yu, Z.; Cho, G.; Javey, A. Large-Area Compliant Tactile Sensors Using Printed Carbon Nanotube Active-Matrix Backplanes. Adv. Mater. 2015, 27, 1561–1566. [Google Scholar] [CrossRef]
- Vijayan, V.; Connolly, J.P.; Condell, J.; McKelvey, N.; Gardiner, P. Review of Wearable Devices and Data Collection Considerations for Connected Health. Sensors 2021, 21, 5589. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon Nanomaterials against Pathogens; the Antimicrobial Activity of Carbon Nanotubes, Graphene/Graphene Oxide, Fullerenes, and Their Nanocomposites. Adv. Colloid. Interface. Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Qayyum, A.; Shar, G.A.; Soomro, G.A.; Nazir, A.; Munir, B.; Iqbal, M. Zn-Doped SiO2 Nanoparticles Preparation and Characterization under the Effect of Various Solvents: Antibacterial, Antifungal and Photocatlytic Performance Evaluation. J. Photochem. Photobiol. B 2018, 185, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bauman, L.; Nogueira, C.L.; Aucoin, M.G.; Anderson, W.A.; Zhao, B. Antimicrobial Polymeric Composites for High-Touch Surfaces in Healthcare Applications. Curr. Opin. Biomed. Eng. 2022, 22, 100395. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, L.; Millians, W.; Wu, T.; Ming, W. Dual-Functional Antifogging/Antimicrobial Polymer Coating. ACS Appl. Mater. Interfaces 2016, 8, 8737–8742. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef]
- Zhang, W.; Piao, S.; Lin, L.; Yin, Y.; Guo, J.; Jiang, Z.; Cho, Y.; Li, R.; Gao, J.; Pang, H.; et al. Wearable and Antibacterial HPMC-Anchored Conductive Polymer Composite Strain Sensor with High Gauge Factors under Small Strains. Chem. Eng. J. 2022, 435, 135068. [Google Scholar] [CrossRef]
- Charnley, M.; Textor, M.; Acikgoz, C. Designed Polymer Structures with Antifouling–Antimicrobial Properties. React. Funct. Polym. 2011, 71, 329–334. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/Nanosilver Composite Coatings for Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial Properties of a Novel Copper-Based Composite Coating with Potential for Use in Healthcare Facilities. Antimicrob. Resist. Infect. Control. 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Recio-Sánchez, G.; Segura, A.; Benito-Gómez, N.; Lancellotti, M.; Hernandez-Montelongo, J. Composite Thin Films of Nanoporous Silicon/Green Synthesized Silver Nanoparticles as Antibacterial Surface. Mater. Lett. 2022, 324, 132734. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Hoque, J.; Yadav, V.; Prakash, R.G.; Sanyal, K.; Haldar, J. Dual-Function Polymer–Silver Nanocomposites for Rapid Killing of Microbes and Inhibiting Biofilms. ACS Biomater. Sci. Eng. 2019, 5, 81–91. [Google Scholar] [CrossRef]
