Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PVA-EG and PVA-FT Gels
2.2.2. Characterizations
2.2.3. Mechanical Tests
3. Results and Discussion
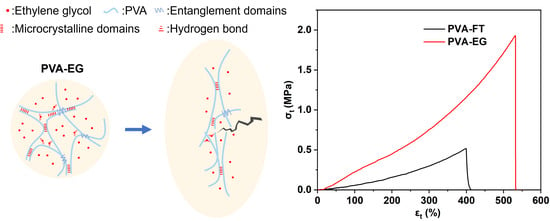
3.1. Preparation Mechanism
3.2. Structural Characterization
3.2.1. FTIR and DSC Analysis
3.2.2. XRD Analysis
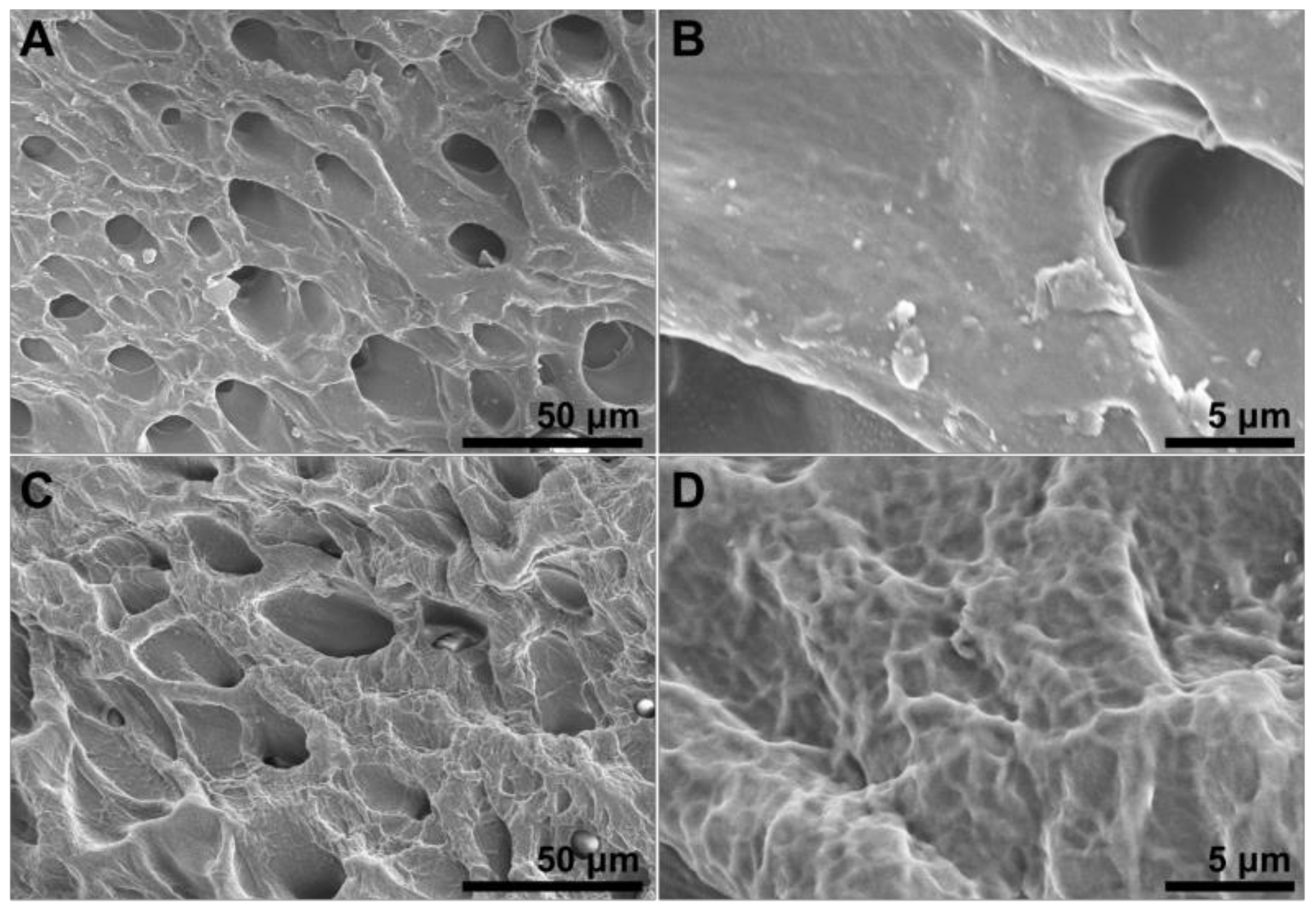
3.2.3. SEM Analysis
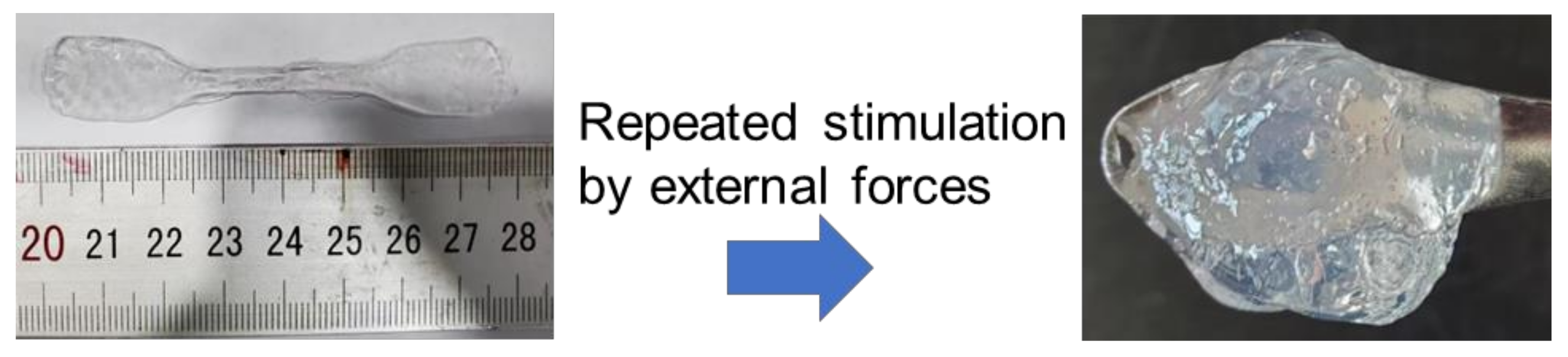
3.3. Mechanical Properties
3.4. EG-Induced Strengthening Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, H.; Lei, M.; Zhang, P.; Leng, J.; Zheng, Z.; Yu, Y. Orthogonal photochemistry-assisted printing of 3D tough and stretchable conductive hydrogels. Nat. Commun. 2021, 12, 2082. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Wu, J. Conductive hydrogel-and-oganohydrogel-based stretchable sensors. ACS Appl. Mater. Interfaces. 2021, 13, 2128–2144. [Google Scholar] [CrossRef]
- Tran, H.D.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar]
