Encapsulation of Methanotrophs within a Polymeric Matrix Containing Copper- and Iron-Based Nanoparticles to Enhance Methanol Production from a Simulated Biogas
Abstract
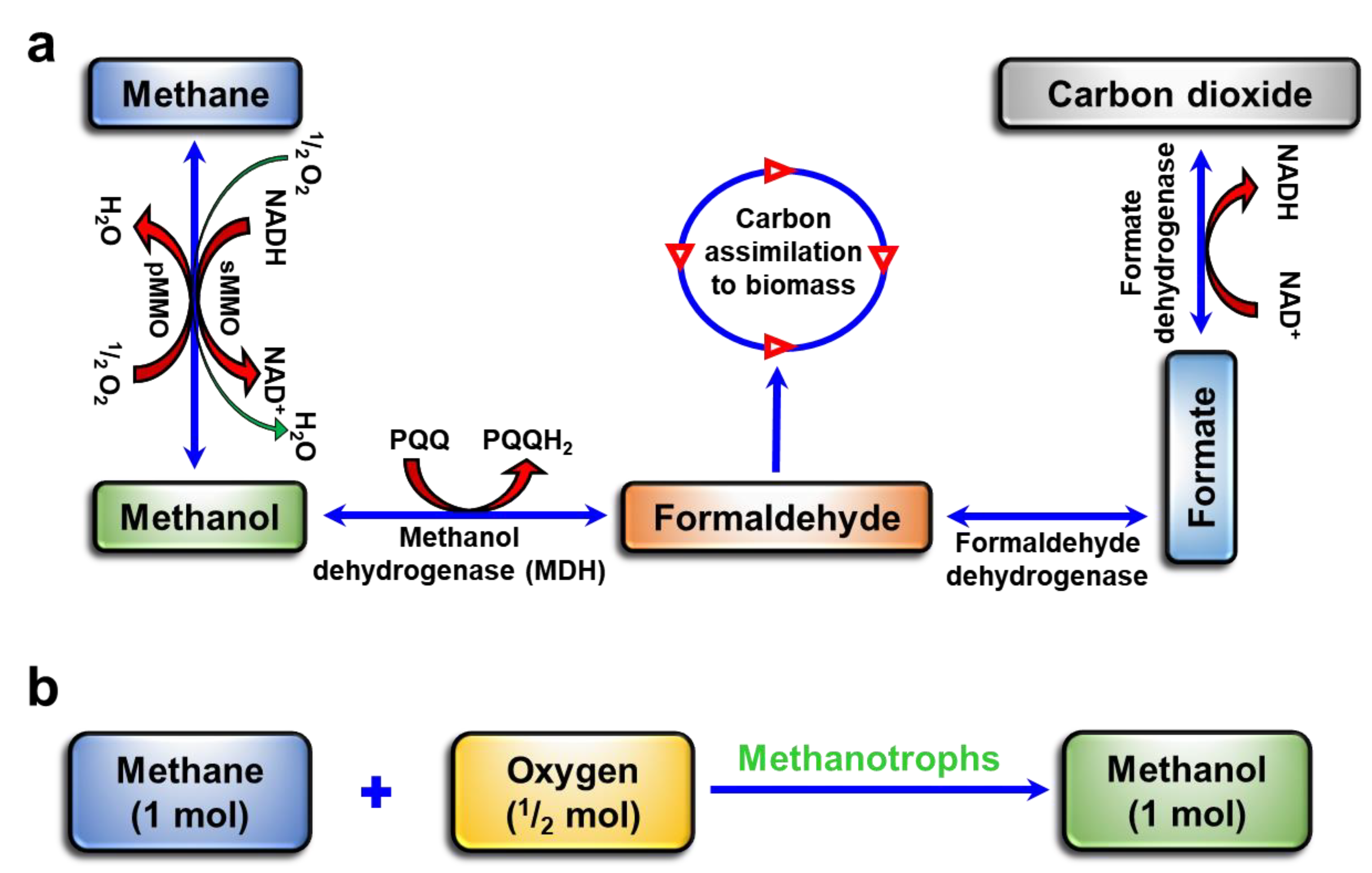
1. Introduction
2. Materials and Methods
2.1. Materials and Cultures
2.2. Influence of NPs on the Growth of Methanotrophs for Methanol Production
2.3. Encapsulation of Whole Cells in Polymeric Matrix Containing Nanoparticles for Methanol Production and Biocatalytic Activity
2.4. Influence of Process Parameters on Methanol Production by Encapsulated Methanotrophs
2.5. Reusability Measurements
2.6. Influence of CH4 Vectors on Methanol Production by Encapsulated M. bryophila
2.7. Instrumental Analysis
3. Results and Discussion
3.1. Influence of NPs on the Growth of Methanotrophs to Enhance Methanol Production
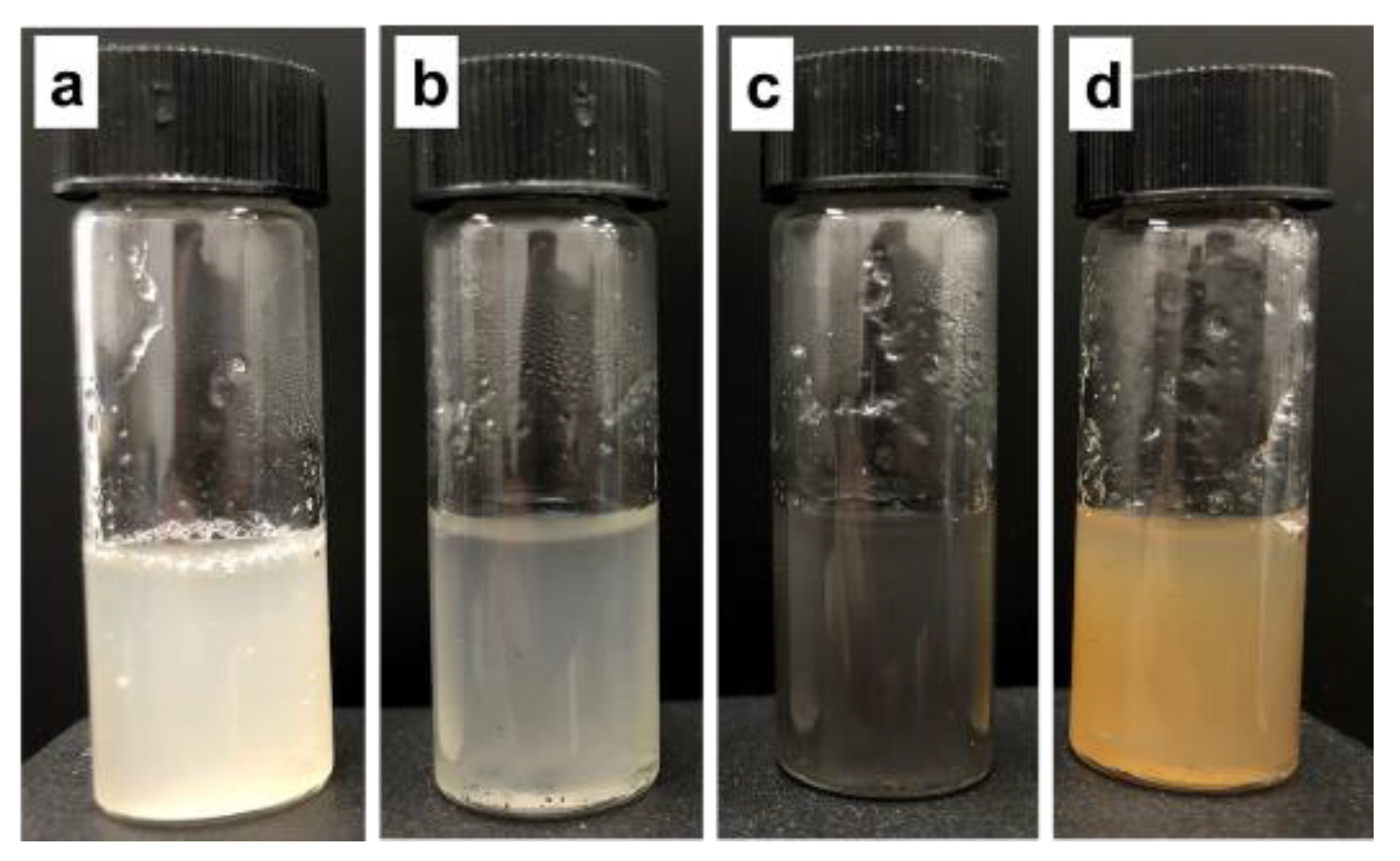
3.2. Encapsulation of Methanotrophs within Polymeric Matrix Containing NPs
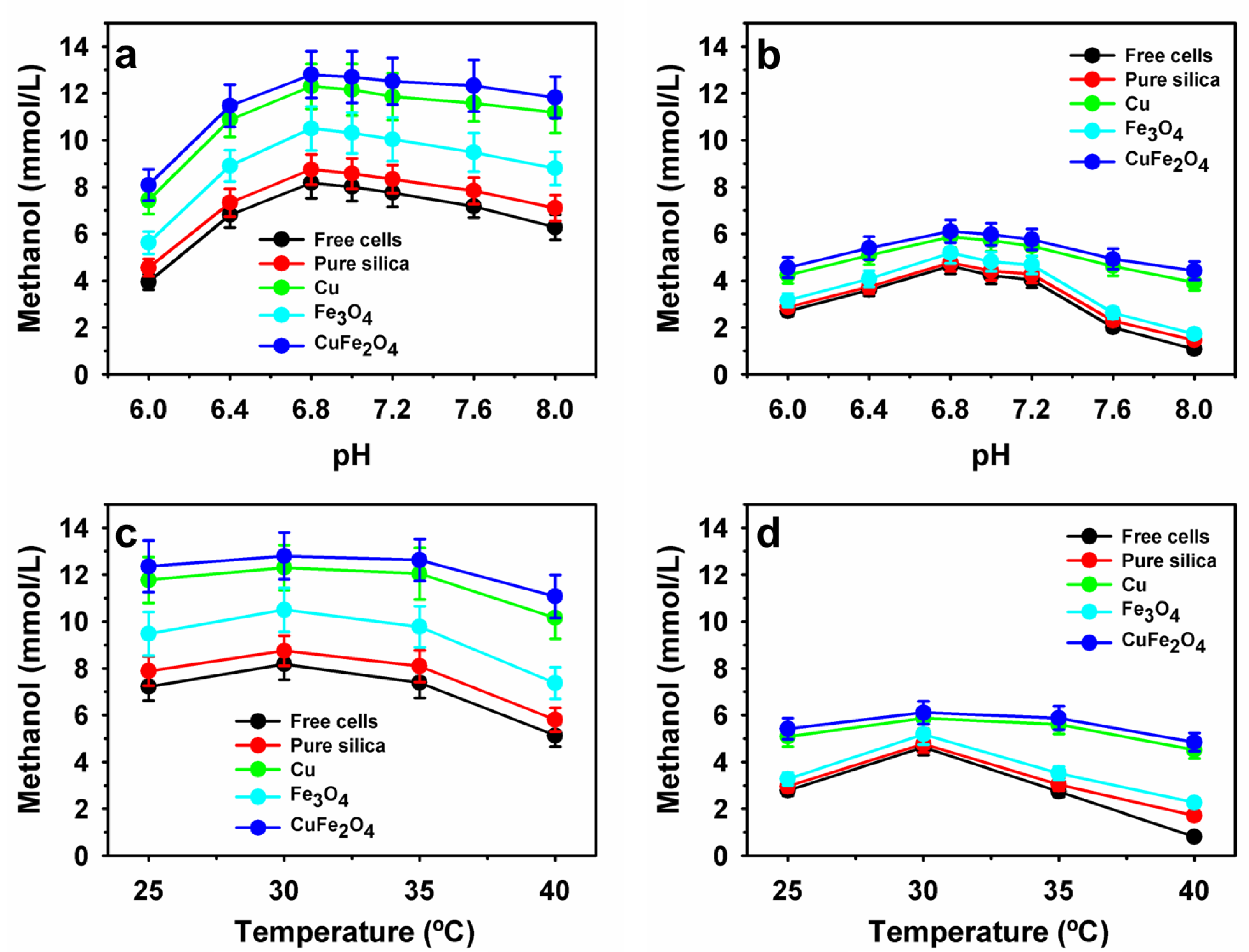
3.3. Influence of Physiological Parameters on Methanol Production by Encapsulated Methanotrophs
3.4. Production Profiles and Recycling of Encapsulated M. bryophila within Polymeric Matrix Containing CuFe2O4
3.5. Influence of CH4 Vectors for Enhanced Methanol Production by Encapsulated M. bryophila
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: Current state of art and future prospects. Front. Microbiol. 2021, 12, 636486. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; García-Depraect, O.; Santos-Beneit, F.; Bordel, S.; Lebrero, R.; Muñoz, R. Recent trends and advances in biogas upgrading and methanotrophs-based valorization. Chem. Eng. J. Adv. 2022, 11, 100325. [Google Scholar] [CrossRef]
