Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles—An Electro-Optical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electric Light Scattering
2.2.2. Potentiometric Acid-Base Titration
2.2.3. Microelectrophoresis
2.2.4. Dynamic Light Scattering
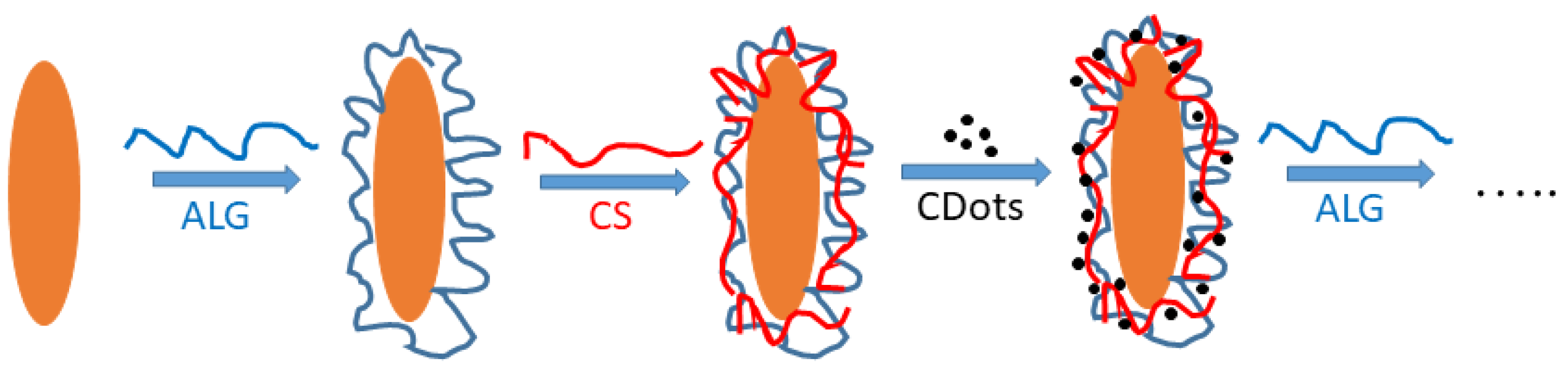
2.2.5. Formation of the Composite Multilayer Film
2.2.6. An Estimation of the Hydrodynamic Thickness of Each Polymer Layer
2.2.7. UV-Vis Spectroscopy
3. Results
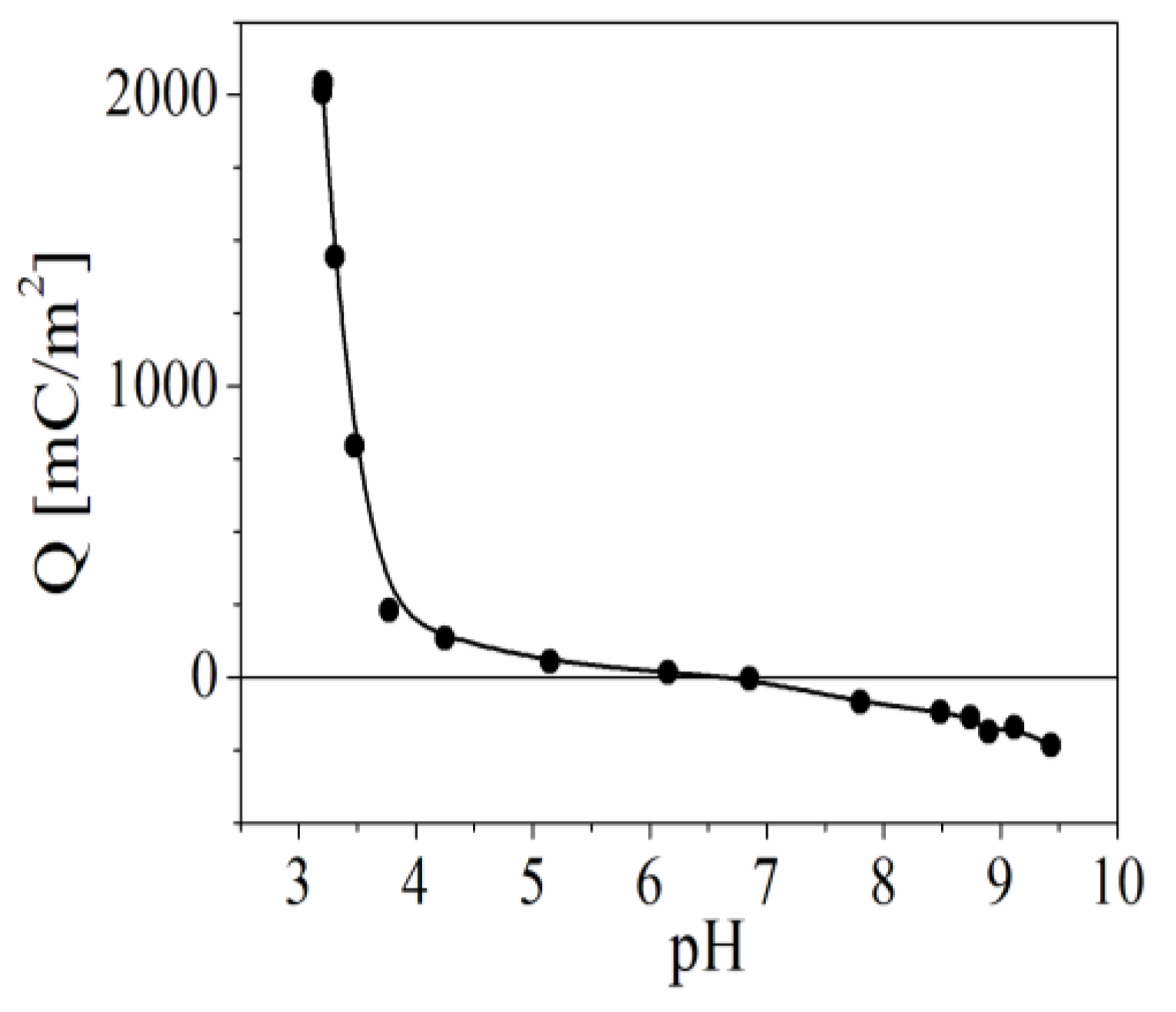
3.1. Surface Charge Density of the Model Oxide β-FeOOH Particles
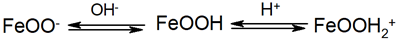
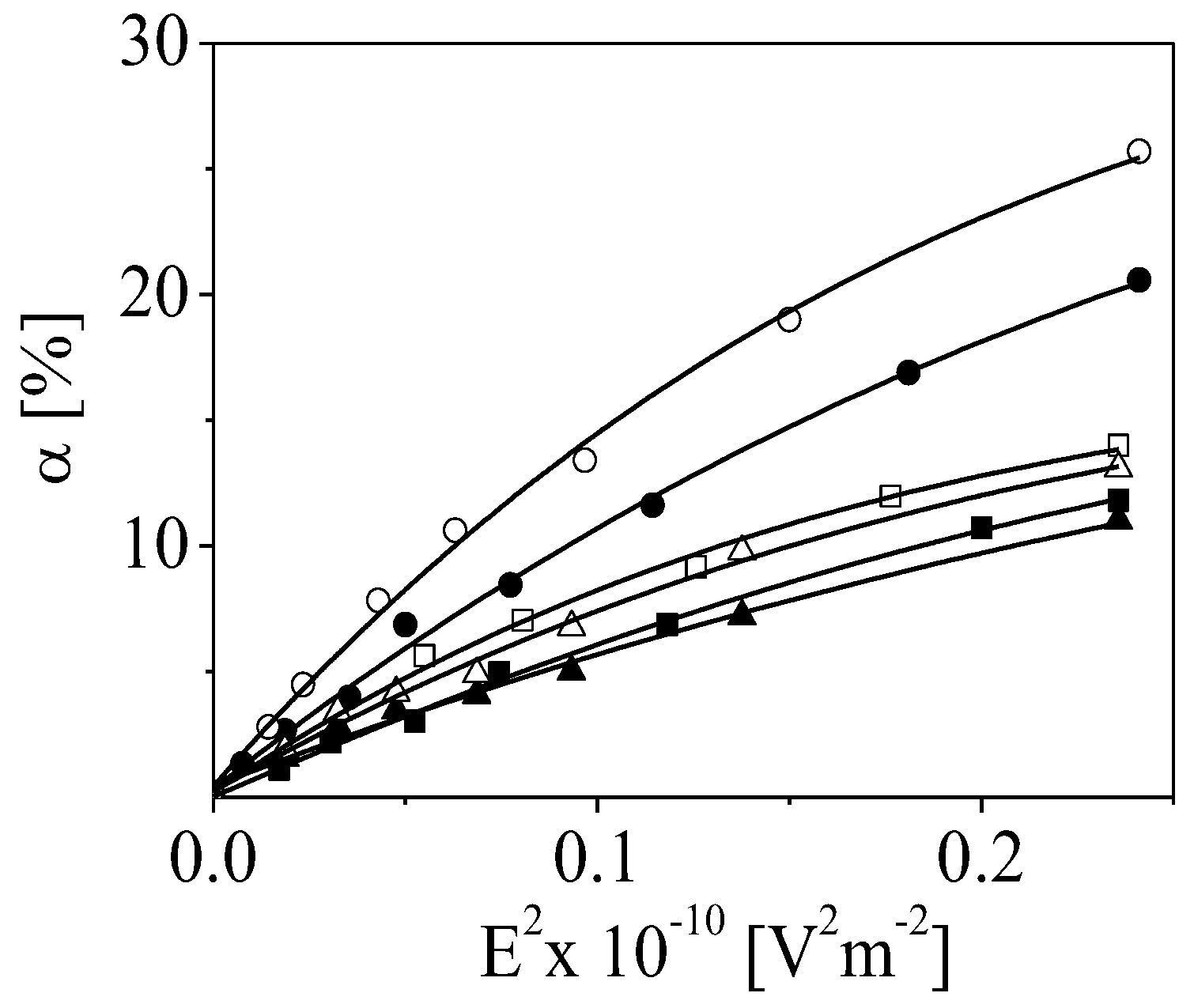
3.2. Characterization of Produced Colloid–Polymer Complexes
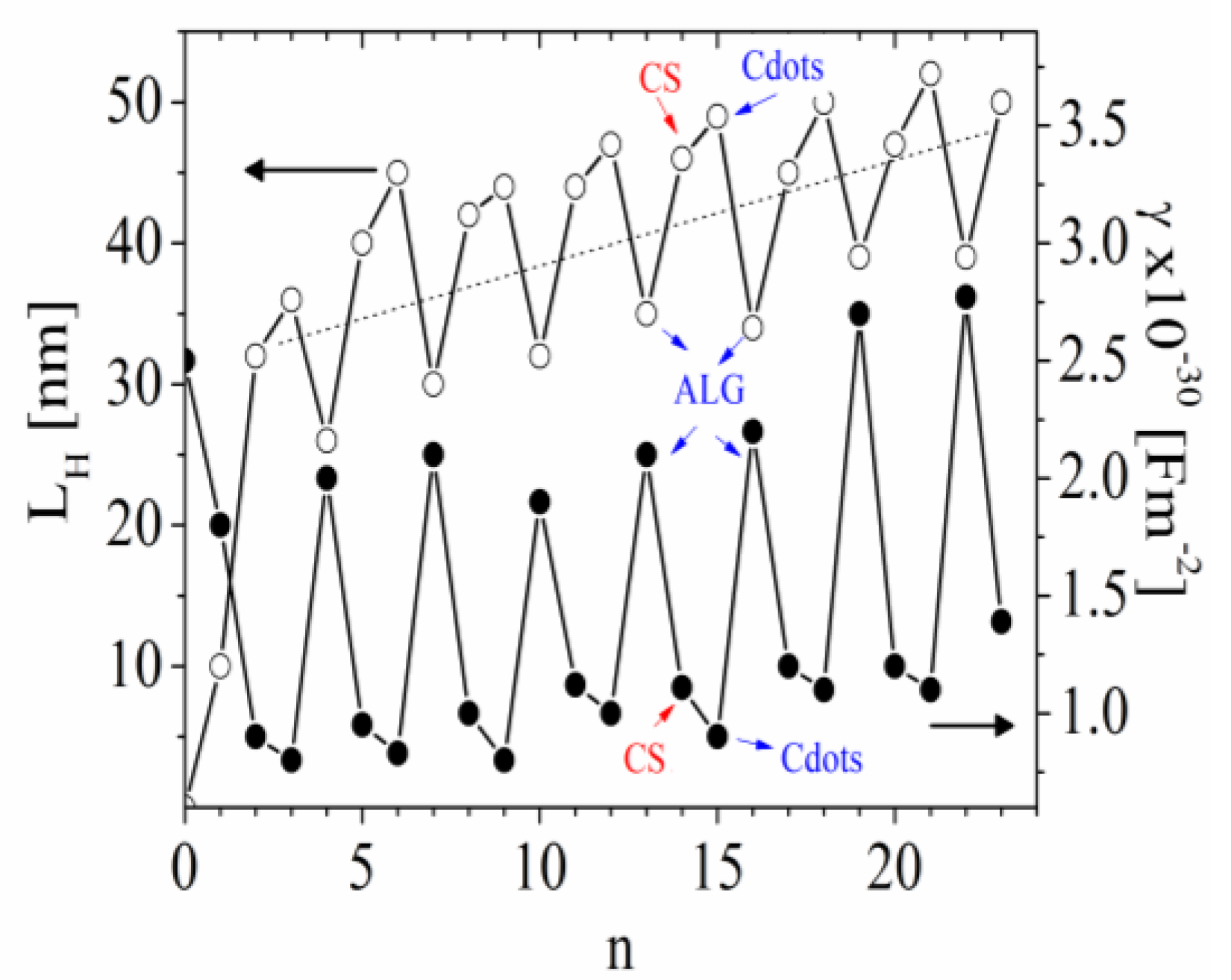
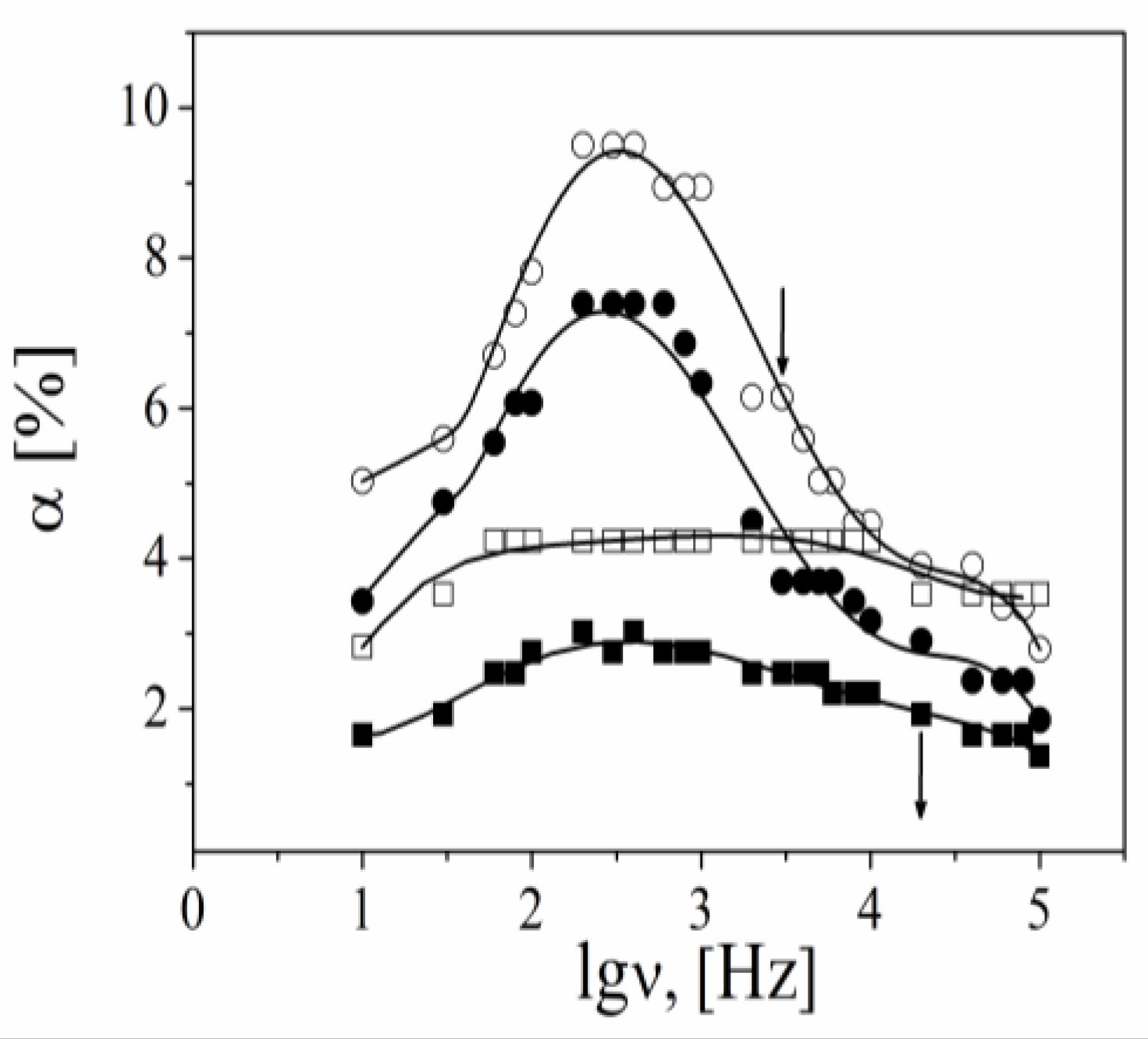
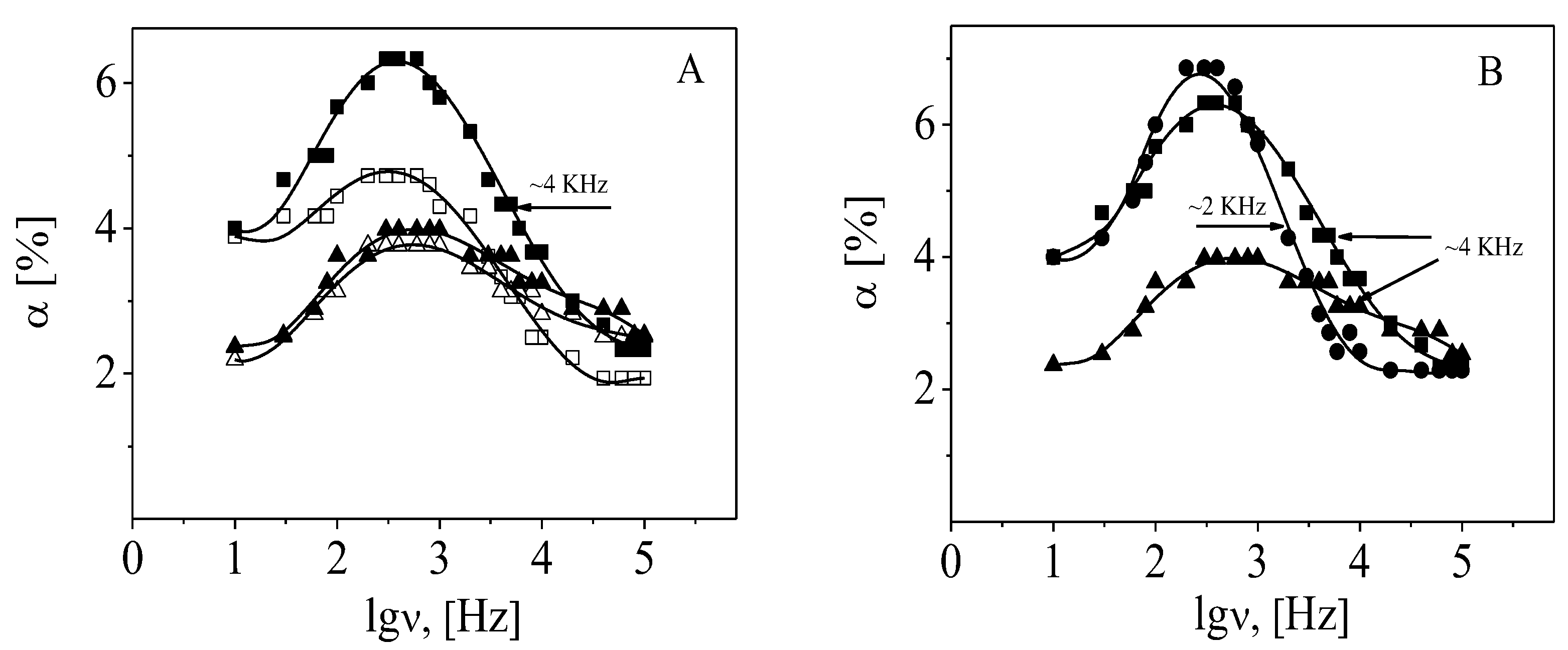
3.3. Dynamics of the Counterions
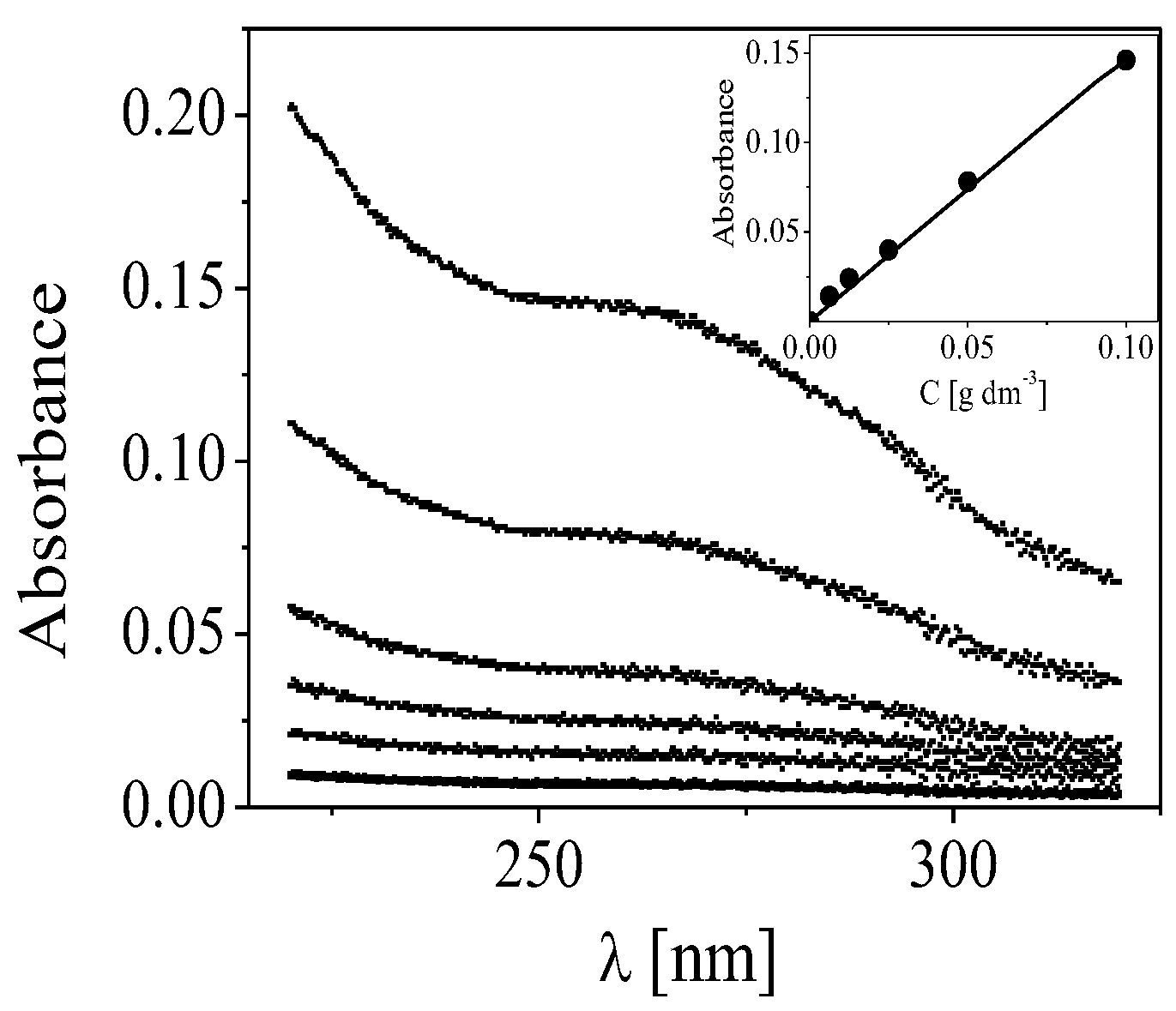
3.4. Estimation of the Concentration of Cdots in the Film
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cyu, J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem. Cell Press 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Iakobson, O.; Ivankova, E.; Shevchenko, N. Photonic Crystal Films Based on Polymer Particles with a Core/Shell Structure Responding to Ethanol. Langmuir 