Aqueous Cationic Fluorinated Polyurethane for Application in Novel UV-Curable Cathodic Electrodeposition Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
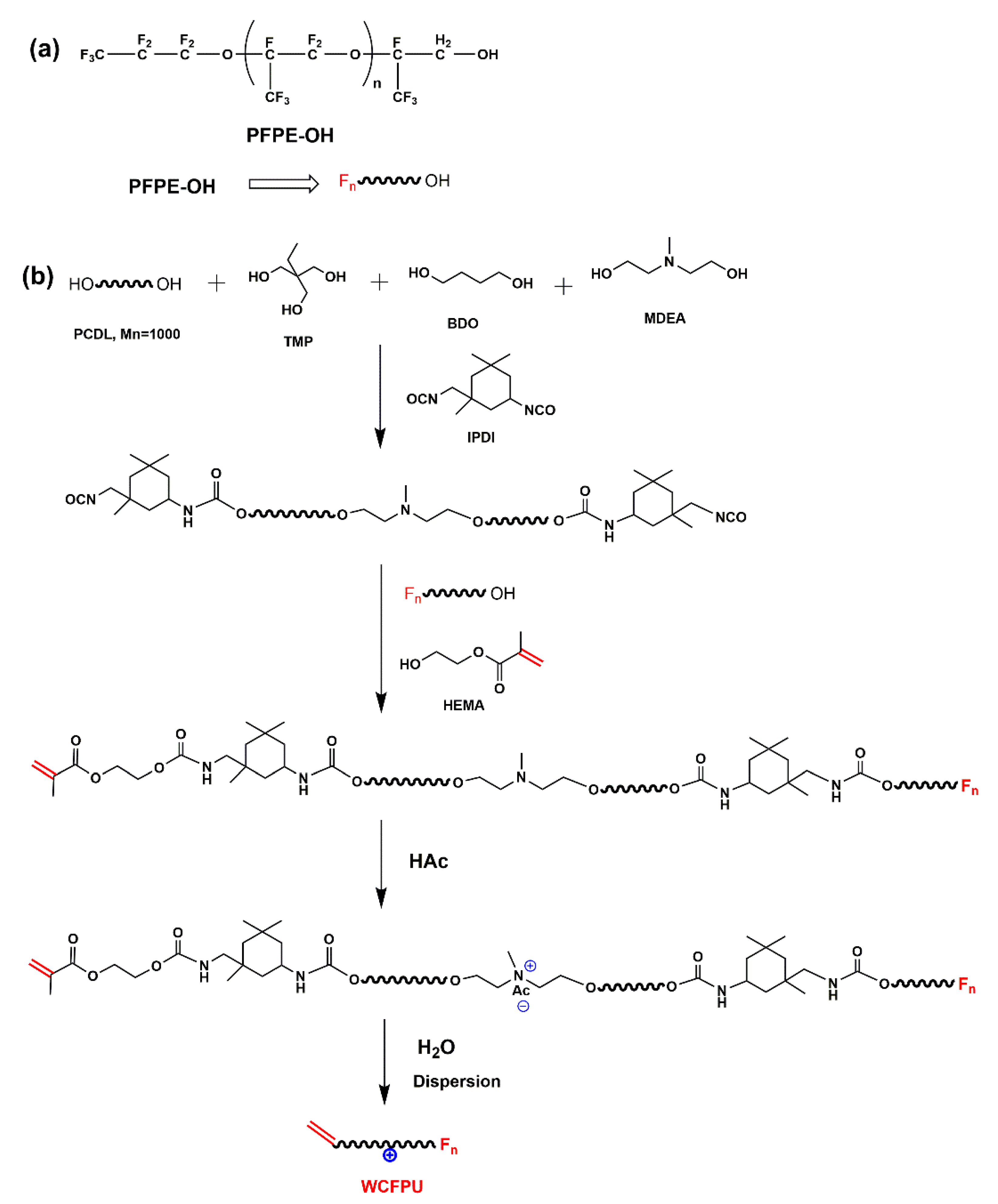
2.2. Cationic Aqueous Fluorinated Polyurethane Emulsion Preparation (WCFPU)
2.3. UV-Cured Cathodic Electrodeposition Coating Preparation
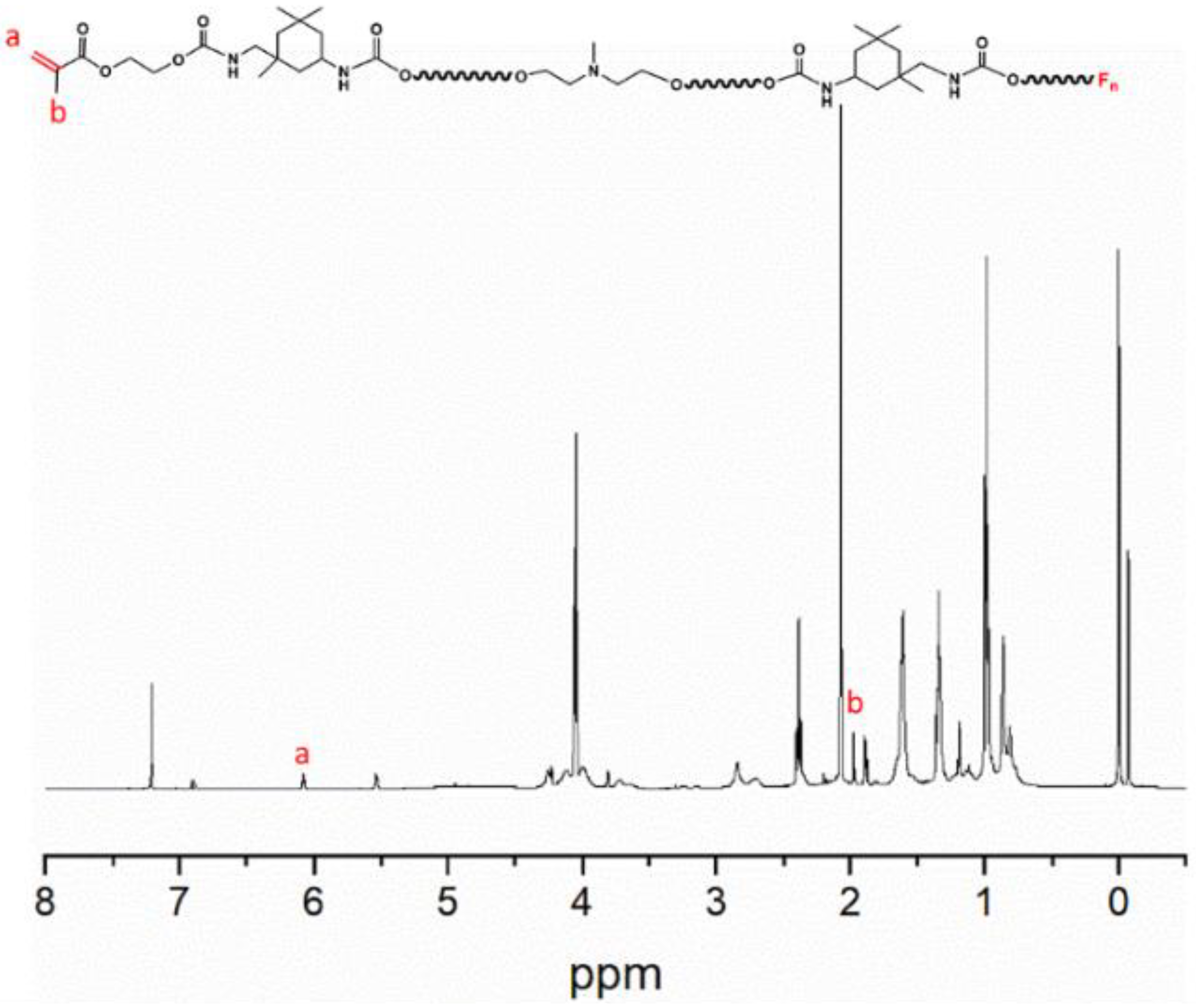
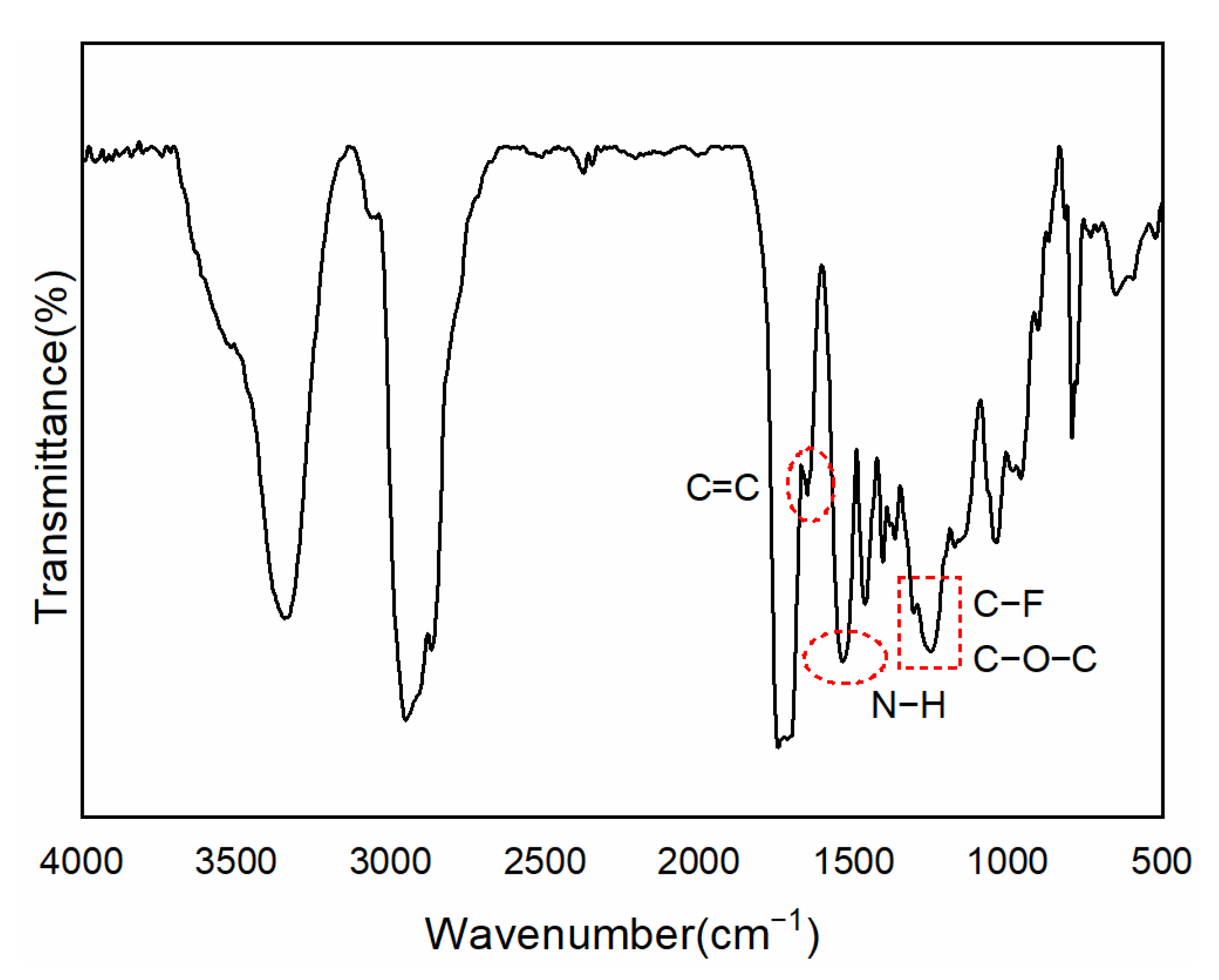
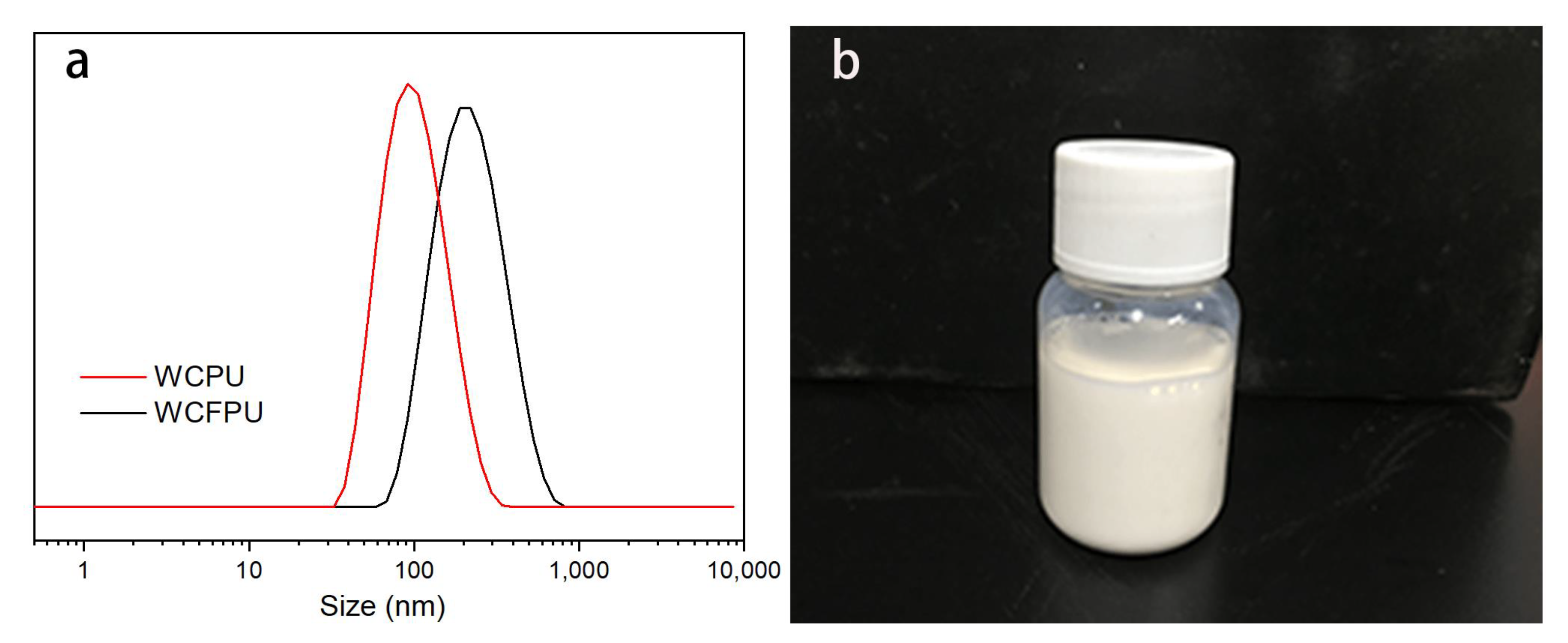
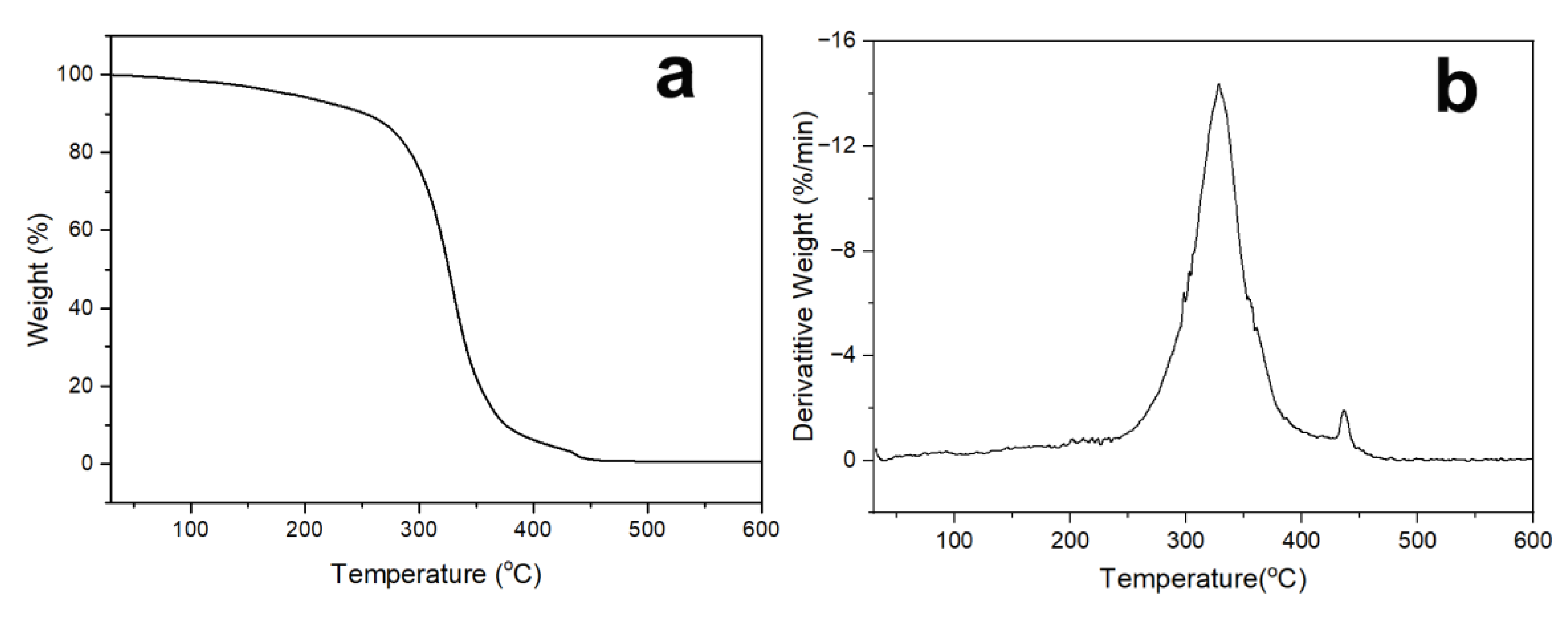
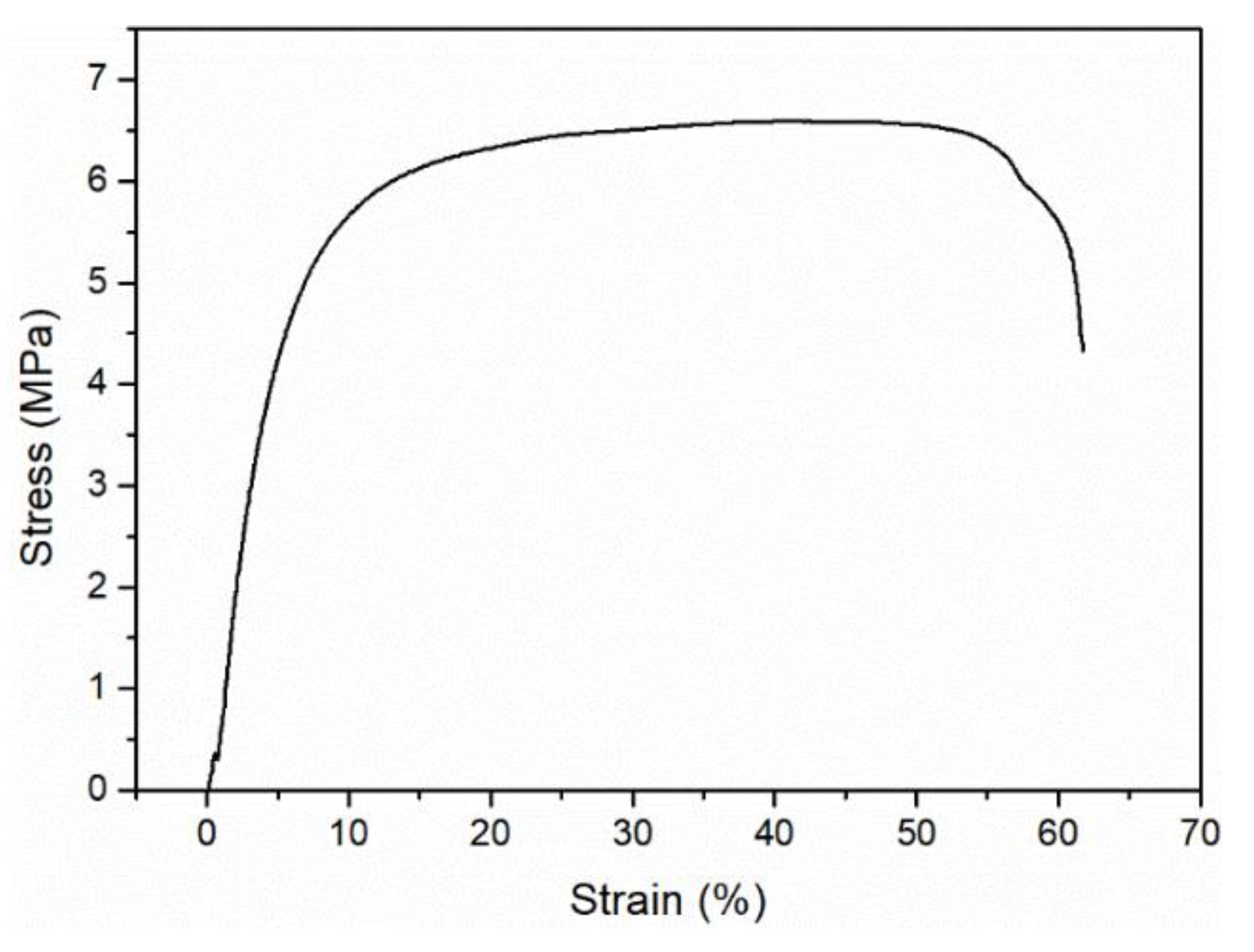
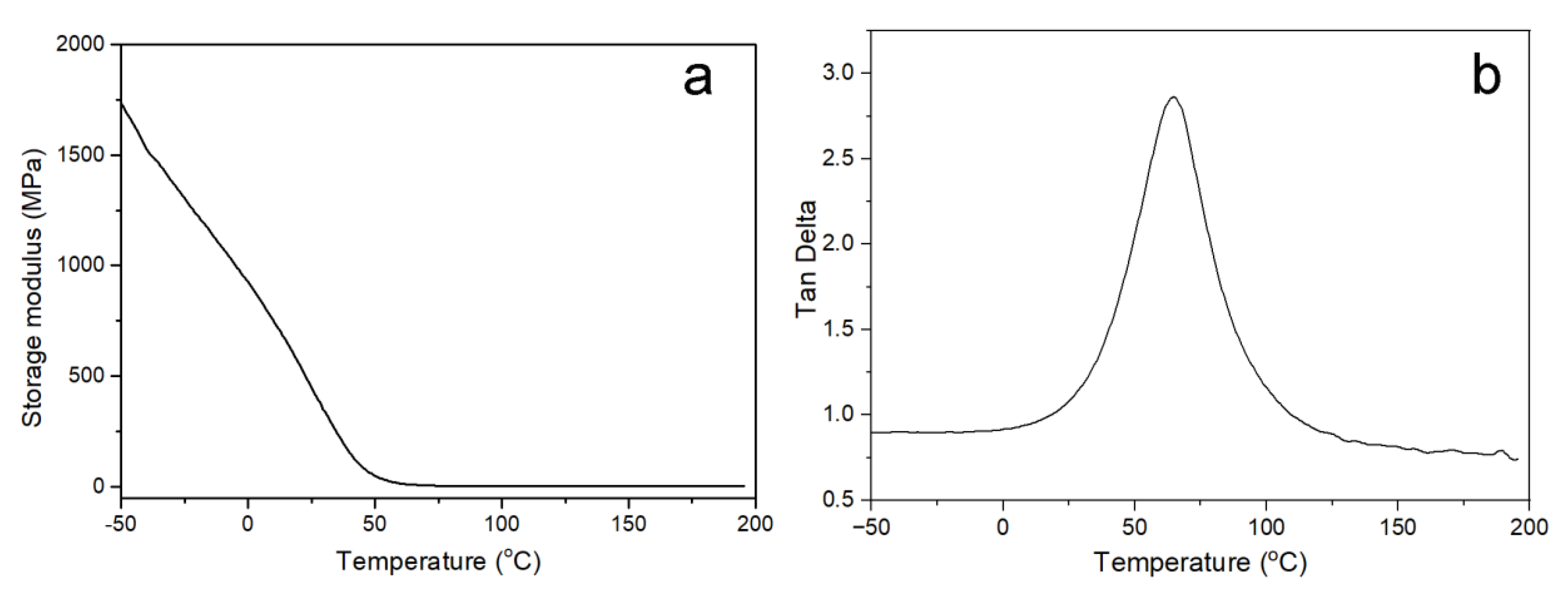
2.4. Characterization
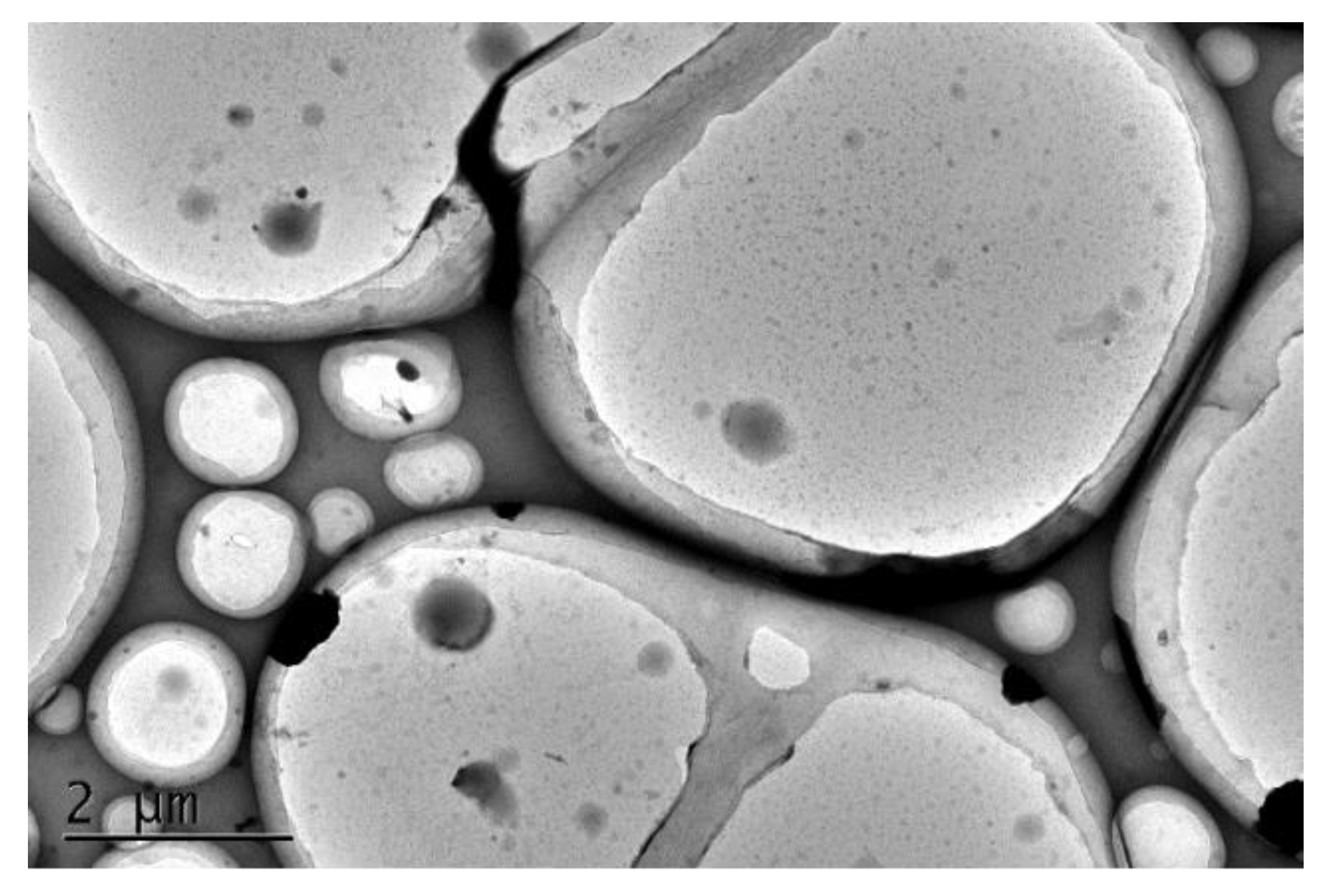
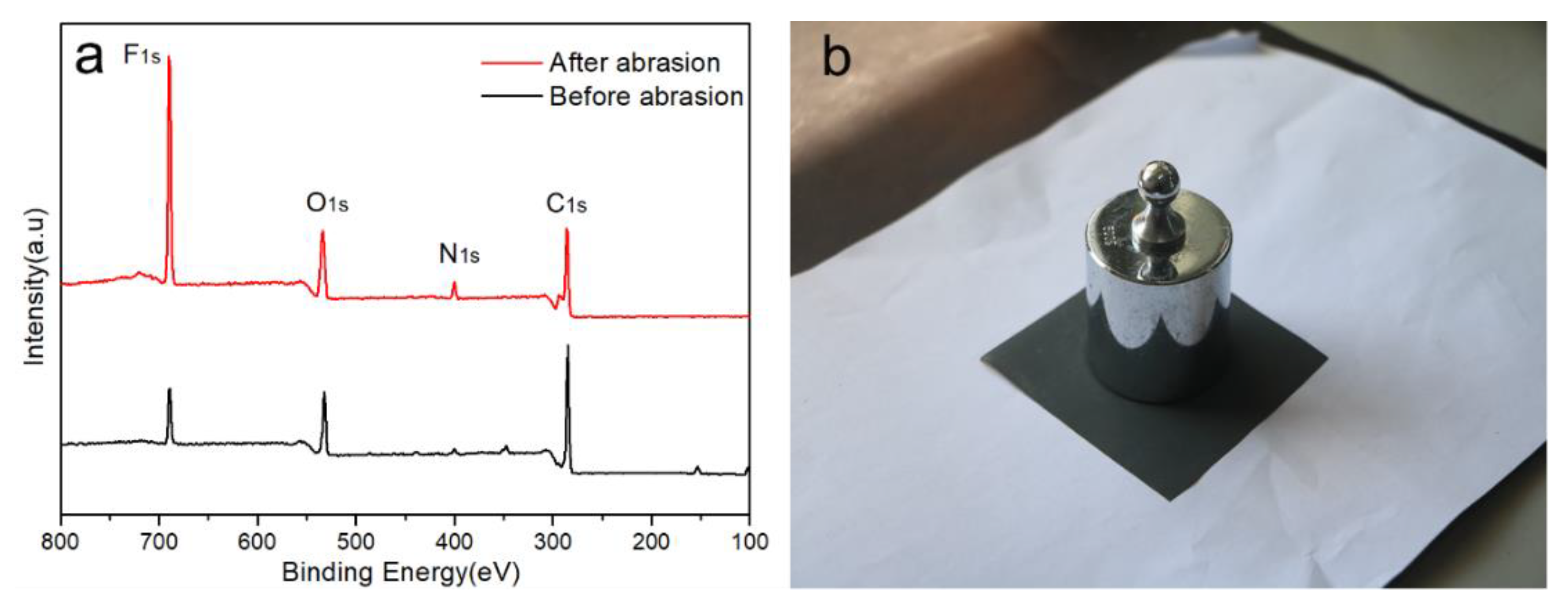
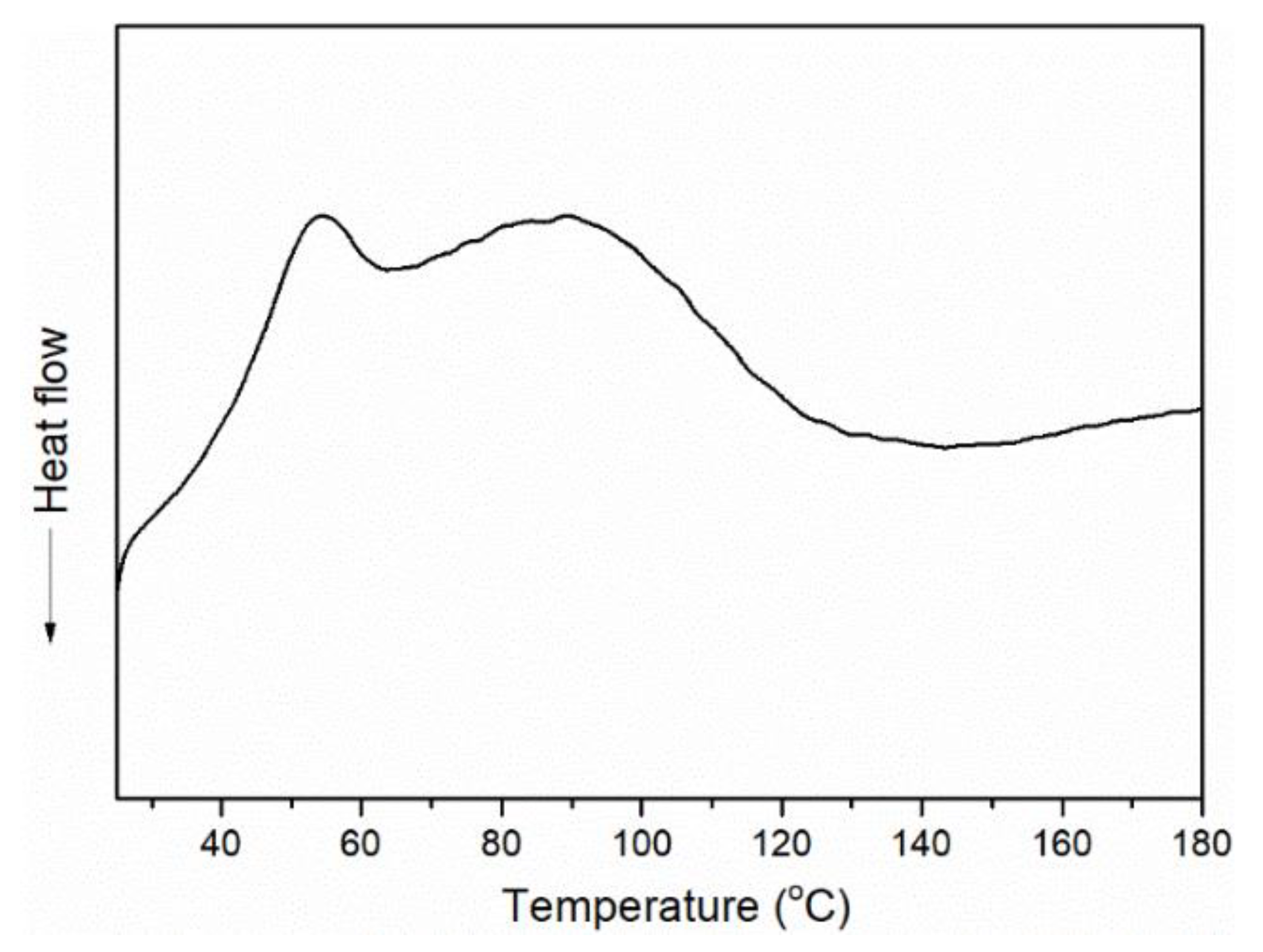
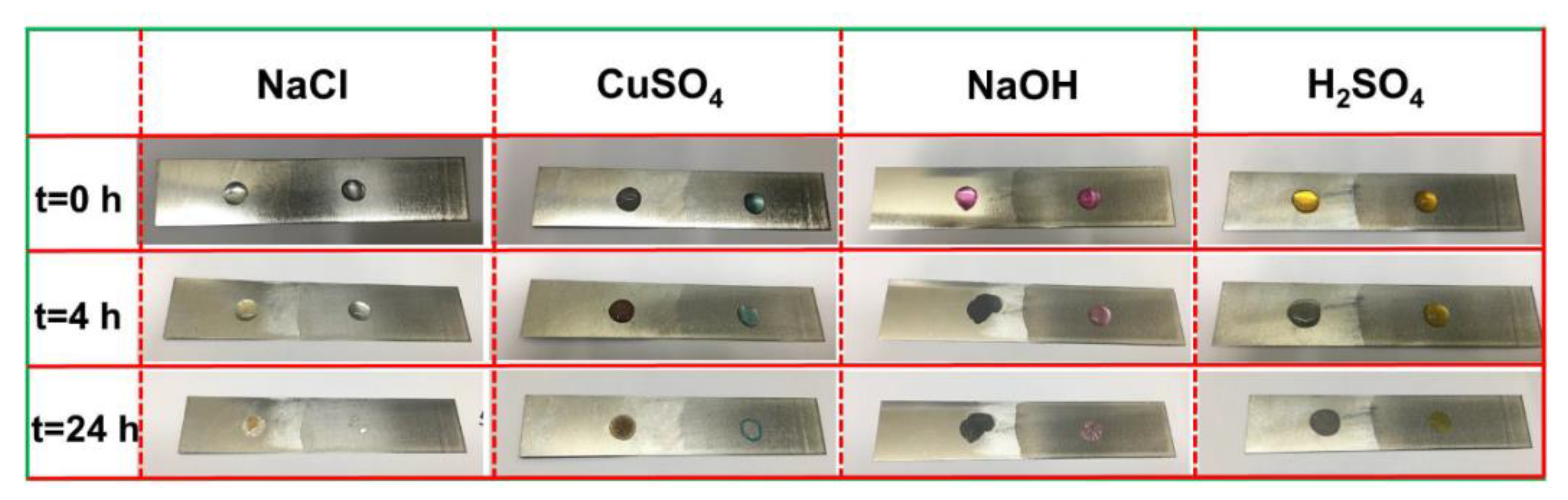
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Larock, R.C. Soybean oil-based, aqueous cationic polyurethane dispersions: Synthesis and properties. Prog. Org. Coat. 2010, 69, 31–37. [Google Scholar] [CrossRef]
- Tzoumani, I.; Soto Beobide, A.; Iatridi, Z.; Tzoumani, I.; Soto Beobide, A.; Iatridi, Z.; Voyiatzis, G.A.; Bokias, G.; Kallitsis, J.K. Glycidyl Methacrylate-Based Copolymers as Healing Agents of Waterborne Polyurethanes. Int. J. Mol. Sci. 2022, 23, 8118. [Google Scholar] [CrossRef]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Tatiya, P.D.; Hedaoo, R.K.; Mahulikar, P.P.; Gite, V.V. Novel Polyurea Microcapsules Using Dendritic Functional Monomer: Synthesis, Characterization, and Its Use in Self-healing and Anticorrosive Polyurethane Coatings. Ind. Eng. Chem. Res. 2013, 52, 1562–1570. [Google Scholar] [CrossRef]