- Bae, J.; Jin, S.; Kang, S.; Cho, B.-K.; Oh, M.-K. Recent progress in the engineering of C1-utilizing microbes. Curr. Opin. Biotechnol. 2022, 78, 102836. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, W.; Zhang, C.; Ning, P.; Li, J.; Liu, Y.; Du, G.; Liu, L. C1-based biomanufacturing: Advances, challenges and perspectives. Bioresour. Technol. 2023, 367, 128259. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Guarnieri, M.T.; Tao, L.; Laurens, L.M.L.; Dowe, N.; Pienkos, P.T. Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 2014, 32, 596–614. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kondaveeti, S.; Otari, S.V.; Kumar, A.; Kalia, V.C.; Lee, J.-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour. Technol. 2020, 315, 123791. [Google Scholar] [CrossRef]
- Sheets, J.P.; Ge, X.; Li, Y.-F.; Yu, Z.; Li, Y. Biological conversion of biogas to methanol using methanotrophs isolated from solid-state anaerobic digestate. Bioresour. Technol. 2016, 201, 50–57. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Sinha, A.; Das, D. Process engineering strategy for improved methanol production in Methylosinus trichosporium through enhanced mass transfer and solubility of methane and carbon dioxide. Bioresour. Technol. 2023, 371, 128603. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Hana, J.-S.; Ahn, C.-M.; Kim, C.-G. Comparative enzyme inhibitive methanol production by Methylosinus sporium from simulated biogas. Environ. Technol. 2015, 36, 983–991. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Khonde, V.K.; Deshpande, M.S.; Sabale, T.R.; Kumbhar, P.S.; Ghosalkar, A.R. Selection of methanotrophic platform for methanol production using methane and biogas. J. Biosci. Bioeng. 2021, 132, 460–468. [Google Scholar] [CrossRef]
- Taylor, A.; Molzahn, P.; Bushnell, T.; Cheney, C.; LaJeunesse, M.; Azizian, M.; Semprni, L. Immobilization of Methylosinus trichosporium OB3b for methanol production. J. Ind. Microbiol. Biotechnol. 2018, 45, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yoshimori, K.; Ishikawa, M.; Hori, K.; Kamachi, T. Switching between methanol accumulation and cell growth by expression control of methanol dehydrogenase in Methylosinus trichosporium OB3b mutant. Front. Microbiol. 