2023, 39, 9952–9962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, S.; He, W.; Gong, S.; Zhang, Y.; Zhao, X.; Fu, Y.; Zhu, Z. Modeling and characterization of the electrical conductivity on metal nanoparticles/carbon nanotube/polymer composites. Sci. Rep. 2022, 12, 10448. [Google Scholar] [CrossRef] [PubMed]
- Decher, J.D.; Hong, J.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Sol. Film. 1992, 210/211, 831–835. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Coll. Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Abubakre, O.K.; Medupin, R.O.; Akintunde, I.B.; Jimoh, O.T.; Abdulkareem, A.S.; Muriana, R.A.; James, J.A.; Ukoba, K.O.; Jen, T.-C.; Yoro, K.O. Carbon nanotube-reinforced polymer nanocomposites for sustainable biomedical applications: A review. J. Sci. Adv. Mater. Devices 2023, 8, 100557. [Google Scholar] [CrossRef]
- Mirabello, G.; Steinmetz, L.; Geers, C.; Rothen-Ruthishauser, B.; Bonmarin, M.; Petri-Fink, A.; Lattuada, M. Quantification of nanoparticles’ concentration inside polymer films using lock-in thermography. Nanoscale Adv. 2023, 5, 2963–2972. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 26, 12736–12737. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6727–6744. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2015, 15, 382–393. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Sapelkin, A.V.; Sukhorukov, G.B. Carbon nanodots: Mechanism of photoluminescence and principles of application. Trends Anal. Chem. 2017, 7, 27–37. [Google Scholar] [CrossRef]
- Roy, P.; Chen, P.-C.; Periasamy, A.P.; Chen, Y.-N.; Chang, H.-T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. NBM 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhourukov, G.B. In Situ Synthesis of Fluorescent carbon dots/polyelectrolyte nanocomposite microcapsules with reduced permeability and ultrasound sensitivity. Am. Chem. Soc. Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef]
- Yang, X.; Peng, L.; Zong, J.; Zhu, Y. Preparation of photoluminescent carbon dots-embedded polyelectrolyte microcapsules. Particuology 2013, 11, 334–339. [Google Scholar] [CrossRef]
- Chen, B.; Feng, J. White-Light-Emitting Polymer Composite Film Based on Carbon Dots and Lanthanide Complexes. J. Phys. Chem. C 2015, 119, 7865–7872. [Google Scholar] [CrossRef]
- Bazazi, S.; Hosseini, S.P.; Hashemi, E.; Rashidzadeh, B.; Liu, Y.; Saeb, M.R.; Xiao, H.; Seidi, F. Polysaccharide-based C-dots and polysaccharide/C-dot nanocomposites: Fabrication strategies and applications. Nanoscale 2023, 15, 3630–3650. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Natural Chelating Polymers: Alginic Acid, Chitin and Chitosan; Pergamon Press: Oxford, UK, 1973; p. 254. [Google Scholar]