- Xu, J.; Gao, F.; Wang, H.; Dai, R.; Dong, S.; Wang, H. Organic/inorganic hybrid waterborne polyurethane coatings with self-healing properties for anticorrosion application. Prog. Org. Coat. 2023, 174, 107244. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-based polyurethane: Recent advances and future perspectives. Macromol. Rapid Commun. 2021, 42, 2000492. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, Z.; Xu, C.; Zhang, A.; Xiang, J.; Fan, H. A self-matting waterborne polyurethane coating with admirable abrasion-resistance. RSC Adv. 2021, 11, 27620–27626. [Google Scholar] [CrossRef]
- Wang, C.; Mu, C.; Lin, W.; Xiao, H. Functional-modified polyurethanes for rendering surfaces antimicrobial: An overview. Adv. Colloid Interface Sci. 2020, 283, 102235. [Google Scholar] [CrossRef]
- Xu, D.; Liang, G.; Qi, Y.; Gong, R.; Zhang, X.; Zhang, Y.; Liu, B.; Kong, L.; Dong, X.; Li, Y. Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers 2022, 14, 5456. [Google Scholar] [CrossRef]
- Liu, X.; Hong, W.; Chen, X. Continuous production of water-borne polyurethanes: A review. Polymers 2020, 12, 2875. [Google Scholar] [CrossRef]
- Gite, V.V.; Tatiya, P.D.; Marathe, R.J.; Mahulikar, P.P.; Hundiwale, D.G. Microencapsulation of quinoline as a corrosion inhibitor in polyurea microcapsules for application in anticorrosive PU coatings. Prog. Org. Coat. 