2021, 12, 639266. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Xia, S.W.; Shen, R.N.; Xia, C.G.; Li, S.B. Methanol biosynthesis by methanotrophic bacterial cells—Effects of various immobilization methods on biocatalytic activity of immobilized cells. Ann. N. Y. Acad. Sci. 1998, 864, 609–615. [Google Scholar] [CrossRef]
- Mehta, P.K.; Mishra, S.; Ghose, T.K. Methanol biosynthesis by covalently immobilized cells of Methylosinus trichosporium: Batch and continuous studies. Biotechnol. Bioeng. 1991, 37, 551–556. [Google Scholar] [CrossRef]
- Sheets, J.P.; Lawson, K.; Ge, X.; Yu, Z.; Li, Y. Development and evaluation of a trickle bed bioreactor for enhanced mass transfer and methanol production from biogas. Biochem. Eng. J. 2017, 122, 103–114. [Google Scholar] [CrossRef]
- Takeguchi, M.; Okura, I. Role of iron and copper in particulate methane monooxygenase of Methylosinus trichosporium OB3b. Catal. Surv. Asia 2000, 4, 51–63. [Google Scholar] [CrossRef]
- Bidir, M.G.; Millerjothi, N.K.; Adaramola, M.S.; Hagos, F.Y. The role of nanoparticles on biofuel production and as an additive in ternary blend fuelled diesel engine: A review. Energy Rep. 2021, 7, 3614–3627. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Z.; Li, Y.; Zhao, Z.; Liu, J.; Jiang, L.; Xu, H.; Li, Z. Fish-in-net,” A novel method for cell immobilization of Zymomonas mobilis. PLoS ONE 2013, 8, e79569. [Google Scholar] [CrossRef][Green Version]
- Patel, S.K.S.; Kalia, V.C.; Joo, J.B.; Kang, Y.C.; Lee, J.-K. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour. Technol. 2020, 297, 122433. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Lee, J.-K. Integration of biogas derived from dark fermentation and anaerobic digestion of biowaste to enhance methanol production by methanotrophs. Bioresour. Technol. 