Multi-Physically Cross-Linked Hydrogels for Flexible Sensors with High Strength and Self-Healing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Multi-Physically Cross-Linked Hydrogels
2.3. FTIR Spectroscopy
2.4. X-ray Diffraction Analysis
2.5. Scanning Electron Microscopy
2.6. Rheological Properties
2.7. Uniaxial Tensile Tests
2.8. Energy Dissipation Performance
2.9. Swelling Performance Test
2.10. Conductivity Tests
3. Results and Discussion
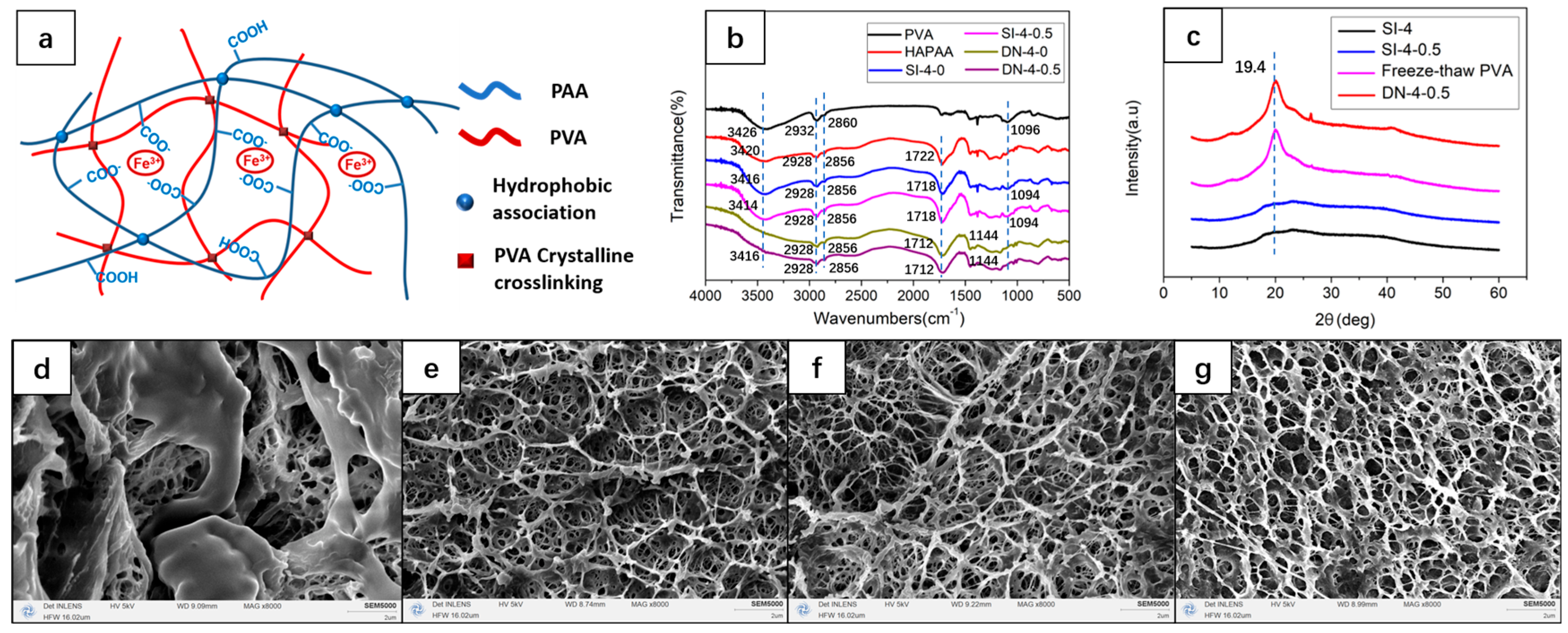
3.1. Network Structure and Swelling Properties
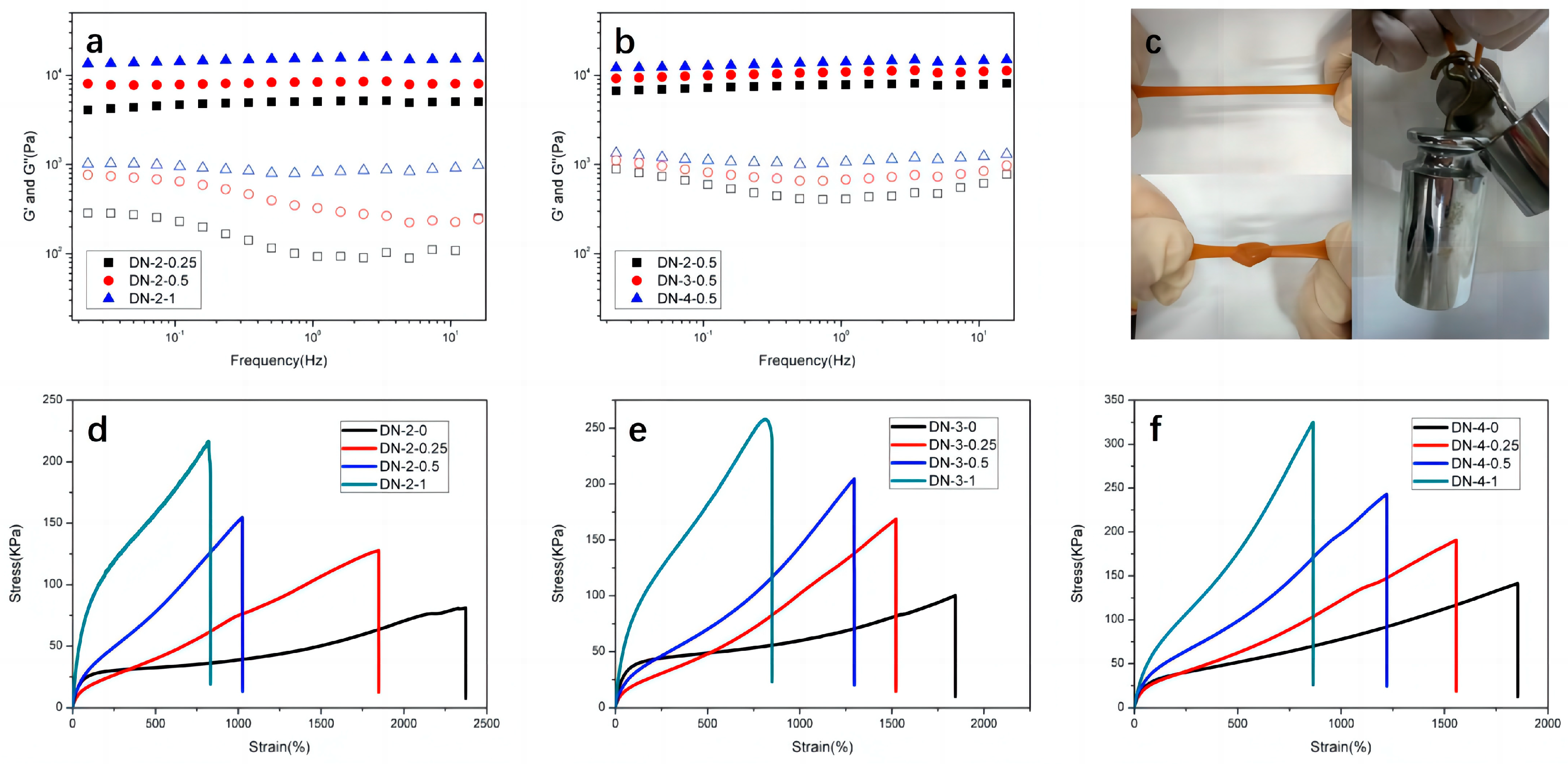
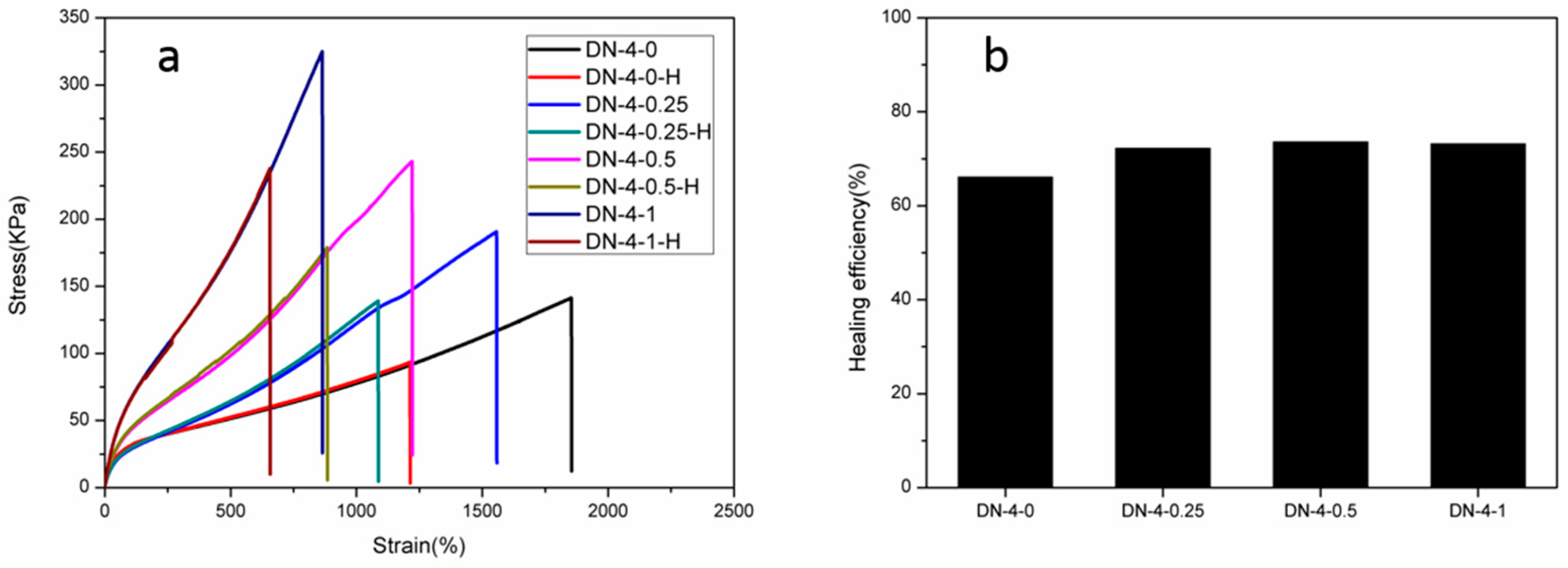
3.2. Mechanical Properties
3.3. Conductive Sensing Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Emam, H.E.; Shaheen, T.I. Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef] [PubMed]
- Zu, S.; Wang, Z.; Zhang, S.; Guo, Y.; Chen, C.; Zhang, Q.; Wang, Z.; Liu, T.; Liu, Q.; Zhang, Z. A bioinspired 4D printed hydrogel capsule for smart controlled drug release. Mater. Today Chem. 2022, 24, 100789. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Zheng, M.; Yue, O.; Huang, M.; Zou, X.; Cui, B.; Xie, L.; Dong, S.; Shang, J.; et al. Mechanically Robust and Transparent Organohydrogel-Based E-Skin Nanoengineered from Natural Skin. Adv. Funct. Mater. 2023, 33, 2212856. [Google Scholar] [CrossRef]
- Bian, S.; Hao, L.; Qiu, X.; Wu, J.; Chang, H.; Kuang, G.-M.; Zhang, S.; Hu, X.; Dai, Y.; Zhou, Z.; et al. An Injectable Rapid-Adhesion and Anti-Swelling Adhesive Hydrogel for Hemostasis and Wound Sealing. Adv. Funct. Mater. 2022, 32, 2207741. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Yang, D.; Wu, P. A Multi-Scale Structural Engineering Strategy for High-Performance MXene Hydrogel Supercapacitor Electrode. Adv. Sci. 2021, 8, 2101664. [Google Scholar] [CrossRef]
- Lu, H.; Li, M.; Wang, X.; Wang, Z.; Pi, M.; Cui, W.; Ran, R. Recyclable physical hydrogels as durable and efficient solar-driven evaporators. Chem. Eng. J. 2022, 450, 138257. [Google Scholar] [CrossRef]