- Liu, H.L.; Dai, S.A.; Fu, K.Y.; Hsu, S.H. Antibacterial Properties of Silver Nanoparticles in Three Different Sizes and Their Nanocomposites with a New Waterborne Polyurethane. Int. J. Nanomed. 2010, 5, 1017–1028. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Yu, D. Synthesis of Waterborne Polyurethane–Silver Nanoparticle Antibacterial Coating for Synthetic Leather. J. Coat. Technol. Res. 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Mohammadi, A.; Doctorsafaei, A.H.; Burujeny, S.B.; Rudbari, H.A.; Kordestani, N.; Ayati Najafabadi, S.A. Silver(I) Complex with a Schiff Base Ligand Extended Waterborne Polyurethane: A Developed Strategy to Obtain a Highly Stable Antibacterial Dispersion Impregnated with in Situ Formed Silver Nanoparticles. Chem. Eng. J. 2020, 381, 122776. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-Polymer Nanocomposites: An Excellent and Cost-Effective Biocide for Use on Antibacterial Surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Daina, S.; Sadocco, P.; Neto, C.P.; Trindade, T. Antibacterial Activity of Nanocomposites of Copper and Cellulose. Biomed. Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Castaño, J.; Valdebenito-Rolack, E.; Sanhueza, F.; Toro, R.; Bello-Toledo, H.; Uarac, P.; Saez, L. Copper-Polyurethane Composite Materials: Particle Size Effect on the Physical-Chemical and Antibacterial Properties. Polymers 2020, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Lugo-Uribe, L.E.; Pérez-Alvarez, M.; Tavizón, S.F.; Santillán, G.D.J.S. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021, 13, 1694. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Seidi, F. Anti-Bacterial Activity of Gold Nanocomposites as a New Nanomaterial Weapon to Combat Photogenic Agents: Recent Advances and Challenges. RSC Adv. 2021, 11, 34688–34698. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS. Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Podschun, R.; Schürmann, U.; Kulkarni, A.; Faupel, F. Physico-Chemical and Antimicrobial Properties of Co-Sputtered Ag–Au/PTFE Nanocomposite Coatings. Nanotechnology 2006, 17, 4904–4908. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Riquelme, S.A.; Sánchez-Sanhueza, G.; Medina, C.; Solís-Pomar, F.; Rojas, D.; Montalba, C.; Melendrez, M.F.; Pérez-Tijerina, E. Comparative Study of the Antimicrobial Effect of Nanocomposites and Composite Based on Poly(Butylene Adipate-Co-Terephthalate) Using Cu and Cu/Cu2O Nanoparticles and CuSO4. Nanoscale Res. Lett. 2019, 14, 158. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Birhanu, R.; Afrasa, M.A.; Hone, F.G. Recent Progress of Advanced Metal-Oxide Nanocomposites for Effective and Low-Cost Antimicrobial Activates: A Review. J. Nanomater. 2023, 2023, 1–25. [Google Scholar] [CrossRef]