2023, 369, 128427. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Kalia, V.C.; Lee, J.-K. Synthetic design of methanotroph co-cultures and their immobilization within polymers containing magnetic nanoparticles to enhance methanol production from wheat straw-based biogas. Bioresour. Technol. 2022, 364, 128032. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Shanmugam, R.; Kalia, V.C.; Lee, J.-K. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour. Technol. 2020, 304, 123022. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, C.; Pol, A.; Nuijten, G.H.L.; Op den Camp, H.J.M. Methanol production by “Methylacidiphilum fumariolicum” SolV under different growth conditions. Appl. Environ. Microbiol. 2020, 86, e01188-20. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Datta, S.; Goswami, G.; Das, D. Two-stage integrated process for bio-methanol production coupled with methane and carbon dioxide sequestration: Kinetic modelling and experimental validation. J. Environ. Manag. 2022, 301, 113927. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, D.O.; Pybus, V.; Wilson, R. Metal ion-mediated accumulation of alcohols during alkane oxidation by whole cells of Methylosinus trichosporium. Enzym. Microb. Technol. 1990, 12, 343–348. [Google Scholar] [CrossRef]
- Xin, J.-Y.; Cui, J.-R.; Niu, J.-Z.; Hua, S.-F.; Xia, C.-G.; Li, S.-B.; Zhu, L.-M. Biosynthesis of methanol from CO2 and CH4 by methanotrophic bacteria. Biotechnology 2004, 3, 67–71. [Google Scholar]
- Senko, O.; Makhlis, T.; Bihovsky, M.; Podmasterev, V.; Efremenko, E.; Razumovsky, S.; Varfolomeyev, S. Methanol production in the flow system with immobilized cells Methylosinus sporium. In Proceedings of the XV International Workshop on Bioencapsulation, Vienna, Austria, 6–8 September 2007; P2–6. pp. 1–4. [Google Scholar]