- Su, X.; Hao, D.; Sun, M.; Wei, T.; Xu, D.; Ai, X.; Guo, X.; Zhao, T.; Jiang, L. Nature Sunflower Stalk Pith with Zwitterionic Hydrogel Coating for Highly Efficient and Sustainable Solar Evaporation. Adv. Funct. Mater. 2022, 32, 2108135. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Huang, G.; Xie, Y.; Zheng, M.; Feng, J.; Kan, K.; Shen, J. Novel Uracil-Functionalized Poly(ionic liquid) Hydrogel: Highly Stretchable and Sensitive as a Direct Wearable Ionic Skin for Human Motion Detection. ACS Appl. Mater. Interfaces 2023, 15, 11062–11075. [Google Scholar] [CrossRef]
- Kim, Y.; Yuk, H.; Zhao, R.; Chester, S.A.; Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.; Wu, W.; Bai, D.; Liang, Y.; Hu, S.; Yu, W.; He, P.; Zhang, J. Ionic liquid–polymer thermochromic electrolytes with a wide and tunable LCST for application in multi-stimuli-responsive optical modulation. J. Mater. Chem. A 2023, 11, 9626–9634. [Google Scholar] [CrossRef]
- Liang, J.; Li, B.; Gai, X.; Li, N.; Wang, J.; Zhang, Y.; Zhou, Q.; Sun, Y. Photochromic/electrochromic strain sensor with a fast and reversible light-printing ability. J. Mater. Chem. C 2023, 11, 3634–3643. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive polymer based hydrogels and their application in wearable sensors: A review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Cao, J.; Zhang, X.; Zhang, Y.; Yin, S.; Jia, L.; Guo, Q.; Zhang, X.; Zhang, J.; Zhou, T. Human-tissue-inspired anti-fatigue-fracture hydrogel for a sensitive wide-range human–machine interface. J. Mater. Chem. A 2020, 8, 2074–2082. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Li, M.; Yan, B.; Ran, R. Near-Infrared Laser “Weldable” Hydrogen-Bonded Hydrogel Sensor Based on Photothermal Gel–Sol Transition. ACS Sustain. Chem. Eng. 2021, 9, 16241–16250. [Google Scholar] [CrossRef]
- Su, G.; Zhang, Y.; Zhang, X.; Feng, J.; Cao, J.; Zhang, X.; Zhou, T. Soft yet Tough: A Mechanically and Functionally Tissue-like Organohydrogel for Sensitive Soft Electronics. Chem. Mater. 2022, 34, 1392–1402. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Feng, J.; Peng, J.; Zhou, Y.; Zhao, Y.; Zhao, Y.; Lu, Z.; Sun, M.; Wu, C.; et al. A mechanically soft-tissue-like organohydrogel with multi-functionalities for sensitive soft ionotronics. Chem. Eng. J. 2023, 466, 143087. [Google Scholar] [CrossRef]