- Kasi, G.; Gnanasekar, S.; Zhang, K.; Kang, E.T.; Xu, L.Q. Polyurethane-based Composites with Promising Antibacterial Properties. J. Appl. Polym. Sci. 2022, 139, 52181. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Kaprou, G.D.; Papavieros, G.; Mastellos, D.C.; Constantoudis, V.; Tserepi, A.; Gogolides, E. Enhanced Antibacterial Activity of ZnO-PMMA Nanocomposites by Selective Plasma Etching in Atmospheric Pressure. Micro. Nano. Eng. 2021, 13, 100098. [Google Scholar] [CrossRef]
- Rashid, S.; Vita, G.M.; Persichetti, L.; Iucci, G.; Battocchio, C.; Daniel, R.; Visaggio, D.; Marsotto, M.; Visca, P.; Bemporad, E.; et al. Biocompatibility and Antibacterial Properties of TiCu(Ag) Thin Films Produced by Physical Vapor Deposition Magnetron Sputtering. Appl. Surf. Sci. 2022, 573, 151604. [Google Scholar] [CrossRef]
- Haider, A.; Kwak, S.; Gupta, K.C.; Kang, I.-K. Antibacterial Activity and Cytocompatibility of PLGA/CuO Hybrid Nanofiber Scaffolds Prepared by Electrospinning. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Mizwari, Z.M.; Oladipo, A.A.; Yilmaz, E. Chitosan/Metal Oxide Nanocomposites: Synthesis, Characterization, and Antibacterial Activity. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 383–391. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial Effect of Carbon Nanomaterials: Nanotubes, Carbon Nanofibers, Nanodiamonds, and Onion-like Carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Imani, R.; Shao, W.; Emami, S.H.; Faghihi, S.; Prakash, S. Improved Dispersibility of Nano-Graphene Oxide by Amphiphilic Polymer Coatings for Biomedical Applications. RSC Adv. 2016, 6, 77818–77829. [Google Scholar] [CrossRef]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial Graphene Polymer (PVK-GO) Nanocomposite Films. Chem. Commun. 2011, 47, 8892. [Google Scholar] [CrossRef]
- Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Ciric, L.; Edirisinghe, M. The Effect of Graphene–Poly(Methyl Methacrylate) Fibres on Microbial Growth. Interface Focus 2018, 8, 20170058. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial Applications of Electroactive PVK-SWNT Nanocomposites. Env. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- De Vaan, A.J.S.M. Competing Display Technologies for the Best Image Performance. J. Soc. Inf. Disp. 2007, 15, 657. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, A.; Fujii, H.; Hotta, A.; Asanuma, R.; Irie, H. Prevalence of Bacterial Contamination of Touchscreens and Posterior Surfaces of Smartphones Owned by Healthcare Workers: A Cross-Sectional Study. BMC Infect. Dis. 2021, 21, 681. [Google Scholar] [CrossRef]