- Razumovsky, S.D.; Efremenko, E.N.; Makhlis, T.A.; Senko, O.V.; Bikhovsky, M.Y.; Podmaster’ev, V.V.; Varfolomeev, S.D. Effect of immobilization on the main dynamic characteristics of the enzymatic oxidation of methane to methanol by bacteria Methylosinus sporium B-2121. Russ. Chem. Bull. Int. Ed. 2008, 57, 1633–1636. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Shrestha, N.; David, A.; Basotra, N.; Johnson, G.R.; Chadha, B.S.; Gadhamshetty, V.; Sani, R.K. Producing methane, methanol and electricity from organic waste of fermentation reaction using novel microbes. Bioresour. Technol. 2018, 258, 270–278. [Google Scholar] [CrossRef]
- Kim, I.-K. Simultaneous denitrification and bio-methanol production for sustainable operation of biogas plants. Sustainability 2019, 11, 6658. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Liu, T.; Wang, Z.; Guo, J.; Yuan, Z.; Zheng, M. Feasibility of methane bioconversion to methanol by acid-tolerant ammonia-oxidizing bacteria. Water Res. 2021, 197, 117077. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Shokri, Z.; Galehban, M.H. The immobilized Ni(II)-thiourea complex on silica-layered copper ferrite: A novel and reusable nanocatalyst for one-pot reductive-acetylation of nitroarenes. Appl. Organometal. Chem. 2019, 33, e4771. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, X.; Tang, L.; Zhang, B.; Mao, H. Synthesis and formation mechanism of amorphous silica particles via sol–gel process with tetraethylorthosilicate. Ceram. Int. 2019, 45, 7673–7680. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, X.; Li, Y.-F.; Yu, Z.; Li, Y. Isolation of a methanotroph from a hydrogen sulfide-rich anaerobic digester for methanol production from biogas. Process Biochem. 2016, 51, 838–844. [Google Scholar] [CrossRef]
- Blanchette, C.D.; Knipe, J.M.; Stolaroff, J.K.; DeOtte, J.R.; Oakdale, J.S.; Maiti, A.; Lenhardt, J.M.; Sirajuddin, S.; Rosenzweig, A.C.; Banker, S.E. Printable enzyme-embedded materials for methane to methanol conversion. Nat. Commun. 2016, 7, 11900. [Google Scholar] [CrossRef]