- Dong, X.; Guo, X.; Liu, Q.; Zhao, Y.; Qi, H.; Zhai, W. Strong and Tough Conductive Organo-Hydrogels via Freeze-Casting Assisted Solution Substitution. Adv. Funct. Mater. 2022, 32, 2203610. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, W.; Wen, J.; Wu, Z.; Zhang, Y.; Zhang, H.; Wu, H.; Yao, H.; Xue, H.; Gao, J. Mixed solvent exchange enabled high-performance polymeric gels. Polymer 2023, 267, 125661. [Google Scholar] [CrossRef]
- Wu, P.; Qin, Z.; Dassanayake, R.; Sun, Z.; Cao, M.; Fu, K.; Zhou, Y.; Liu, Y. Antimicrobial MXene-based conductive alginate hydrogels as flexible electronics. Chem. Eng. J. 2023, 455, 140546. [Google Scholar] [CrossRef]
- Su, G.; Yin, S.; Guo, Y.; Zhao, F.; Guo, Q.; Zhang, X.; Zhou, T.; Yu, G. Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications. Mater. Horiz. 2021, 8, 1795–1804. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-Strength, Self-Healable, Temperature-Sensitive, MXene-Containing Composite Hydrogel as a Smart Compression Sensor. ACS Appl. Mater. Interfaces 2019, 11, 47350–47357. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Liu, Z.; Xie, R.; Zhang, Y.; Yang, Y.; Zhao, X.; Zhang, Y.; Yang, A.; Cheng, Y. Nanocomposite conductive hydrogels with Robust elasticity and multifunctional responsiveness for flexible sensing and wound monitoring. Mater. Horiz. 2023, 10, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors. Small 2022, 18, 2101518. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, K.; Fan, C.; Zhao, X.; Gao, J.; Jing, W.; Zhang, X.; Li, J.; Li, Y.; Yang, J.; et al. An Ultrasoft Self-Fused Supramolecular Polymer Hydrogel for Completely Preventing Postoperative Tissue Adhesion. Adv. Mater. 2021, 33, 2008395. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, L.; Li, Y.; Shi, Z.; Wei, Q.; Ma, G.; Zhang, W.; Guo, Y.; Wu, P.; Hu, Z. Double Hydrogen-bonding Reinforced High-Performance Supramolecular Hydrogel Thermocell for Self-powered Sensing Remote-Controlled by Light. Adv. Funct. Mater. 2023, 33, 2211720. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, Y.; Que, X.; Zhang, Z.; Peng, J.; Li, J.; Zhai, M. Super Adhesive MXene-based Nanocomposite Hydrogel with Self-Healable and Conductivity Properties via Radiation Synthesis. Adv. Eng. Mater. 2022, 24, 2101692. [Google Scholar] [CrossRef]
- Huang, S.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2110140. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, W.; Wang, L.; Yao, C.; Wang, C.; Gu, B.; Li, D.; He, J. Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property. Polymers 2022, 14, 3908. [Google Scholar] [CrossRef]
- Cai, S.; Niu, B.; Ma, X.; Wan, S.; He, X. High strength, recyclable, anti-swelling and shape-memory hydrogels based on crystal microphase crosslinking and their application as flexible sensor. Chem. Eng. J. 2022, 430, 132957. [Google Scholar] [CrossRef]
- Hao, X.P.; Zhang, C.W.; Zhang, X.N.; Hou, L.X.; Hu, J.; Dickey, M.D.; Zheng, Q.; Wu, Z.L. Healable, Recyclable, and Multifunctional Soft Electronics Based on Biopolymer Hydrogel and Patterned Liquid Metal. Small 2022, 18, 2201643. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Wang, X.; Zou, X.; Zheng, S.; Liu, Z.; Li, Q.; Xia, Q.; Yan, F. Ultra-Tough and Recyclable Ionogels Constructed by Coordinated Supramolecular Solvents. Angew. Chem. Int. Ed. 2022, 61, e202212512. [Google Scholar] [CrossRef]
- Shi, C.; Yang, F.; Hu, L.; Wang, H.; Wang, Y.; Wang, Z.; Pan, S.; Chen, J. Construction of polysaccharide based physically crosslinked double-network antibacterial hydrogel. Mater. Lett. 2022, 316, 132048. [Google Scholar] [CrossRef]