- Julian, T.R.; Leckie, J.O.; Boehm, A.B. Virus Transfer between Fingerpads and Fomites. J. Appl. Microbiol. 2010, 109, 1868–1874. [Google Scholar] [CrossRef]
- Katsuse Kanayama, A.; Takahashi, H.; Yoshizawa, S.; Tateda, K.; Kaneko, A.; Kobayashi, I. Staphylococcus Aureus Surface Contamination of Mobile Phones and Presence of Genetically Identical Strains on the Hands of Nursing Personnel. Am. J. Infect. Control. 2017, 45, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Ippili, S.; Kim, B.; Jella, V.; Jung, J.-S.; Vuong, V.-H.; Yoon, S.-G. Antireflective, Transparent, Water-Resistant, and Antibacterial Zn-Doped Silicon Oxide Thin Films for Touchscreen-Based Display Applications. ACS Sustain. Chem. Eng. 2022, 10, 2136–2147. [Google Scholar] [CrossRef]
- Robrecht, M.J. Antimicrobial Solutions for Touchscreens in Times of Pandemic. Inf. Disp. 2021, 37, 28–31. [Google Scholar] [CrossRef]
- Orphanides, A.K.; Nam, C.S. Touchscreen Interfaces in Context: A Systematic Review of Research into Touchscreens across Settings, Populations, and Implementations. Appl. Erg. 2017, 61, 116–143. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 Nanocomposite Coatings for Surfaces, Dental and Orthopaedic Implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wu, S.; Cui, Z.; Zheng, Y.; Li, Z.; Jiang, H.; Zhu, S.; Liu, X. Superlattice Nanofilm on a Touchscreen for Photoexcited Bacteria and Virus Killing by Tuning Electronic Defects in the Heterointerface. Adv. Mater. 2023, 35, 2300380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv. 2013, 3, 12003. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Ippili, S.; Jella, V.; Lee, J.M.; Jung, J.-S.; Lee, D.-H.; Yang, T.-Y.; Yoon, S.-G. ZnO–PTFE-Based Antimicrobial, Anti-Reflective Display Coatings and High-Sensitivity Touch Sensors. J. Mater. Chem. A Mater. 2022, 10, 22067–22079. [Google Scholar] [CrossRef]
- Canama, G.J.C.; Delco, M.C.L.; Talandron, R.A.; Tan, N.P. Synthesis of Chitosan-Silver Nanocomposite and Its Evaluation as an Antibacterial Coating for Mobile Phone Glass Protectors. ACS Omega 2023, 8, 17699–17711. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, B.J.; Eom, J.H.; Choi, M.J.; Yoon, S.G. Achieving Antifingerprinting and Antibacterial Effects in Smart-Phone Panel Applications Using ZnO Thin Films without a Protective Layer. ACS Appl. Mater. Interfaces 2016, 8, 997–1003. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, Characterization, and Antimicrobial Properties of Copper Nanoparticles. Int. J. Nanomed. 2013, 8, 4467. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The Antibacterial Effects of Silver, Titanium Dioxide and Silica Dioxide Nanoparticles Compared to the Dental Disinfectant Chlorhexidine on Streptococcus Mutans Using a Suite of Bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Seo, S.