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Z.Y.; Liu, W.T.; Liu, H.; Zhu, C. Synthesis and molecular interaction of tung oil-based anionic waterborne polyurethane dispersion. J. Appl. Polym. Sci. 2020, 137, 49383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wang, X.; Dong, Q.; Zeng, X.; Quirino, R.L.; Lu, Q.; Wang, Q.; Zhang, C. Waterborne polyurethanes from castor oil-based polyols for next generation of environmentally-friendly hair-styling agents. Prog. Org. Coat. 2020, 142, 105588. [Google Scholar] [CrossRef]
- Stefanović, I.S.; Džunuzović, J.V.; Džunuzović, E.S.; Brzić, S.J.; Jasiukaitytė-Grojzdek, E.; Basagni, A.; Marega, C. Tailoring the properties of waterborne polyurethanes by incorporating different content of poly(dimethylsiloxane). Prog. Org. Coat. 2021, 161, 106474. [Google Scholar] [CrossRef]
- Gite, V.V.; Chaudhari, A.B. Incorporation of Modified Nano Montmorillonite (MMT) in Polyurethane Coatings Based on Acrylic Copolymer and Trimer of Isophorone Diisocyanate. Mater. Sci. Forum 2013, 757, 99–109. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S.; Osman Al Edrus, S.S. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795. [Google Scholar] [CrossRef]
- Borah, K.; Palanisamy, A.; Narayan, R.; Satapathy, S.; Dileepkumar, V.; Misra, S. Structure-property relationship of thermoplastic polyurethane cationomers carrying quaternary ammonium groups. React. Funct. Polym. 2022, 181, 105447. [Google Scholar] [CrossRef]
- Fakhar, A.; Maghami, S.; Sameti, E.; Shekari, M.; Sadeghi, M. Gas separation through polyurethane—ZnO mixed matrix membranes and mathematical modeling of the interfacial morphology. SPE Polym. 2020, 1, 113–124. [Google Scholar] [CrossRef]