- Hu, L.; Guo, S.; Wang, B.; Fu, R.; Fan, D.; Jiang, M.; Fei, Q.; Gonzalez, R. Bio-valorization of C1 gaseous substrates into bioalcohols: Potentials and challenges in reducing carbon emissions. Biotechnol. Adv. 2022, 59, 107954. [Google Scholar] [CrossRef] [PubMed]




| NPs | Feed a | Specific Growth Rate (h−1) | Methanol Production (mmol/L) b | ||
|---|---|---|---|---|---|
| M. bryophila | M. stellata | M. bryophila | M. stellata | ||
| Control | CH4 | 0.013 ± 0.001 | 0.008 ± 0.001 | 4.90 ± 0.37 | 3.11 ± 0.26 |
| CH4 + CO2 | 0.015 ± 0.001 | 0.009 ± 0.001 | 5.45 ± 0.43 | 3.32 ± 0.22 | |
| Cu | CH4 | 0.016 ± 0.002 | 0.010 ± 0.001 | 6.60 ± 0.48 | 3.76 ± 0.25 |
| CH4 + CO2 | 0.019 ± 0.002 | 0.012 ± 0.001 | 7.25 ± 0.54 | 4.23 ± 0.33 | |
| Fe3O4 | CH4 | 0.015 ± 0.001 | 0.009 ± 0.001 | 5.75 ± 0.49 | 3.42 ± 0.27 |
| CH4 + CO2 | 0.017 ± 0.002 | 0.010 ± 0.001 | 6.08 ± 0.52 | 3.54 ± 0.26 | |
| CuFe2O4 | CH4 | 0.019 ± 0.002 | 0.012 ± 0.001 | 7.39 ± 0.56 | 4.26 ± 0.28 |
| CH4 + CO2 | 0.021 ± 0.002 | 0.015 ± 0.002 | 8.18 ± 0.67 | 4.63 ± 0.34 | |
| Culture | Methanol Production (mmol/L) | MMO Activity (nmol/min/mg) | ||
|---|---|---|---|---|
| M. bryophila | M. stellata | M. bryophila | M. stellata | |
| Free cells | 8.18 ± 0.67 | 4.63 ± 0.34 | 3.82 ± 0.26 | 1.39 ± 0.09 |
| Pure polymeric matrix | 8.75 ± 0.64 | 4.77 ± 0.37 | 4.43 ± 0.33 | 1.51 ± 0.11 |
| Cu–polymeric matrix | 11.3 ± 0.87 | 5.60 ± 0.42 | 5.54 ± 0.37 | 1.71 ± 0.12 |
| Fe3O4–polymeric matrix | 10.1 ± 0.76 | 5.05 ± 0.38 | 5.01 ± 0.38 | 1.58 ± 0.11 |
| CuFe2O4–polymeric matrix | 11.5 ± 0.85 | 5.79 ± 0.44 | 5.65 ± 0.42 | 1.85 ± 0.13 |
| NPs | NP Conc. (mg/mL) | Methanol Production (mmol/L) | MMO Activity (nmol/min/mg) | ||
|---|---|---|---|---|---|
| M. bryophila | M. stellata | M. bryophila | M. stellata | ||