-Y.; Jung, J.-S.; Yoon, S.-G. Water-Resistant and Antibacterial Zinc Aluminate Films: Application of Antibacterial Thin Film Capacitors. ACS Appl. Electron. Mater. 2021, 3, 1429–1436. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Choi, H.-J.; Park, B.-J.; Um, J.-H.; Jung, H.-J.; Jeong, J.-R.; Yoon, S.-G. Effect of Protective Layer on Enhanced Transmittance, Mechanical Durability, Anti-Fingerprint, and Antibacterial Activity of the Silver Nanoparticles Deposited on Flexible Substrate. Sens. Actuators A Phys. 2015, 221, 131–138. [Google Scholar] [CrossRef]
- Bodas, D.S.; Mandale, A.B.; Gangal, S.A. Deposition of PTFE Thin Films by RF Plasma Sputtering on 〈100〉 Silicon Substrates. Appl. Surf. Sci. 2005, 245, 202–207. [Google Scholar] [CrossRef]
- Cho, E.; Kim, S.H.; Kim, M.; Park, J.-S.; Lee, S.-J. Super-Hydrophobic and Antimicrobial Properties of Ag-PPFC Nanocomposite Thin Films Fabricated Using a Ternary Carbon Nanotube-Ag-PTFE Composite Sputtering Target. Surf. Coat. Technol. 2019, 370, 18–23. [Google Scholar] [CrossRef]
- Pahlevanneshan, Z.; Deypour, M.; Kefayat, A.; Rafienia, M.; Sajkiewicz, P.; Esmaeely Neisiany, R.; Enayati, M.S. Polyurethane-Nanolignin Composite Foam Coated with Propolis as a Platform for Wound Dressing: Synthesis and Characterization. Polymers 2021, 13, 3191. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef]
- Cheng, K.; Huang, Z.; Wang, P.; Sun, L.; Ghasemi, H.; Ardebili, H.; Karim, A. Antibacterial Flexible Triboelectric Nanogenerator via Capillary Force Lithography. J. Colloid. Interface Sci. 2023, 630, 611–622. [Google Scholar] [CrossRef]
- Jella, V.; Ippili, S.; Eom, J.-H.; Pammi, S.V.N.; Jung, J.-S.; Tran, V.-D.; Nguyen, V.H.; Kirakosyan, A.; Yun, S.; Kim, D.; et al. A Comprehensive Review of Flexible Piezoelectric Generators Based on Organic-Inorganic Metal Halide Perovskites. Nano Energy 2019, 57, 74–93. [Google Scholar] [CrossRef]
- Ippili, S.; Kim, J.H.; Jella, V.; Behera, S.; Vuong, V.-H.; Jung, J.-S.; Cho, Y.; Ahn, J.; Kim, I.-D.; Chang, Y.H.; et al. Halide Double Perovskite-Based Efficient Mechanical Energy Harvester and Storage Devices for Self-Charging Power Unit. Nano Energy 2023, 107, 108148. [Google Scholar] [CrossRef]
- Vuong, V.-H.; Pammi, S.V.N.; Ippili, S.; Jella, V.; Nguyen Thi, T.; Sairam Pasupuleti, K.; Kim, M.-D.; Ji Jeong, M.; Jeong, J.-R.; Sik Chang, H.; et al. Flexible, Stable, and Self-Powered Photodetectors Embedded with Chemical Vapor Deposited Lead-Free Bismuth Mixed Halide Perovskite Films. Chem. Eng. J. 2023, 458, 141473. [Google Scholar] [CrossRef]
