A Study of Isosorbide Synthesis from Sorbitol for Material Applications Using Isosorbide Dimethacrylate for Enhancement of Bio-Based Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods for Structural Characterization
2.2.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.2. Mass Spectrometry (MS)
2.2.3. X-ray Diffraction Analysis (XRD)
2.2.4. Nuclear Magnetic Resonance (NMR)
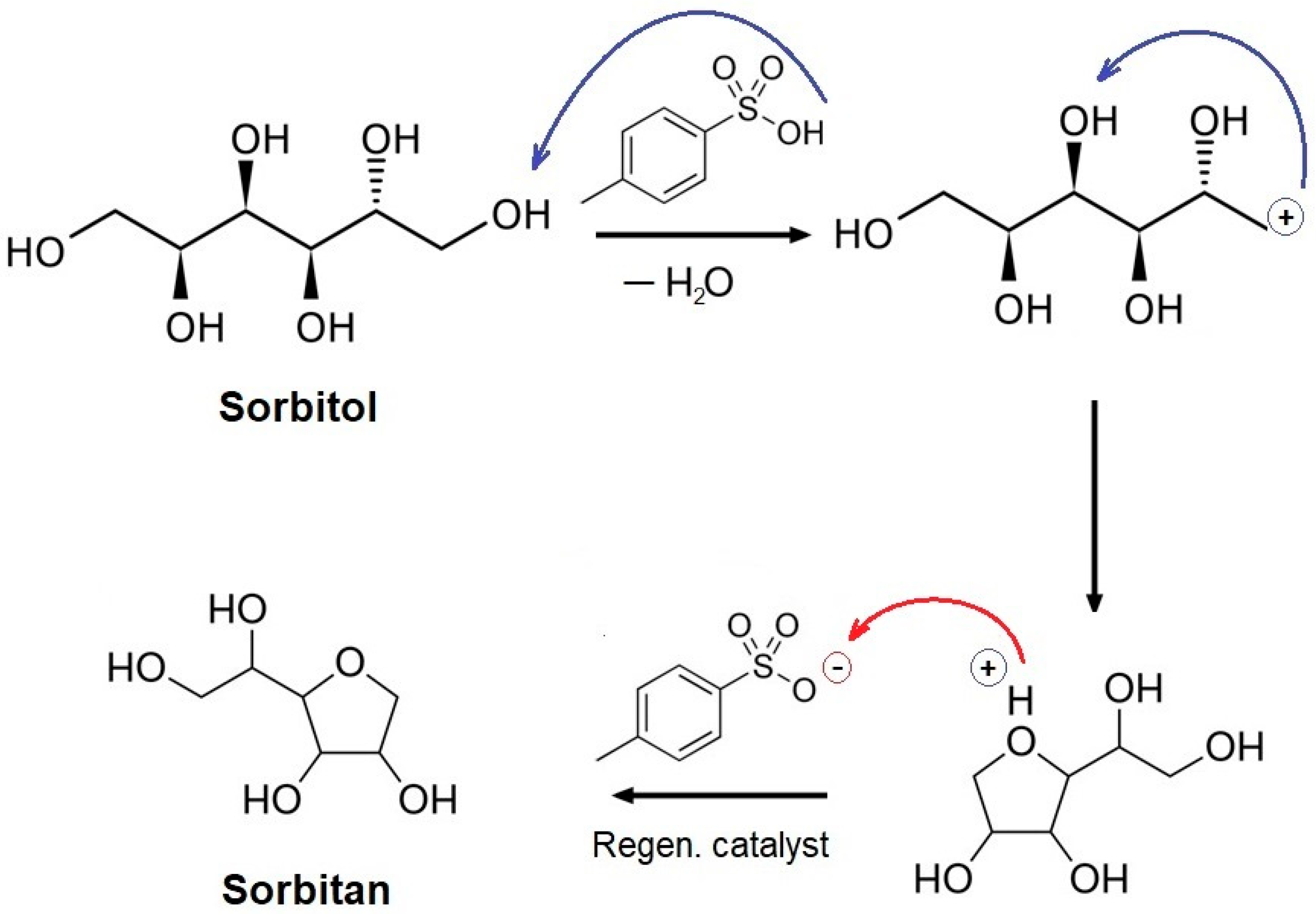