- Qian, J.; Xiao, R.; Su, F.; Guo, M.; Liu, D. 3D wet-spinning printing of wearable flexible electronic sensors of polypyrrole@ polyvinyl formate. J. Ind. Eng. Chem. 2022, 111, 490–498. [Google Scholar] [CrossRef]
- Wang, R.; Sui, J.; Wang, X. Natural piezoelectric biomaterials: A biocompatible and sustainable building block for biomedical devices. ACS Nano 2022, 16, 17708–17728. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High strength astringent hydrogels using protein as the building block for physically cross-linked multi-network. ACS Appl. Mater. Interfaces 2017, 10, 7593–7601. [Google Scholar] [CrossRef]
- El Shafee, E.; Naguib, H. Water sorption in cross-linked poly (vinyl alcohol) networks. Polymer 2003, 44, 1647–1653. [Google Scholar] [CrossRef]
- Ceylan, S.; Gokturk, D.; Bolgen, N. Effect of crosslinking methods on the structure and biocompatibility of polyvinyl alcohol/gelatin cryogels. Biomed. Mater. Eng. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Xiao, C. Self-healable and pH-sensitive high-strength water-soluble chitosan/chemically cross-linked polyvinyl alcohol semi-IPN hydrogel. Int. J. Biol. Macromol. 