- Thomas, A.M.; Yoon, C.; Ippili, S.; Jella, V.; Yang, T.-Y.; Yoon, G.; Yoon, S.-G. High-Performance Flexible Ultraviolet Photodetectors Based on Facilely Synthesized Ecofriendly ZnAl:LDH Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 61434–61446. [Google Scholar] [CrossRef]
- Ippili, S.; Jella, V.; Thomas, A.M.; Yoon, S.-G. The Recent Progress on Halide Perovskite-Based Self-Powered Sensors Enabled by Piezoelectric and Triboelectric Effects. Nanoenergy Adv. 2021, 1, 3–31. [Google Scholar] [CrossRef]
- Ippili, S.; Jella, V.; Kim, J.; Hong, S.; Kim, H.-S.; Yoon, S.-G. High-Power Nanogenerator of 2D-Layered Perovskite in a Polymer Matrix for Self-Charging Battery-Powered Electronics. Nano Energy 2022, 103, 107781. [Google Scholar] [CrossRef]
- Ippili, S.; Jella, V.; Thomas, A.M.; Yoon, C.; Jung, J.-S.; Yoon, S.-G. ZnAl–LDH-Induced Electroactive β-Phase and Controlled Dielectrics of PVDF for a High-Performance Triboelectric Nanogenerator for Humidity and Pressure Sensing Applications. J. Mater. Chem. A Mater. 2021, 9, 15993–16005. [Google Scholar] [CrossRef]
- Yoon, C.; Ippili, S.; Jella, V.; Thomas, A.M.; Jung, J.-S.; Han, Y.; Yang, T.-Y.; Yoon, S.-G.; Yoon, G. Synergistic Contribution of Flexoelectricity and Piezoelectricity towards a Stretchable Robust Nanogenerator for Wearable Electronics. Nano Energy 2022, 91, 106691. [Google Scholar] [CrossRef]
- Ippili, S.; Jella, V.; Eom, S.; Hong, S.; Yoon, S.-G. Light-Driven Piezo- and Triboelectricity in Organic–Inorganic Metal Trihalide Perovskite toward Mechanical Energy Harvesting and Self-Powered Sensor Application. ACS Appl. Mater. Interfaces 2020, 12, 50472–50483. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Liu, J.; Gu, L.; Bai, S.; Chen, X.; Qin, Y. Wearable Triboelectric Generator for Powering the Portable Electronic Devices. ACS Appl. Mater. Interfaces 2015, 7, 18225–18230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, Z. Piezoelectric Based Touch Sensing for Interactive Displays—A Short Review. Materials 2021, 14, 5698. [Google Scholar] [CrossRef]
- Huang, R.; Cai, X.; Du, J.; Lian, J.; Hui, P.; Gu, M.; Li, F.; Wang, J.; Chen, W. Bioinspired Plasmonic Nanosensor for On-Site Antimicrobial Susceptibility Testing in Urine Samples. ACS Nano 2022, 16, 19229–19239. [Google Scholar] [CrossRef]
- Ferreira, A.; Fernandes, M.M.; Souza, A.L.R.; Correa, M.A.; Lanceros-Mendez, S.; Vaz, F. Flexible TiCux Thin Films with Dual Antimicrobial and Piezoresistive Characteristics. ACS Appl. Bio. Mater. 2022, 5, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, D.; Hua, Z.; Wang, J.; Cao, S.; Ma, X. Cellulose Paper Modified by a Zinc Oxide Nanosheet Using a ZnCl2-Urea Eutectic Solvent for Novel Applications. Nanomaterials 2021, 11, 1111. [Google Scholar] [CrossRef]