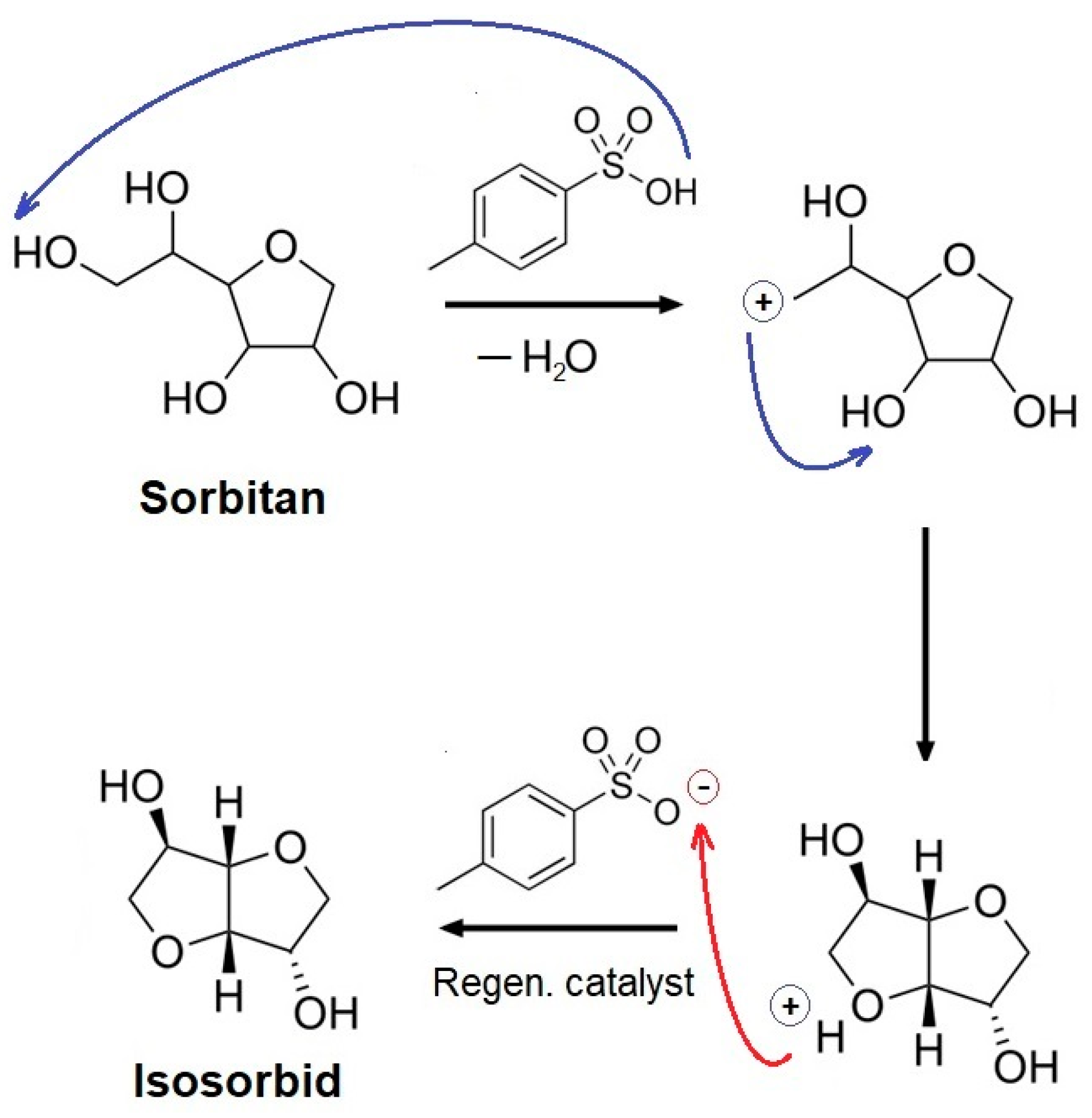
2.3. Synthesis of Isosorbide form Sorbitol
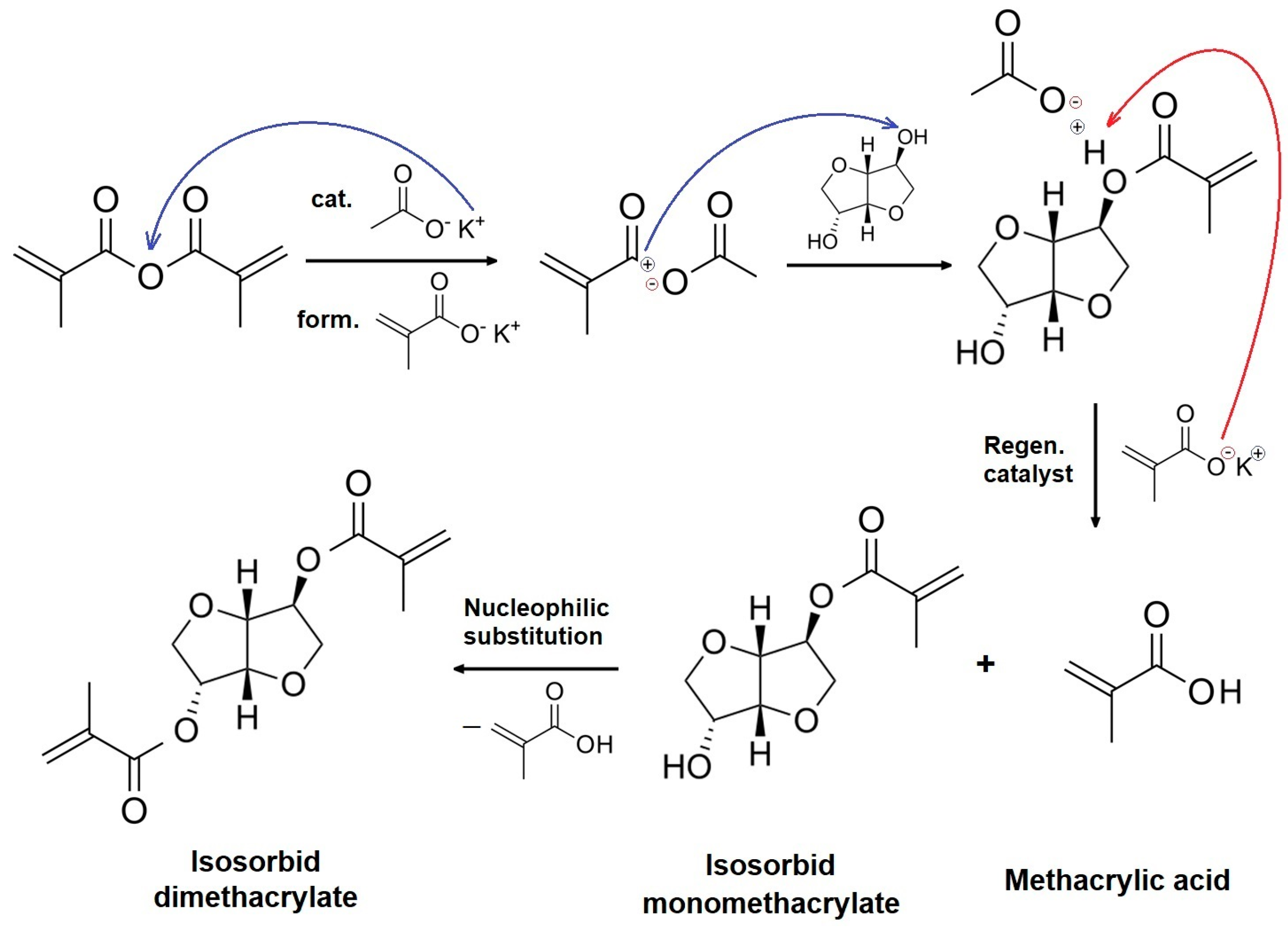
2.4. Synthesis of Isosorbide Dimethacrylate