| Control (free cells) | 0 | 8.18 ± 0.67 | 4.63 ± 0.34 | 3.82 ± 0.26 | 1.39 ± 0.09 |
| Cu | 0.010 | 11.3 ± 0.87 | 5.60 ± 0.42 | 5.54 ± 0.37 | 1.71 ± 0.12 |
| 0.025 | 12.3 ± 0.96 | 5.88 ± 0.45 | 6.15 ± 0.48 | 1.90 ± 0.15 | |
| 0.050 | 11.9 ± 0.88 | 5.69 ± 0.39 | 5.81 ± 0.46 | 1.74 ± 0.14 | |
| Fe3O4 | 0.010 | 10.1 ± 0.76 | 5.05 ± 0.38 | 5.01 ± 0.38 | 1.58 ± 0.11 |
| 0.025 | 10.5 ± 0.83 | 5.19 ± 0.44 | 5.08 ± 0.44 | 1.63 ± 0.13 | |
| 0.050 | 10.4 ± 0.79 | 5.14 ± 0.41 | 5.04 ± 0.39 | 1.58 ± 0.12 | |
| CuFe2O4 | 0.010 | 11.5 ± 0.85 | 5.79 ± 0.45 | 5.65 ± 0.42 | 1.85 ± 0.13 |
| 0.025 | 12.8 ± 0.99 | 6.11 ± 0.49 | 6.38 ± 0.48 | 1.99 ± 0.15 | |
| 0.050 | 12.4 ± 0.91 | 5.88 ± 0.48 | 6.11 ± 0.45 | 1.89 ± 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.K.S.; Gupta, R.K.; Kim, I.-W.; Lee, J.-K. Encapsulation of Methanotrophs within a Polymeric Matrix Containing Copper- and Iron-Based Nanoparticles to Enhance Methanol Production from a Simulated Biogas. Polymers 2023, 15, 3667. https://doi.org/10.3390/polym15183667
Patel SKS, Gupta RK, Kim I-W, Lee J-K. Encapsulation of Methanotrophs within a Polymeric Matrix Containing Copper- and Iron-Based Nanoparticles to Enhance Methanol Production from a Simulated Biogas. Polymers. 2023; 15(18):3667. https://doi.org/10.3390/polym15183667
Chicago/Turabian StylePatel, Sanjay K. S., Rahul K. Gupta, In-Won Kim, and Jung-Kul Lee. 2023. "Encapsulation of Methanotrophs within a Polymeric Matrix Containing Copper- and Iron-Based Nanoparticles to Enhance Methanol Production from a Simulated Biogas" Polymers 15, no. 18: 3667. https://doi.org/10.3390/polym15183667
APA StylePatel, S. K. S., Gupta, R. K., Kim, I.-W., & Lee, J.-K. (2023). Encapsulation of Methanotrophs within a Polymeric Matrix Containing Copper- and Iron-Based Nanoparticles to Enhance Methanol Production from a Simulated Biogas. Polymers, 15(18), 3667. https://doi.org/10.3390/polym15183667