- Gong, R.; Cao, H.; Zhang, H.; Qiao, L.; Wang, F.; Wang, X. Terminal Hydrophilicity-Induced Dispersion of Cationic Waterborne Polyurethane from CO2-Based Polyol. Macromolecules 2020, 53, 6322–6330. [Google Scholar] [CrossRef]
- Sukhawipat, N.; Raksanak, W.; Kalkornsurapranee, E.; Saetung, A.; Saetung, N. A new hybrid waterborne polyurethane coating synthesized from natural rubber and rubber seed oil with grafted acrylate. Prog. Org. Coat. Int. Rev. J. 2020, 141, 105554. [Google Scholar] [CrossRef]
- Król, P.; Pielichowska, K.; Król, B.; Nowicka, K.; Walczak, M.; Kowal, M. Polyurethane cationomers containing fluorinated soft segments with hydrophobic properties. Colloid Polym. Sci. 2021, 299, 1011–1029. [Google Scholar] [CrossRef]
- Zou, C.Y.; Zhang, H.M.; Qiao, L.J.; Wang, X.H.; Wang, F. Near neutral waterborne cationic polyurethane from CO2-polyol, a compatible binder to aqueous conducting polyaniline for eco-friendly anti-corrosion purposes. Green Chem. 2020, 22, 7823–7831. [Google Scholar] [CrossRef]
- Gong, R.; Cao, H.; Zhang, H.; Qiao, L.; Wang, X. UV-curable cationic waterborne polyurethane from CO2-polyol with excellent water resistance. Polym. Int. J. Sci. Technol. Polym. 2021, 218, 123536. [Google Scholar] [CrossRef]
- Saji, V.S. Electrophoretic-deposited superhydrophobic coatings. Chem. Asian J. 2021, 16, 474–491. [Google Scholar] [CrossRef]