- Ailincai, D.; Turin Moleavin, I.-A.; Sarghi, A.; Fifere, A.; Dumbrava, O.; Pinteala, M.; Balan, G.G.; Rosca, I. New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers 2023, 15, 2669. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, X.; Tian, C.; Zhong, Y.; Yan, M.; Miao, C.; Wu, T.; Zhou, X. Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels. Gels 2023, 9, 484. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Dao, L.; Liu, L.; Yang, Y.; Liu, J.; Wu, S.; Cheng, Y.; Pang, J. A conductive bio-hydrogel with high conductivity and mechanical strength via physical filling of electrospinning polyaniline fibers. Colloids Surf. A 2022, 637, 128190. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Z.; Huo, Y.; Shen, Y.; Qin, M.; Feng, W. Tetradic double-network physical crosslinking hydrogels with synergistic high stretchable, self-healing, adhesive, and strain-sensitive properties. J. Mater. Sci. Technol. 2022, 98, 169–176. [Google Scholar] [CrossRef]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Hong, L.; Liu, L.; Zhang, Z.; Song, J.; Li, S.; Chen, K.; Gao, G.; Wang, Y. Tough and self-healing hydrogels based on transient crosslinking by nanoparticles. Soft Matter 2022, 18, 1885–1895. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. Developing tough terpolymer hydrogel with outstanding swelling ability by hydrophobic association cross-linking. Polymer 2022, 254, 125037. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Li, M.; Shang, Q.; Xie, R.; Yu, J.; Shen, K.; Zhang, Y.; Cheng, Y. Toughening Double-Network Hydrogels by Polyelectrolytes. Adv. Mater. 2023, 35, e2301551. [Google Scholar] [CrossRef] [PubMed]
- Abolpour Moshizi, S.; Moradi, H.; Wu, S.; Han, Z.J.; Razmjou, A.; Asadnia, M. Biomimetic Ultraflexible Piezoresistive Flow Sensor Based on Graphene Nanosheets and PVA Hydrogel. Adv. Mater. Technol. 2022, 7, 2100783. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Mahmoudpour, M.; Rahimpour, E.; Jouyban, A. Nanomaterial based PVA nanocomposite hydrogels for biomedical sensing: Advances toward designing the ideal flexible/wearable nanoprobes. Adv. Colloid Interface Sci. 2022, 305, 102705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ji, C.; Meng, Q.; Mi, H.; Yang, Q.; Li, Z.; Yang, N.; Qiu, J. Freeze-Tolerant Hydrogel Electrolyte with High Strength for Stable Operation of Flexible Zinc-Ion Hybrid Supercapacitors. Small 2022, 18, 2200055. [Google Scholar] [CrossRef]