2.5. Analytical Methods for Kinetics Study of Syntheses
2.5.1. Liquid Chromatography with Mass Spectrometry (LC-MS)
2.5.2. Gas Chromatography with Flame Ionization Detector (GC-FID)
2.6. Reactivity and Thermal Stability Characterization of Isosorbide Dimethacrylate
2.6.1. Differential Scanning Calorimetry (DSC)
2.6.2. Thermogravimetric Analysis (TGA)
2.7. Thermo-Mechanical Characterization of Synthesized Isosorbide Dimethacrylate Containing Resins
Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
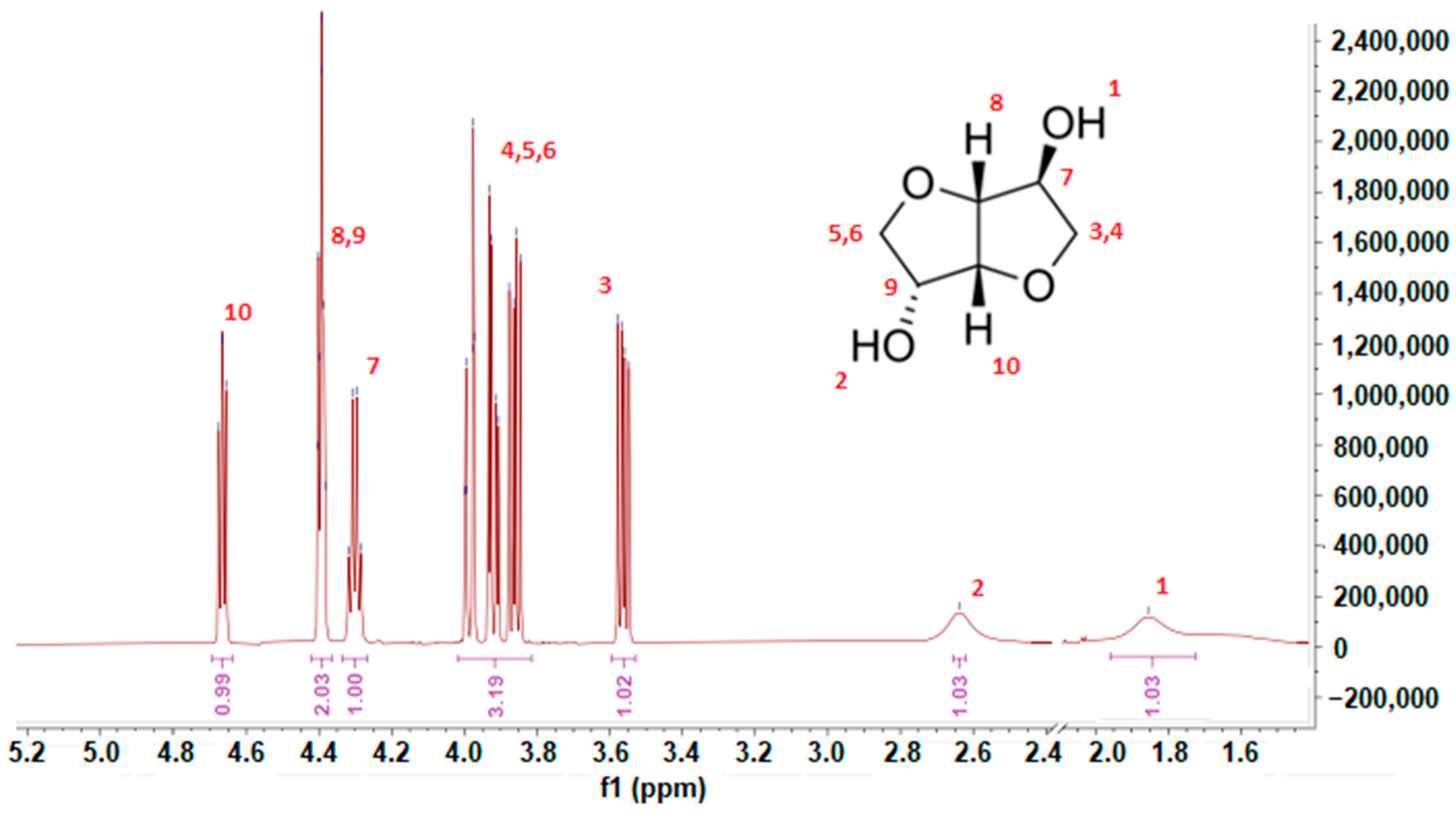
3.1. Isosorbide Synthesis and Characterization
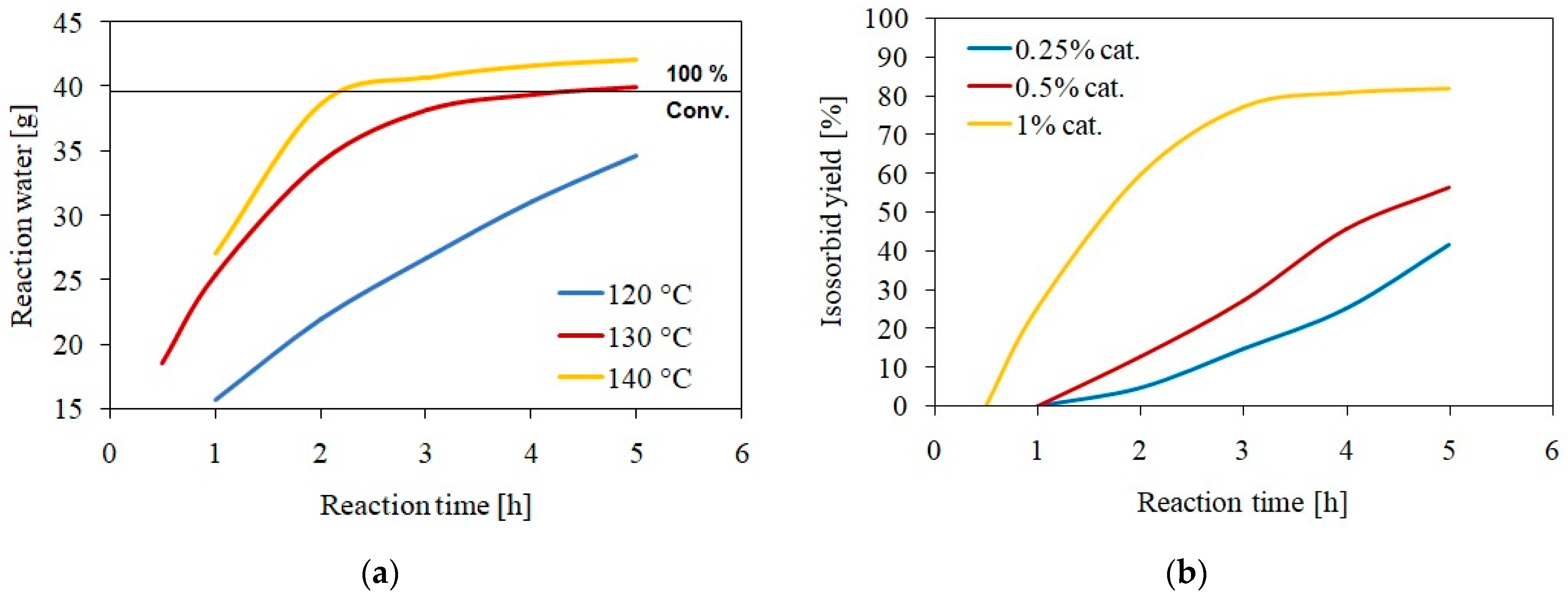