- Radeva, T.; Widmaier, J.; Petkanchin, I. Adsorption of Hydrolyzed Polyacrylamides on Ferric Oxide Particles: Counterion Mobility in Stabilized Suspensions. J. Colloid. Interfaces Sci. 1997, 189, 23–26. [Google Scholar] [CrossRef]
- Radeva, T.; Petkanchin, I. Electric Properties of Adsorbed Polystyrenesulfonate: I: Dependence on the Polyelectrolyte Molecular Weight. J. Colloid. Interfaces Sci. 1999, 220, 112–117. [Google Scholar] [CrossRef]
- Milkova, V. Polyelectrolyte/nanoparticle hybrid films on anisometric colloids studied by electro-optics. Colloids Surf. A. 2014, 455, 156–163. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. Fractionation of alginate by precipitation with calcium and magnesium ions. Acta Chem. Scand. 1965, 19, 1221–1226. [Google Scholar] [CrossRef]
- Donati, I.; Paoletti, S. Material Properties of Alginates. In Alginates: Biology and Applications, Microbiology Monographs; Rehm, B.H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30–35. [Google Scholar] [CrossRef]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The usage and potential uses of alginate for healthcare applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, U.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, J.; Pei, X.; Chen, J.; Wan, Q. Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123246. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Zocher, H.; Heller, W. Schillerschichten als Reaktionsprodukte der langsamen Eisenchlorid-Hydrolyse [Iridescent layers produced by slow hydrolysis of iron chloride]. Z. Anorg. Allg. Chem. 1930, 186, 75–96. [Google Scholar] [CrossRef]
- Stoylov, S.P. Colloid Electro-Optics; Academic Press: London, UK, 1991; pp. 15–27. [Google Scholar]
- O’Konski, C.T.; Yoshioka, K.; Orttung, W.H. Electric Properties of Macromolecules. IV. Determination of Electric and Optical Parameters from Saturation of Electric Birefringence in Solutions. J. Phys. Chem. 1959, 63, 1558–1565. [Google Scholar]
- Perrin, F. Mouvement brownien d’un ellipsoide. I. Dispersion dielectrique pour des molecules ellipsoidales. J. Phys. Radium 1934, 5, 497–511. [Google Scholar]
- Radeva, T.; Milkova, V.; Petkanchin, I. Structure and electrical properties of polyelectrolyte multilayers formed on anisometric colloidal particles. J. Colloid. Interface Sci. 2001, 244, 24–30. [Google Scholar] [CrossRef]