2019, 138, 667–672. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nagano, S.; Hara, M.; Hyon, S.H.; Patel, M.; Matsumura, K. Facile preparation of transparent poly (vinyl alcohol) hydrogels with uniform microcrystalline structure by hot-pressing without using organic solvents. Polym. J. 2017, 49, 535–542. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Gao, H.W.; Yang, R.J.; He, J.Y.; Yang, L. Rheological behaviors of PVA/H2O solutions of high-polymer concentration. J. Appl. Polym. Sci. 2010, 116, 1459–1466. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(vinyl alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, Conductive Self-healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Edit. 2017, 56, 14159–14163. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Liu, Z.; Wu, S.; Hua, M.; He, X. Tuning structural and mechanical anisotropy of PVA hydrogels. Mech. Mater. 2022, 172, 104411. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(vinyl alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wu, H.; Wen, J.; Zhang, S.; Xing, W.; Zhang, H.; Xue, H.; Gao, J.; Mai, Y. Solvent-Exchange-Assisted Wet Annealing: A New Strategy for Superstrong, Tough, Stretchable, and Anti-Fatigue Hydrogels. Adv. Mater. 2023, 35, 2210624. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Wang, Z.; Wang, Z.; Lan, Y.; Ge, Q.; et al. Anisotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2021, 33, 202102011. [Google Scholar] [CrossRef]
- Chen, H.; Chang, H. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloid. Surface. A. 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Yang, J.; Hao, J.; Tang, C.; Guo, Y.; Guo, M.; Li, Z.; Liu, S.; Yu, H.; Qin, G.; Chen, Q. Facile preparation and characterization of tough poly(vinyl alcohol) organohydrogels with low friction and self-cleaning properties. J. Ind. Eng. Chem. 2022, 116, 207–216. [Google Scholar] [CrossRef]