- Clifford, A.; Pang, X.; Zhitomirsky, I. Biomimetically modified chitosan for electrophoretic deposition of composites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 28–34. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Lai, C.; Li, W.; Zhang, D.; Xiong, Z. Cathodic electrophoretic deposition of bismuth oxide (Bi2O3) coatings and their photocatalytic activities. Appl. Surf. Sci. 2015, 331, 455–462. [Google Scholar] [CrossRef]
- Zhitomirsky, D.; Roether, J.; Boccaccini, A.; Zhitomirsky, I. Electrophoretic deposition of bioactive glass/polymer composite coatings with and without HA nanoparticle inclusions for biomedical applications. J. Mater. Process. Technol. 2009, 209, 1853–1860. [Google Scholar] [CrossRef]
- Zhang, X.F.; Jiang, F.; Chen, R.J.; Chen, Y.Q.; Hu, J.M. Robust superhydrophobic coatings prepared by cathodic electrophoresis of hydrophobic silica nanoparticles with the cationic resin as the adhesive for corrosion protection. Corros. Sci. 2020, 173, 108797. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, W.; Chen, L. Synthesis and characterization of cationic acrylic resin used in cathodic electrodeposition coatings. J. Polym. Res. 2022, 29, 303. [Google Scholar] [CrossRef]
- Brewer, G.E.F. Electrophoretic painting. J. Appl. Electrochem. 1983, 13, 269–275. [Google Scholar] [CrossRef]
- Liu, K.; Su, Z.; Miao, S.; Ma, G.; Zhang, S. UV-curable enzymatic antibacterial waterborne polyurethane coating. Biochem. Eng. J. 2016, 113, 107–113. [Google Scholar] [CrossRef]