- Costa, P.; Polícia, R.; Perinka, N.; Alesanco, Y.; Viñuales, A.; Carvalho, E.O.; Pereira, N.; Fernandes, M.M.; Lanceros-Mendez, S. Multifunctional Touch Sensing and Antibacterial Polymer-Based Core-Shell Metallic Nanowire Composites for High Traffic Surfaces. Adv. Mater. Technol. 2022, 7, 2101575. [Google Scholar] [CrossRef]
- Li, M.; Zou, X.; Ding, Y.; Wang, W.; Cheng, Z.; Wang, D.; Wang, Z.; Shao, Y.; Bai, J. Multifunctional Sensors for Respiration Monitoring and Antibacterial Activity Based on Piezoelectric PVDF/BZT-0.5BCT Nanoparticle Composite Nanofibers. Smart Mater. Struct. 2022, 31, 125002. [Google Scholar] [CrossRef]
- Li, L.; Ji, X.; Chen, K. Conductive, Self-Healing, and Antibacterial Ag/MXene-PVA Hydrogel as Wearable Skin-like Sensors. J. Biomater. Appl. 2023, 37, 1169–1181. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Zhai, S.; Cheng, R.; Liu, D.; Gao, X.; Wang, J.; Wang, Z.L. A Breathable, Biodegradable, Antibacterial, and Self-Powered Electronic Skin Based on All-Nanofiber Triboelectric Nanogenerators. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Z.; Zhao, Z.; Liao, Q.; Kang, Z.; Gao, F.; Zhao, X.; Zhang, Y. Self-Powered Flexible Antibacterial Tactile Sensor Based on Triboelectric-Piezoelectric-Pyroelectric Multi-Effect Coupling Mechanism. Nano Energy 2019, 66, 104105. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Z.; Lin, M.; Shen, Q.; Wang, X.; Lin, B.; Fu, L. Strengthened, Antibacterial, and Conductive Flexible Film for Humidity and Strain Sensors. ACS Appl. Mater. Interfaces 2020, 12, 35482–35492. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Kim, T.; Park, C.; Samuel, E.P.; Kim, Y.-I.; An, S.; Yoon, S.S. Wearable Sensors and Supercapacitors Using Electroplated-Ni/ZnO Antibacterial Fabric. J. Mater. Sci. Technol. 2022, 100, 254–264. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, Z.; Liu, Z.; Yao, S.; Li, L. Antibacterial, Antifreezing, Stretchable, and Self-Healing Organohydrogel Electrode Based Triboelectric Nanogenerator for Self-Powered Biomechanical Sensing. Adv. Mater. Interfaces 2022, 9, 2200290. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, R.; Zheng, Y.; Wang, J.; Khatib, M.; Jiang, X.; Zhou, C.; Omar, R.; Saliba, W.; Wu, W.; et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022, 34, 2106842. [Google Scholar] [CrossRef] [PubMed]
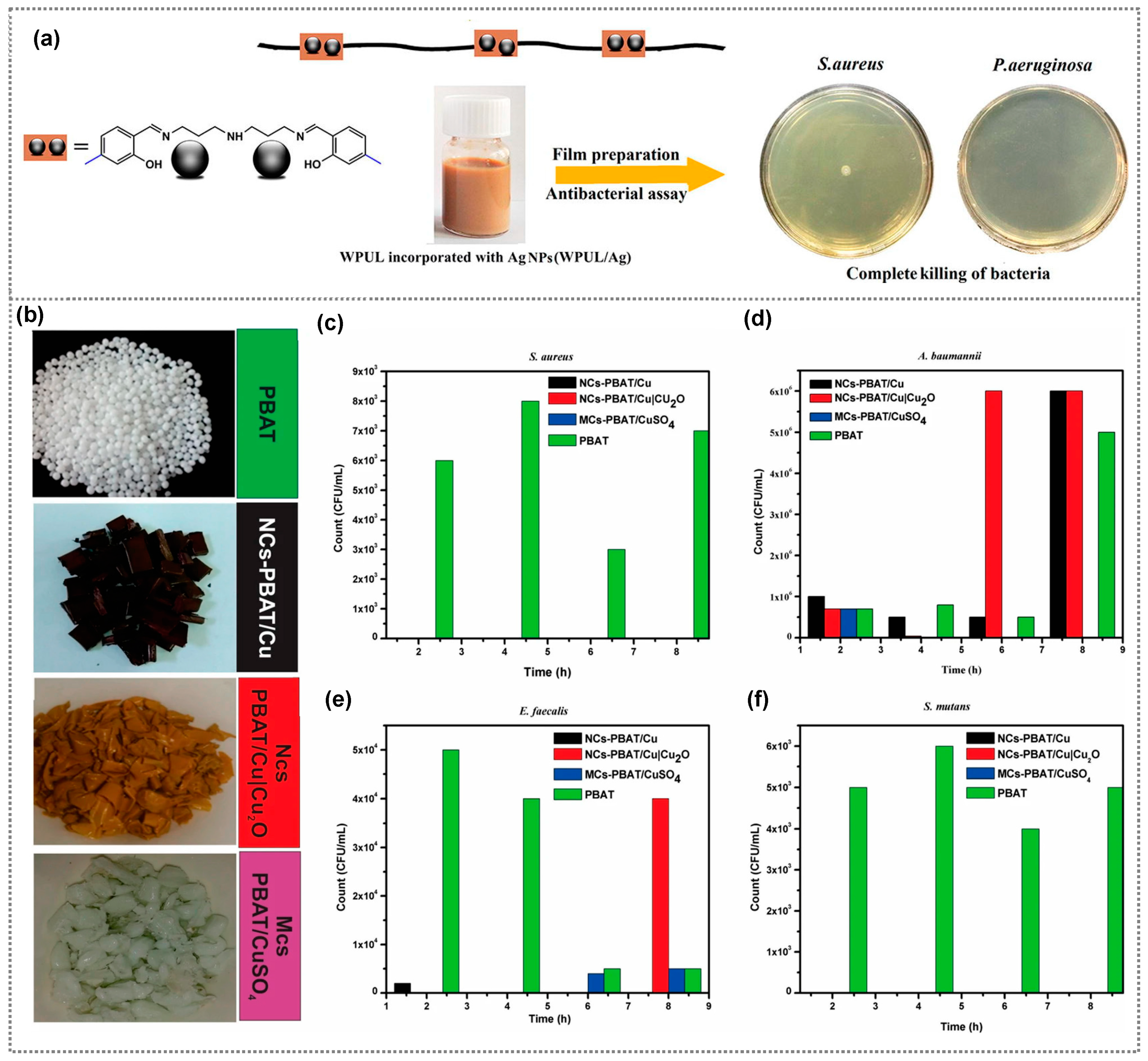

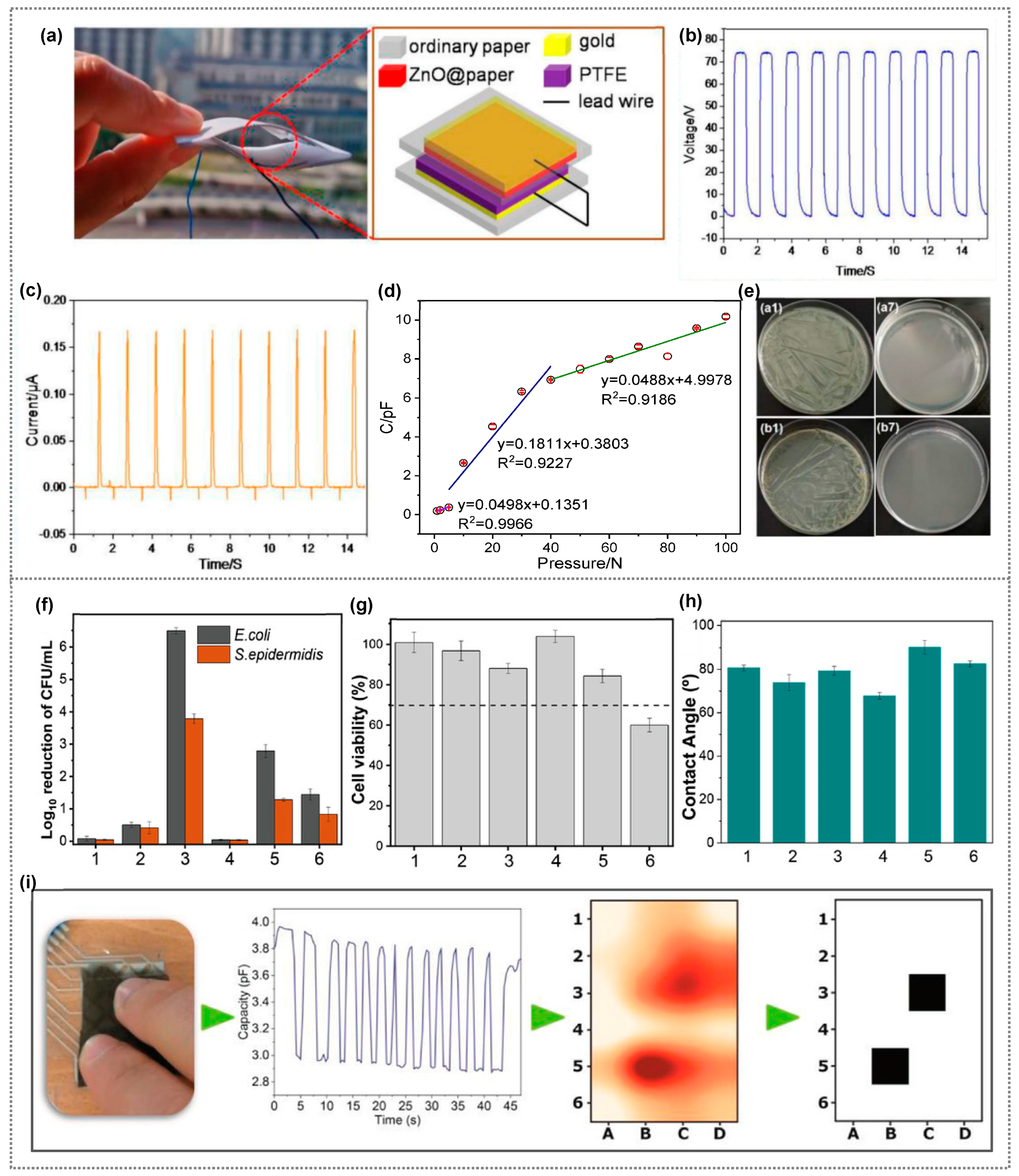


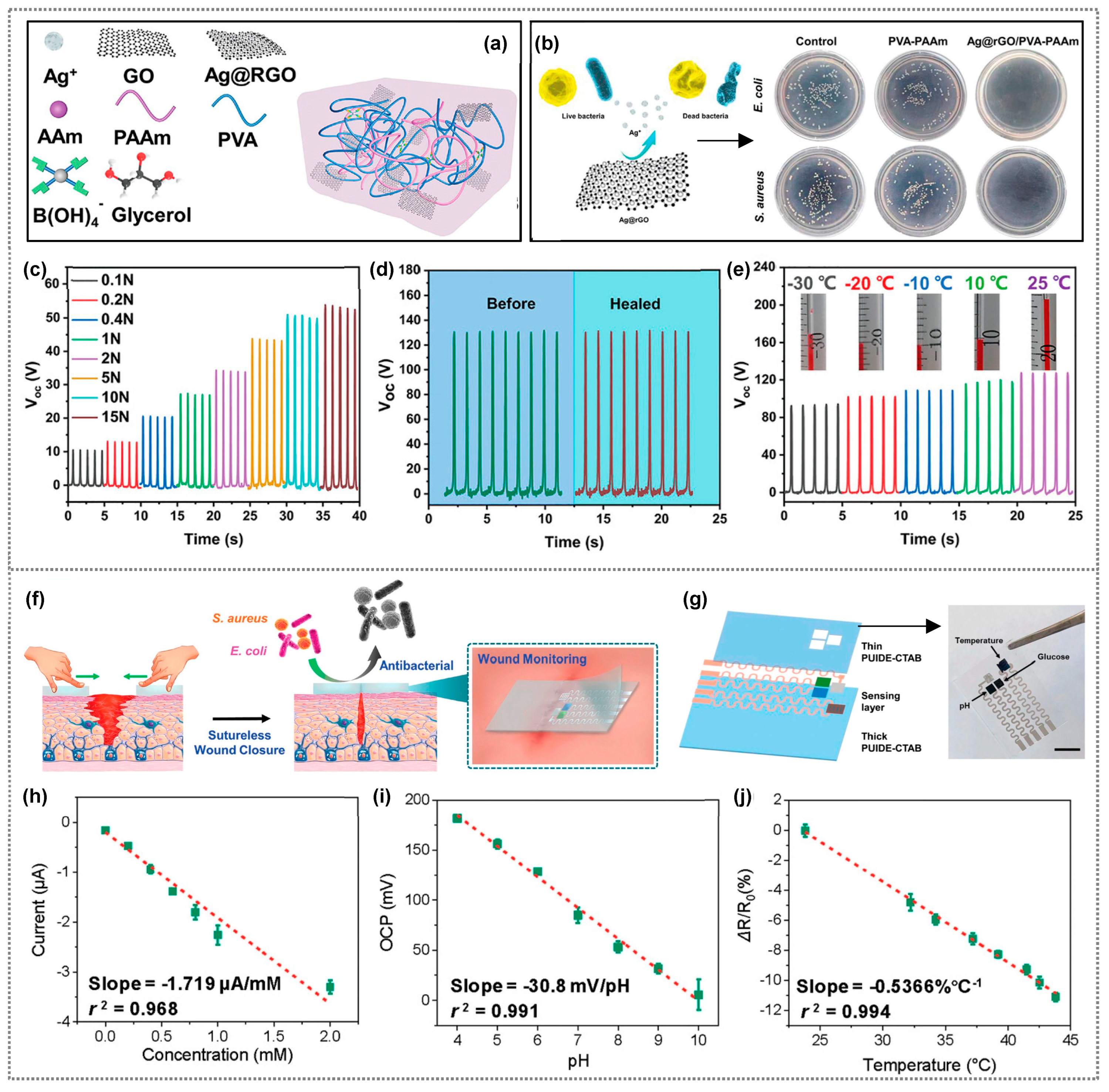
| Composite Material | Bacteria/Virus/Fungi | Antibacterial Activity/Reduction | Ref. |
|---|---|---|---|
| Q-DMHC48/Ag NPs | S. aureus, E. coli, P. aeruginosa, K. pneumoniae, Candida spp., Cryptococcus spp. | >99.99% (S. aureus) 100% (E. coli) | [29] |