- Zou, J.; Wang, L.; Sun, G. Sustainable and Reusable Gelatin-Based Hydrogel “Jelly Ice Cubes” as Food Coolant. II: Ideal Freeze–Thaw Conditions. ACS Sustain. Chem. Eng. 2021, 9, 15365–15374. [Google Scholar] [CrossRef]
- Butylina, S.; Geng, S.; Oksman, K. Properties of as-prepared and freeze-dried hydrogels made from poly (vinyl alcohol) and cellulose nanocrystals using freeze-thaw technique. Eur. Polym. J. 2016, 81, 386–396. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-strength and high-toughness double-cross-linked cellulose hydrogels: A new strategy using sequential chemical and physical cross-linking. Adv. Funct. Mater. 2016, 26, 201601645. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.-N.; He, C.; Wang, H. Facile preparation of hydrogen-bonded supramolecular polyvinyl alcohol-glycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Sugiura, K.; Hashimoto, M.; Matsuzawa, S.; Yamaura, K. Influence of degree of crystallinity and syndiotacticity on infrared spectra of solid PVA. J. Appl. Polym. Sci. 2001, 82, 1291–1298. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Holt, G. Sonication amplitude and processing time influence the cellulose nanocrystals morphology and dispersion. Nanocomposites 2020, 6, 41–46. [Google Scholar] [CrossRef]
- Guo, X.; Dong, X.; Zou, G.; Gao, H.; Zhai, W. Strong and tough fibrous hydrogels reinforced by multiscale hierarchical structures with multimechanisms. Sci. Adv. 2023, 9, 7075. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Li, Y.; Huang, Y.; Li, B.; Jiang, Z.; Gao, G. Anti-freezing, moisturizing, resilient and conductive organohydrogel for sensitive pressure sensors. J. Colloid Interf. Sci. 2021, 594, 584–592. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, K.; Weng, S.; Jiang, X. Super strong and tough anisotropic hydrogels through synergy of directional freeze-casting, metal complexation and salting out. Chem. Eng. J. 2023, 463, 142414. [Google Scholar] [CrossRef]


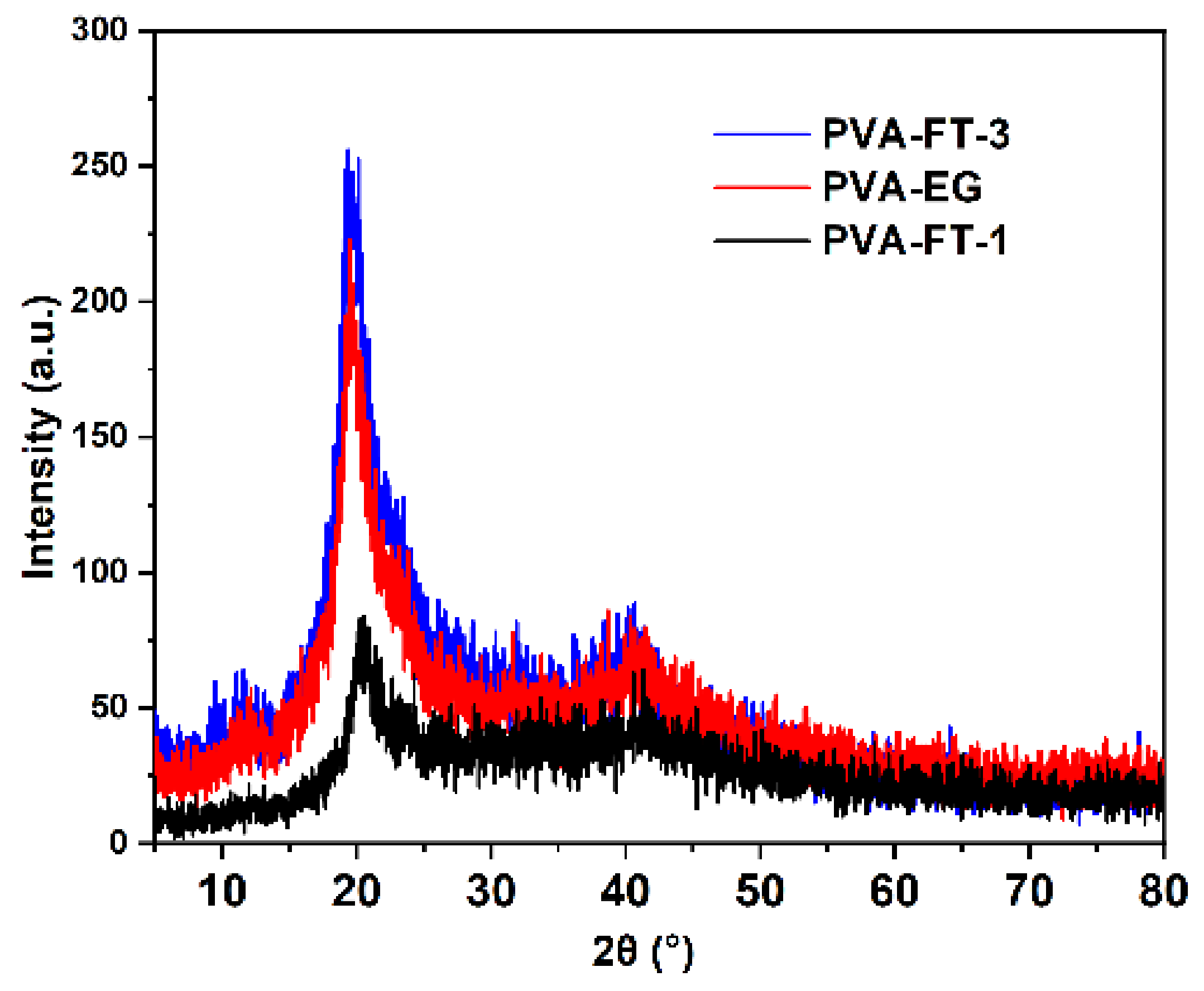