- Ge, J.; Yang, S.; Fu, F.; Wang, J.; Yang, J.; Cui, L.; Ding, B.; Yu, J.; Sun, G. Amphiphobic fluorinated polyurethane composite microfibrous membranes with robust waterproof and breathable performances. RSC Adv. 2013, 3, 2248–2255. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Li, G. Preparation and properties of waterborne cationic fluorinated polyurethane. J. Polym. Res. 2012, 19, 9786. [Google Scholar] [CrossRef]
- Salam, A.; Lucia, L.A.; Jameel, H. Fluorine-based surface decorated cellulose nanocrystals as potential hydrophobic and oleophobic materials. Cellulose 2015, 22, 397–406. [Google Scholar] [CrossRef]
- Wei, C.; Tang, Y.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Facile fabrication of highly omniphobic and self-cleaning surfaces based on water mediated fluorinated nanosilica aggregation. RSC Adv. 2016, 6, 74340–74348. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.; Klier, J.; Schiffman, J.D. High-Performance, UV-Curable Cross-Linked Films via Grafting of Hydroxyethyl Methacrylate Methylene Malonate. Ind. Eng. Chem. Res. 2020, 59, 4542–4548. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Tao, W.; Yang, Y. Preparation of UV curable organic/inorganic hybrid coatings-a review. Prog. Org. Coat. 2020, 145, 105685. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Calvez, I.; Szczepanski, C.R.; Landry, V. Preparation and characterization of low gloss UV-curable coatings based on silica surface modification using an acrylate monomer. Prog. Org. Coat. 2021, 158, 106369. [Google Scholar] [CrossRef]