- Kamburova, K.; Milkova, V.; Petkanchin, I.; Radeva, T. Effect of pectin charge density on formation of multilayer films with chitosan. Biomacromolecules 2008, 9, 1242–1247. [Google Scholar] [CrossRef]
- Radeva, T.; Kamburova, K.; Petkanchin, I. Formation of polyelectrolyte multilayers from polysaccharides at low ionic strength. J. Colloid. Interface Sci. 2006, 298, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Milkova, V.; Radeva, T. Influence of charge density and calcium salt on stiffness of polysaccharides multilayer film. Colloid. Surf. A Physicochem. Eng. Asp. 2015, 481, 13–19. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Mahapatra, D.M. Interfacial properties of two hydrous iron oxides in KNO3 solution. Colloids Surf. A 1989, 42, 173–189. [Google Scholar] [CrossRef]
- Radeva, T.; Petkanchin, I.; Varoqui, R. Electrical and hydrodynamic properties of colloid-polymer surface layers investigated by electro-optics. Langmuir 1993, 9, 170–173. [Google Scholar] [CrossRef]
- Matijevic, E.; Scheiner, P. Ferric hydrous oxide sols: III. Preparation of uniform particles by hydrolysis of Fe(III)-chloride, -nitrate, and -perchlorate solutions. J. Colloid. Interface Sci. 1978, 63, 509–524. [Google Scholar] [CrossRef]
- Fleer, G.; Hoogeveen, N.G.; Cohen Stuart, M.A.; Böhmer, M.R. Formation and stability of multilayers of polyelectrolytes. Langmuir 1996, 12, 3675–3681. [Google Scholar]