| WPUL/Ag | S. aureus, P. aeruginosa | 99.99%, 99.99%, | [32] |
| PBAT/Cu, PBAT/Cu|Cu2O, PBAT/CuSO4 | S. aureus, A. baumanni, E. faecalis, S. mutan | - | [40] |
| Cellulose/Cu nanofillers | S. aureus, K. pneumoniae | - | [34] |
| Polyurethane/Cu | S. aureus, E. coli | 2 Log10 < cell density < 3 Log10, ≥3 Log10 | [35] |
| Polypropylene/Cu NPs | S. aureus, P. aeruginosa | 100% | [36] |
| Chitosan/Au NPs | S. aureus, P. aeruginosa | - | [38] |
| Ag-Au/PTFE | S. aureus, S. epidermidis | - | [39] |
| PMMA/ZnO | E. coli | 1 Log CFU/ml | [45] |
| PLGA/CuO NFs | S. aureus, E. coli | - | [47] |
| Chitosan/NiO-MgO | S. aureus, E. coli | 98%, 92.3% | [48] |
| Cationic Polymers-GO and GR | S. aureus, E. coli, S. epidermidis, P. aeruginosa | - | [54] |
| Polyvinyl N-carbazole/SWNT | E. coli, B. subtilis | 94%, 90% | [56] |
| Polyvinyl N-carbazole/GO | E. coli | 90% | [53] |
| Material | Microorganism | Antibacterial Activity/Reduction | Application | Ref. |
|---|---|---|---|---|
| Zn doped SiO2/PTFE | S. aureus, E. coli | 99.99748%, 99.999947% | Smartphone panel | [62] |
| ZnO-PTFE | S. aureus, E. coli | 4.7, 6.2 | Displays | [69] |
| Chitosan-silver nanoparticles | E. coli | 49.8% | Mobile phone glass protectors | [70] |
| ZnO | S. aureus, E. coli | 99.99999%, 99.99668% | Smartphone panel | [71] |
| ZnAl2O4 | S. aureus, E. coli | 4.5, 6.3 | Surface coating | [74] |
| SiO2-PTFE-coated Ag NPs | S. aureus, E. coli | 4.4, 6.4 | Anti-fingerprint hydrophobic coating | [75] |
| Nano pattern-TPU | E. coli | >99% | Touch sensor, Displays | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippili, S.; Jung, J.-S.; Thomas, A.M.; Vuong, V.-H.; Lee, J.-M.; Sha, M.S.; Sadasivuni, K.K.; Jella, V.; Yoon, S.-G. An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications. Polymers 2023, 15, 3791. https://doi.org/10.3390/polym15183791
Ippili S, Jung J-S, Thomas AM, Vuong V-H, Lee J-M, Sha MS, Sadasivuni KK, Jella V, Yoon S-G. An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications. Polymers. 2023; 15(18):3791. https://doi.org/10.3390/polym15183791
Chicago/Turabian StyleIppili, Swathi, Jang-Su Jung, Alphi Maria Thomas, Van-Hoang Vuong, Jeong-Min Lee, Mizaj Shabil Sha, Kishor Kumar Sadasivuni, Venkatraju Jella, and Soon-Gil Yoon. 2023. "An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications" Polymers 15, no. 18: 3791. https://doi.org/10.3390/polym15183791
APA StyleIppili, S., Jung, J.-S., Thomas, A. M., Vuong, V.-H., Lee, J.-M., Sha, M. S., Sadasivuni, K. K., Jella, V., & Yoon, S.-G. (2023). An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications. Polymers, 15(18), 3791. https://doi.org/10.3390/polym15183791