- Magnoni, F.; Rannée, A.; Marasinghe, L.; El-Fouhaili, B.; Allonas, X.; Croutxé-Barghorn, C. Correlation between the scratch resistance of UV-cured PUA-based coatings and the structure and functionality of reactive diluents. Prog. Org. Coat. 2018, 124, 193–199. [Google Scholar] [CrossRef]
- Tan, J.; Liu, W.; Wang, Z. Preparation and performance of waterborne UV-curable polyurethane containing long fluorinated side chains. J. Appl. Polym. Sci. 2017, 134, 44506. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Lopes, I.M.; Coelho, J.F.J.; Serra, A.C. Synthesis of unsaturated polyesters based on renewable monomers: Structure/properties relationship and crosslinking with 2-hydroxyethyl methacrylate. React. Funct. Polym. 2015, 97, 1–11. [Google Scholar] [CrossRef]
- Peng, K.; Zou, T.; Ding, W.; Wang, R.; Guo, J.; Round, J.J.; Tu, W.; Liu, C.; Hu, J. Development of contact-killing non-leaching antimicrobial guanidyl-functionalized polymers via click chemistry. RSC Adv. 2017, 7, 24903–24913. [Google Scholar] [CrossRef]
- GB 9754-88; Paints and Varnishes—Measurement of Specular Gloss of Non-Metallic Paint Films at 20 Degree, 60 Degree and 85 Degree. China Standard Press: Beijing, China, 1988.
- GB/T6739-1996; Determination of Film Hardness by Pencil Test. China Standard Press: Beijing, China, 1996.
- GB/T1720-1989; Method of Test for Adhesion of Paint Films. China Standard Press: Beijing, China, 1989.
- GB/T 1732-93; Determination of Impact Resistance of Film. China Standard Press: Beijing, China, 1993.
- GB/T 1731-93; Determination of Flexibility of Films. China Standard Press: Beijing, China, 1993.