- Lavalle, P.; Picart, C.; Mutterer, J.; Gergely, C.; Reiss, H.; Voegel, J.C. Modeling the buildup of polyelectrolyte multilayer films having exponential growth. J. Phys. Chem. B 2004, 108, 635–648. [Google Scholar] [CrossRef]
- Sui, Z.; Salloum, D.; Schlenoff, J.B. Effect of molecular weight on the construction of polyelectrolyte multilayers: Stripping versus sticking. Langmuir 2003, 19, 2491. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef]
- Milkova, V.; Radeva, T. Counterion release from adsorbed highly charged polyelectrolyte—an electrooptical study. J. Colloid. Interface Sci. 2006, 289, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P.; Schwarz, G.; Maczuk, J.; Payly, H. On the low-frequency dielectric dispersion of colloidal particles in electrolyte solution. J. Phys. Chem. 1962, 66, 2626–2635. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar]
- Claesson, P.M.; Ninham, B.W. pH-Dependent Interactions between Adsorbed chitosan layers. Langmuir 1992, 8, 1406–1412. [Google Scholar] [CrossRef]
- Arguelles-Mondal, W.; Cabrera, G.; Peniche, C.; Rinaudo, M. Conductimetric study of the interpolyelectrolyte reaction between chitosan and polygalacturonic acid. Polymer 2000, 41, 373–2378. [Google Scholar]