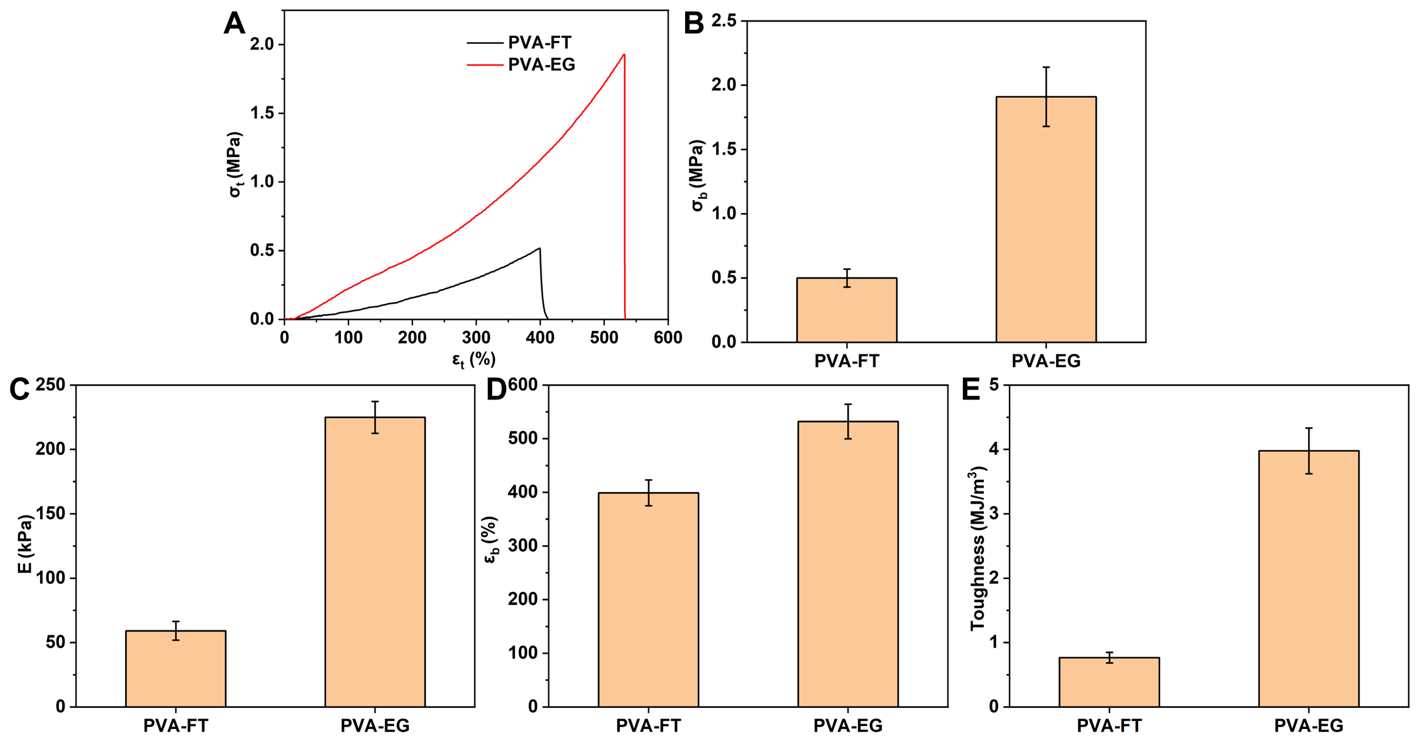
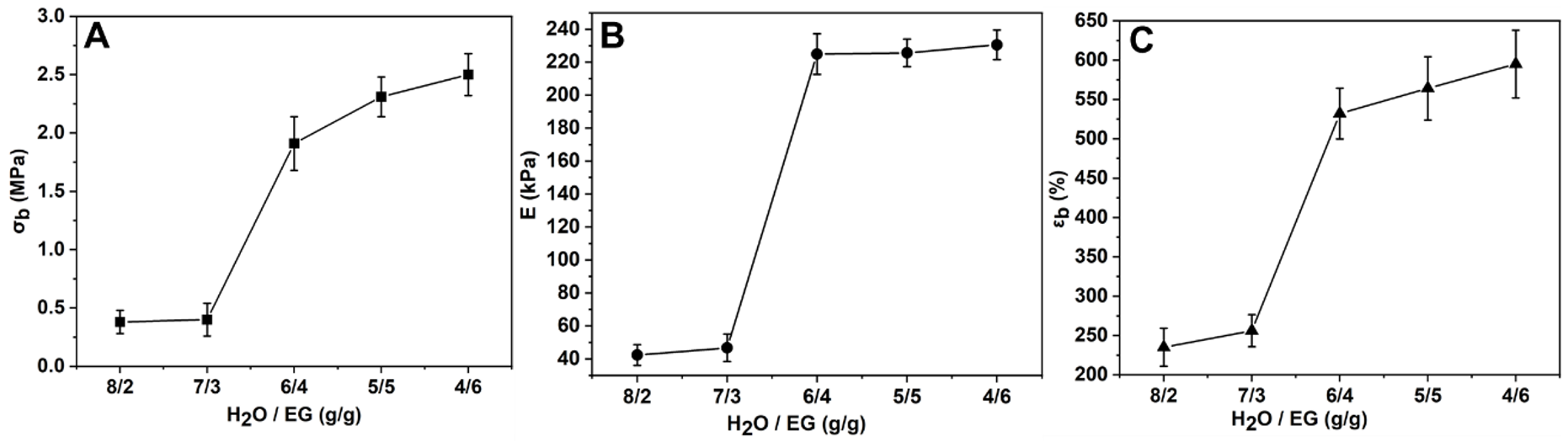

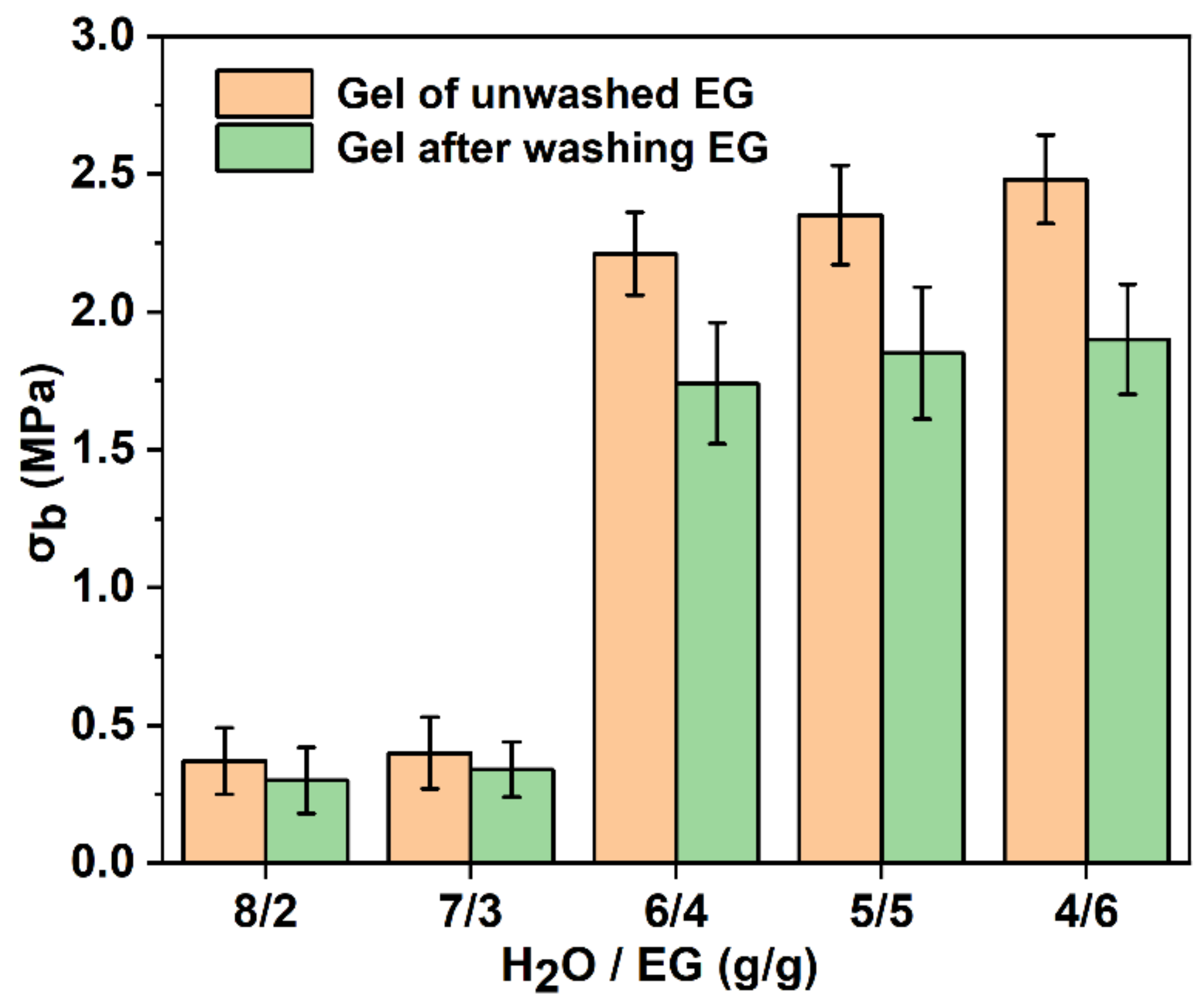
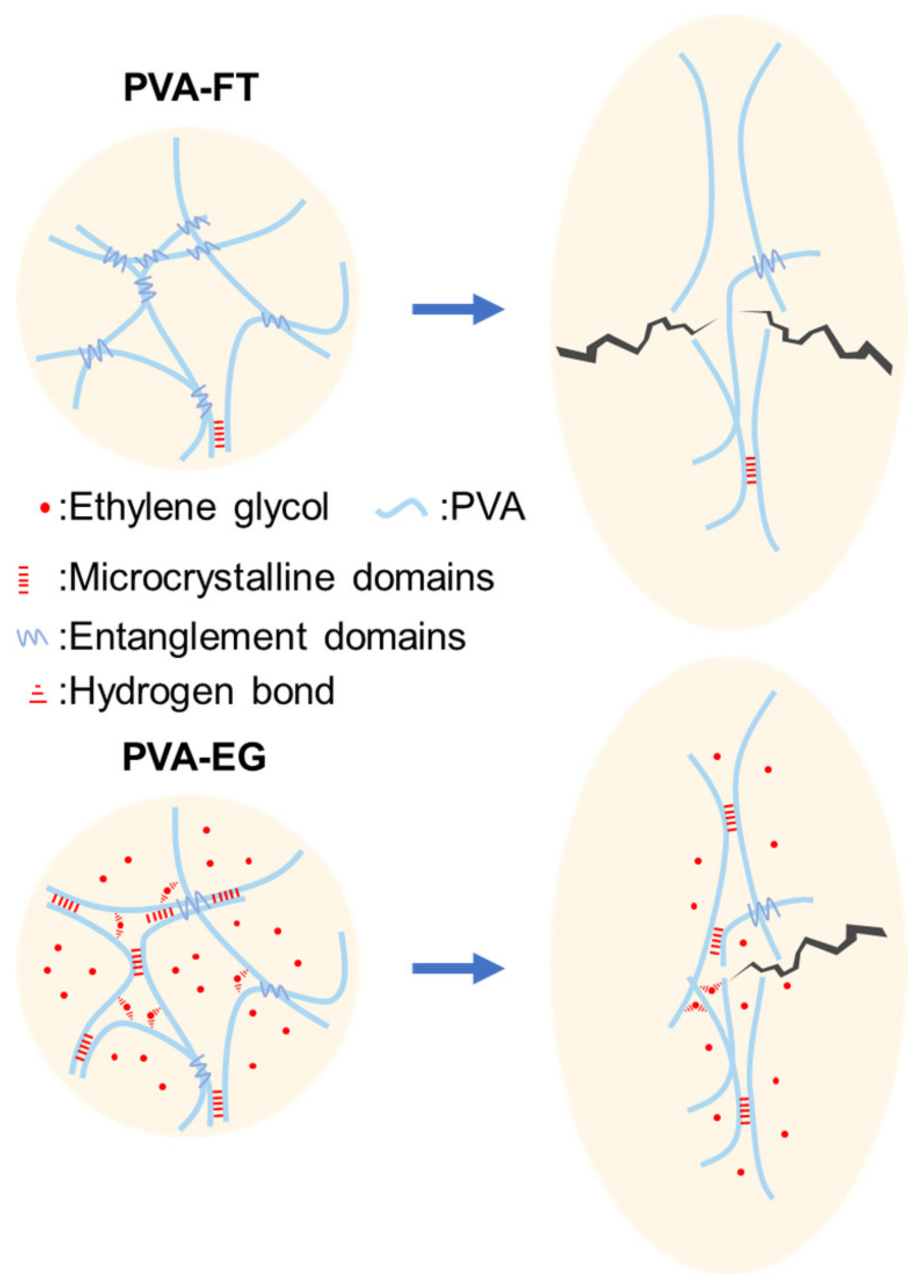
| Samples | Diffraction Peak (2θ) | Crystallite Size (nm) |
|---|---|---|
| PVA-FT-1 | 20.62 | 2.67 |
| PVA-FT-3 | 19.92 | 2.64 |
| PVA-EG | 19.94 | 2.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Gao, J.; Xiang, Y.; Yang, J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers 2023, 15, 3782. https://doi.org/10.3390/polym15183782
Wu F, Gao J, Xiang Y, Yang J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers. 2023; 15(18):3782. https://doi.org/10.3390/polym15183782
Chicago/Turabian StyleWu, Fei, Jianfeng Gao, Yang Xiang, and Jianming Yang. 2023. "Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles" Polymers 15, no. 18: 3782. https://doi.org/10.3390/polym15183782
APA StyleWu, F., Gao, J., Xiang, Y., & Yang, J. (2023). Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers, 15(18), 3782. https://doi.org/10.3390/polym15183782