- Tan, H.; Guo, M.; Du, R.; Xie, X.; Li, J.; Zhong, Y.; Fu, Q. The effect of fluorinated side chain attached on hard segment on the phase separation and surface topography of polyurethanes. Polymer 2004, 45, 1647–1657. [Google Scholar] [CrossRef]
- Campos, R.; Guenthner, A.J.; Haddad, T.S.; Mabry, J.M. Fluoroalkyl-functionalized silica particles: Synthesis, characterization, and wetting characteristics. Langmuir 2011, 27, 10206–10215. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; He, J.; Liu, F. Synthesis and characterization of cathodic electrodeposition coatings based on octadecyl-modified cationic waterborne polyurethanes. J. Coat. Technol. Res. 2020, 17, 1255–1268. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef]
- Gradwell, M.; Hourston, D.; Pabunruang, T.; Schafer, F.-U.; Reading, M. High-resolution thermogravimetric analysis of polyurethane/poly (ethyl methacrylate) interpenetrating polymer networks. J. Appl. Polym. Sci. 1998, 70, 287–295. [Google Scholar] [CrossRef]
- Yang, X.; Shen, Y.; Li, P.Z.; Li, G.H. Waterborne cationic fluorinated polyurethane modified by perfluorinated alkyl long chain. Appl. Eng. Mater. 2011, 287, 2116–2121. [Google Scholar] [CrossRef]
- GB/T 9286-1998; Paints and Varnishes—Cross Cut Test for Films. China Standard Press: Beijing, China, 1998.
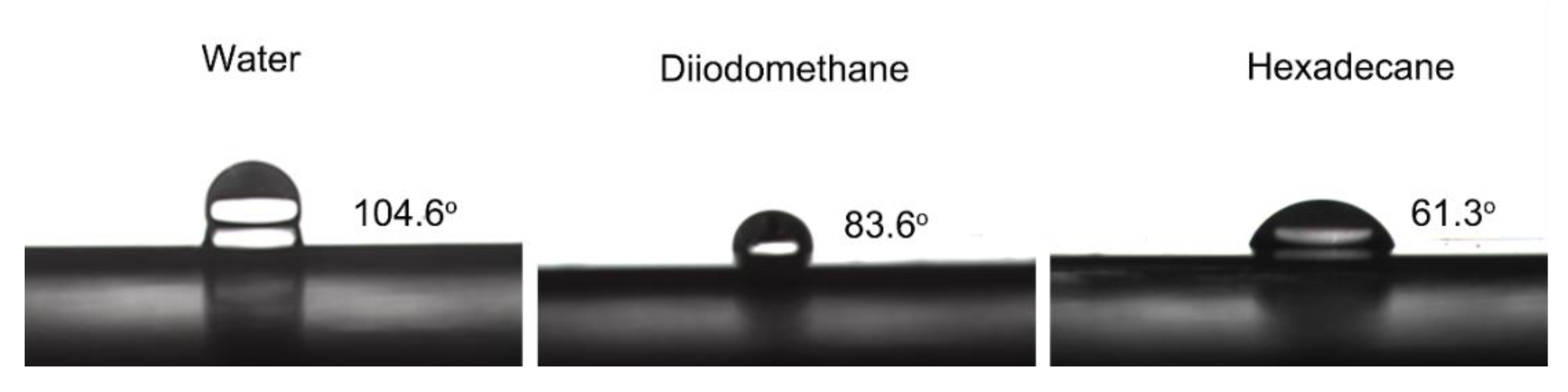
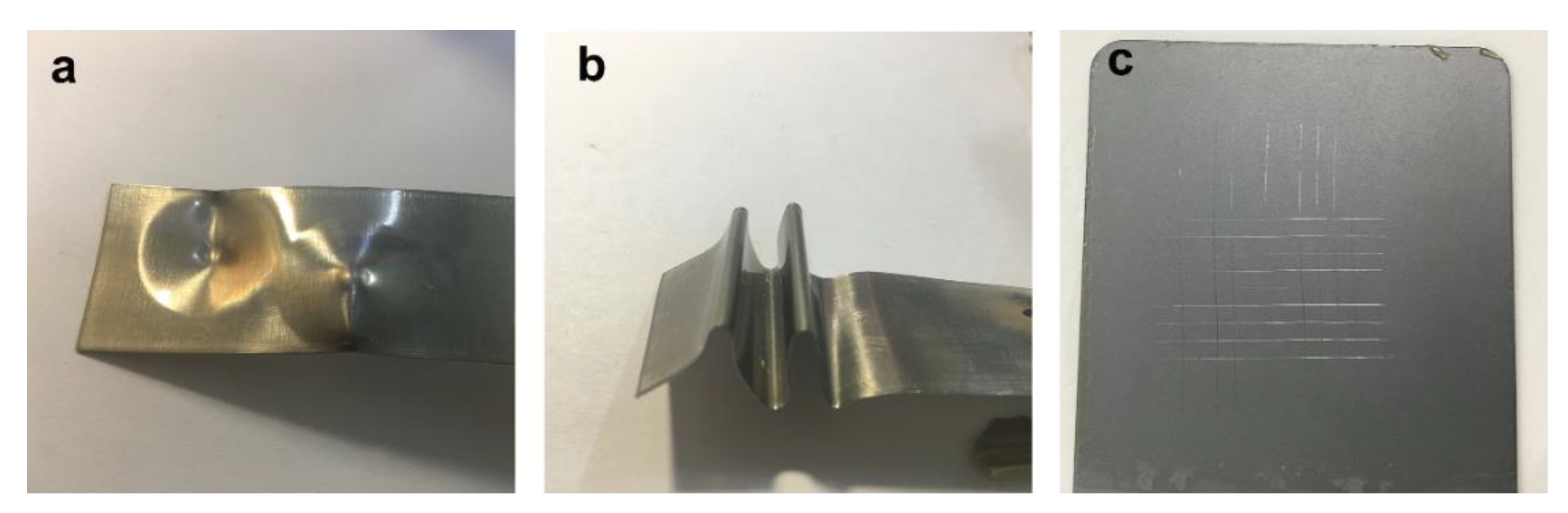
| Test Droplets | Sliding Angle (50 μL) |
|---|---|
| Water | 37.8 ± 1.5° |
| Diiodomethane | 11.2 ± 0.5° |
| Hexadecane | 9.7 ± 0.4° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zeng, Z.; Liu, C.; Wang, X.; Li, S.; Ye, F.; Li, C.; Guan, X. Aqueous Cationic Fluorinated Polyurethane for Application in Novel UV-Curable Cathodic Electrodeposition Coatings. Polymers 2023, 15, 3725. https://doi.org/10.3390/polym15183725
Chen J, Zeng Z, Liu C, Wang X, Li S, Ye F, Li C, Guan X. Aqueous Cationic Fluorinated Polyurethane for Application in Novel UV-Curable Cathodic Electrodeposition Coatings. Polymers. 2023; 15(18):3725. https://doi.org/10.3390/polym15183725
Chicago/Turabian StyleChen, Junhua, Zhihao Zeng, Can Liu, Xuan Wang, Shiting Li, Feihua Ye, Chunsheng Li, and Xiaoxiao Guan. 2023. "Aqueous Cationic Fluorinated Polyurethane for Application in Novel UV-Curable Cathodic Electrodeposition Coatings" Polymers 15, no. 18: 3725. https://doi.org/10.3390/polym15183725
APA StyleChen, J., Zeng, Z., Liu, C., Wang, X., Li, S., Ye, F., Li, C., & Guan, X. (2023). Aqueous Cationic Fluorinated Polyurethane for Application in Novel UV-Curable Cathodic Electrodeposition Coatings. Polymers, 15(18), 3725. https://doi.org/10.3390/polym15183725