- Donati, I.; Cesaro, A.; Paoletti, S. Specific interactions versus counterion condensation. 1. Nongelling ions/polyuronate systems. Biomacromolecules 2006, 7, 281–287. [Google Scholar] [CrossRef]
- Milkova, V. Comparative Electrokinetic Study of Alginate-Coated Colloidal Particles. Gels 2023, 9, 493. [Google Scholar] [CrossRef]
- Ookubo, N.; Hirai, Y.; Ito, K.; Hayakawa, H. Anisotropic counterion polarizations and their dynamics in aqueous polyelectrolytes as studied by frequency domain electric birefringence relaxation spectroscopy. Macromolecules 1989, 22, 1359–1366. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milkova, V. Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles—An Electro-Optical Study. Polymers 2023, 15, 3766. https://doi.org/10.3390/polym15183766
Milkova V. Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles—An Electro-Optical Study. Polymers. 2023; 15(18):3766. https://doi.org/10.3390/polym15183766
Chicago/Turabian StyleMilkova, Viktoria. 2023. "Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles—An Electro-Optical Study" Polymers 15, no. 18: 3766. https://doi.org/10.3390/polym15183766
APA StyleMilkova, V. (2023). Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles—An Electro-Optical Study. Polymers, 15(18), 3766. https://doi.org/10.3390/polym15183766