- Janarthanan, G.; Noh, I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 2021, 63, 35–53. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly(acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]



| Gels | AA (g) | SMA (g) | SDBS (g) | PVA (g) | FeCl3·6H2O (g) | KPS (g) | H2O (g) |
|---|---|---|---|---|---|---|---|
| HAPAA | 3 | 0.22 | 0.45 | 0 | 0 | 0.01 | 11.32 |
| PAA-2-0.25 | 3 | 0.22 | 0.45 | 0.3 | 0.028 | 0.01 | 11 |
| PAA-2-0.5 | 3 | 0.22 | 0.45 | 0.3 | 0.056 | 0.01 | 10.96 |
| PAA-2-1 | 3 | 0.22 | 0.45 | 0.3 | 0.112 | 0.01 | 10.91 |
| PAA-3-0.25 | 3 | 0.22 | 0.45 | 0.45 | 0.028 | 0.01 | 10.85 |
| PAA-3-0.5 | 3 | 0.22 | 0.45 | 0.45 | 0.056 | 0.01 | 10.82 |
| PAA-3-1 | 3 | 0.22 | 0.45 | 0.45 | 0.112 | 0.01 | 10.77 |
| PAA-4-0.25 | 3 | 0.22 | 0.45 | 0.6 | 0.028 | 0.01 | 10.7 |
| PAA-4-0.5 | 3 | 0.22 | 0.45 | 0.6 | 0.056 | 0.01 | 10.67 |
| PAA-4-1 | 3 | 0.22 | 0.45 | 0.6 | 0.112 | 0.01 | 10.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, S.; Tian, Y.; Chen, L.; Du, Y.; Su, G.; Hu, Y. Multi-Physically Cross-Linked Hydrogels for Flexible Sensors with High Strength and Self-Healing Properties. Polymers 2023, 15, 3748. https://doi.org/10.3390/polym15183748
Zhang Y, Wang S, Tian Y, Chen L, Du Y, Su G, Hu Y. Multi-Physically Cross-Linked Hydrogels for Flexible Sensors with High Strength and Self-Healing Properties. Polymers. 2023; 15(18):3748. https://doi.org/10.3390/polym15183748
Chicago/Turabian StyleZhang, Yulin, Shiyu Wang, Yi Tian, Long Chen, Yuhan Du, Gehong Su, and Yu Hu. 2023. "Multi-Physically Cross-Linked Hydrogels for Flexible Sensors with High Strength and Self-Healing Properties" Polymers 15, no. 18: 3748. https://doi.org/10.3390/polym15183748
APA StyleZhang, Y., Wang, S., Tian, Y., Chen, L., Du, Y., Su, G., & Hu, Y. (2023). Multi-Physically Cross-Linked Hydrogels for Flexible Sensors with High Strength and Self-Healing Properties. Polymers, 15(18), 3748. https://doi.org/10.3390/polym15183748