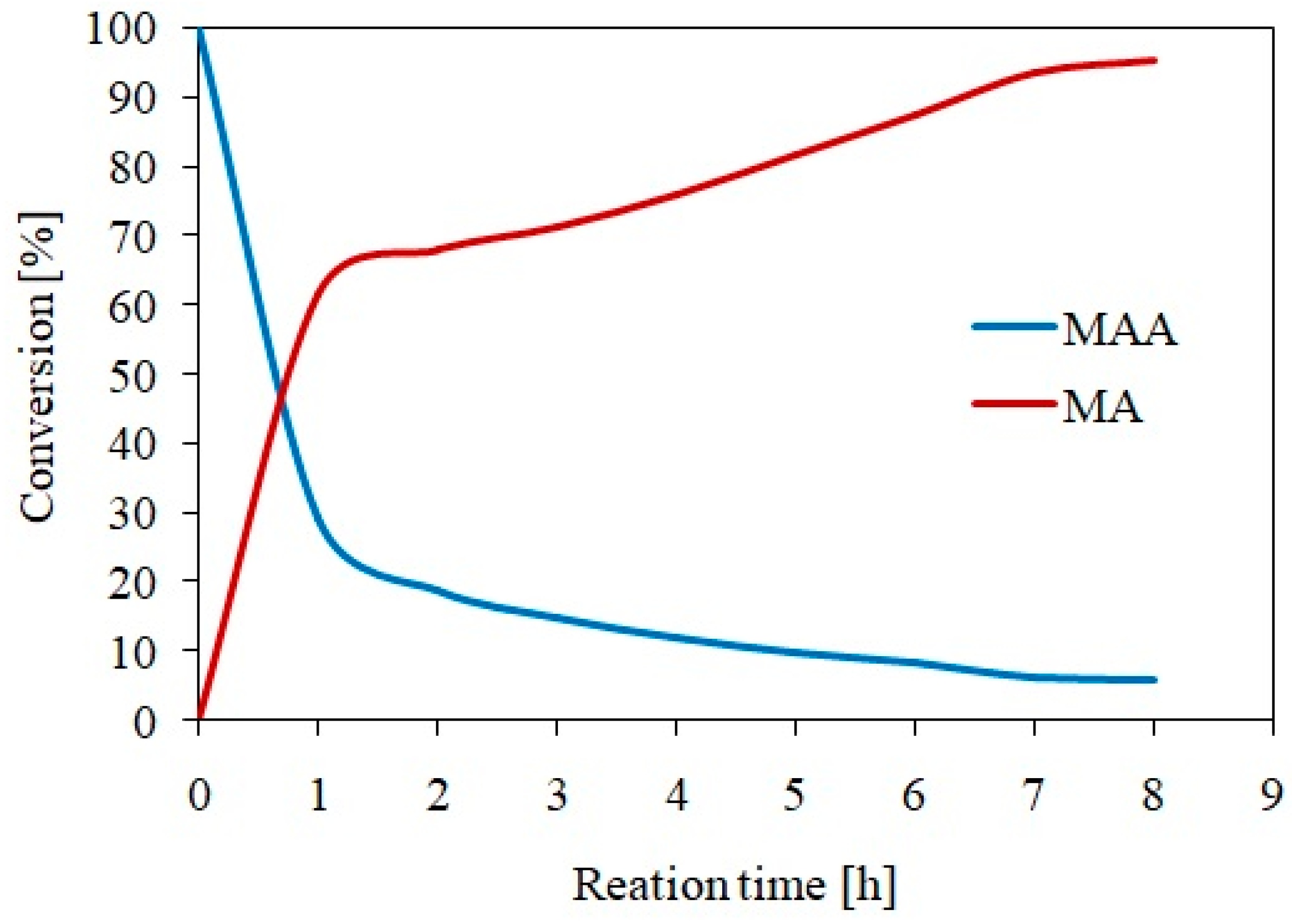
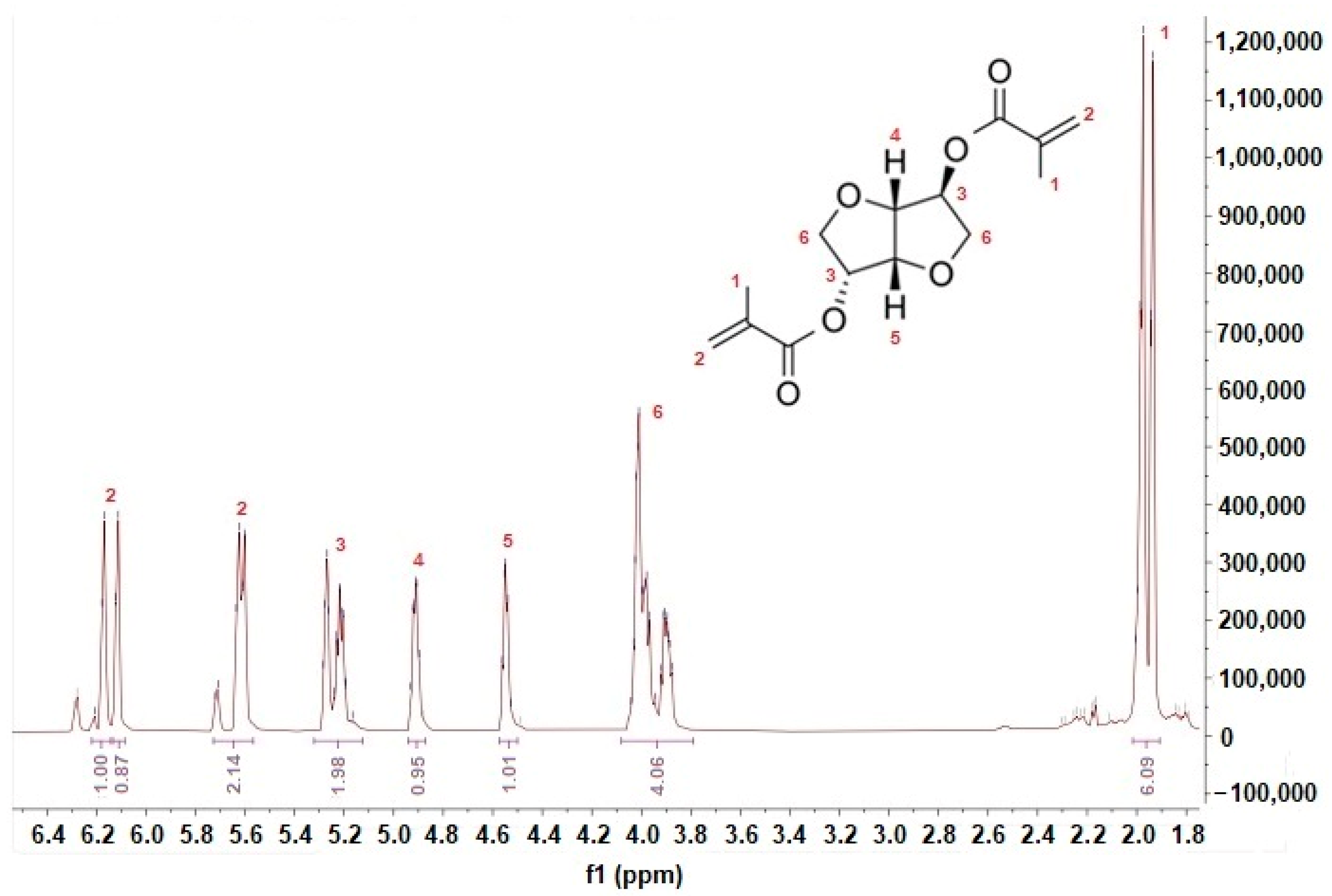
3.2. Isosorbide Dimethacrylate Synthesis and Characterization
3.3. Curability and Thermostability of Synthesized Isosorbide Dimethacrylate
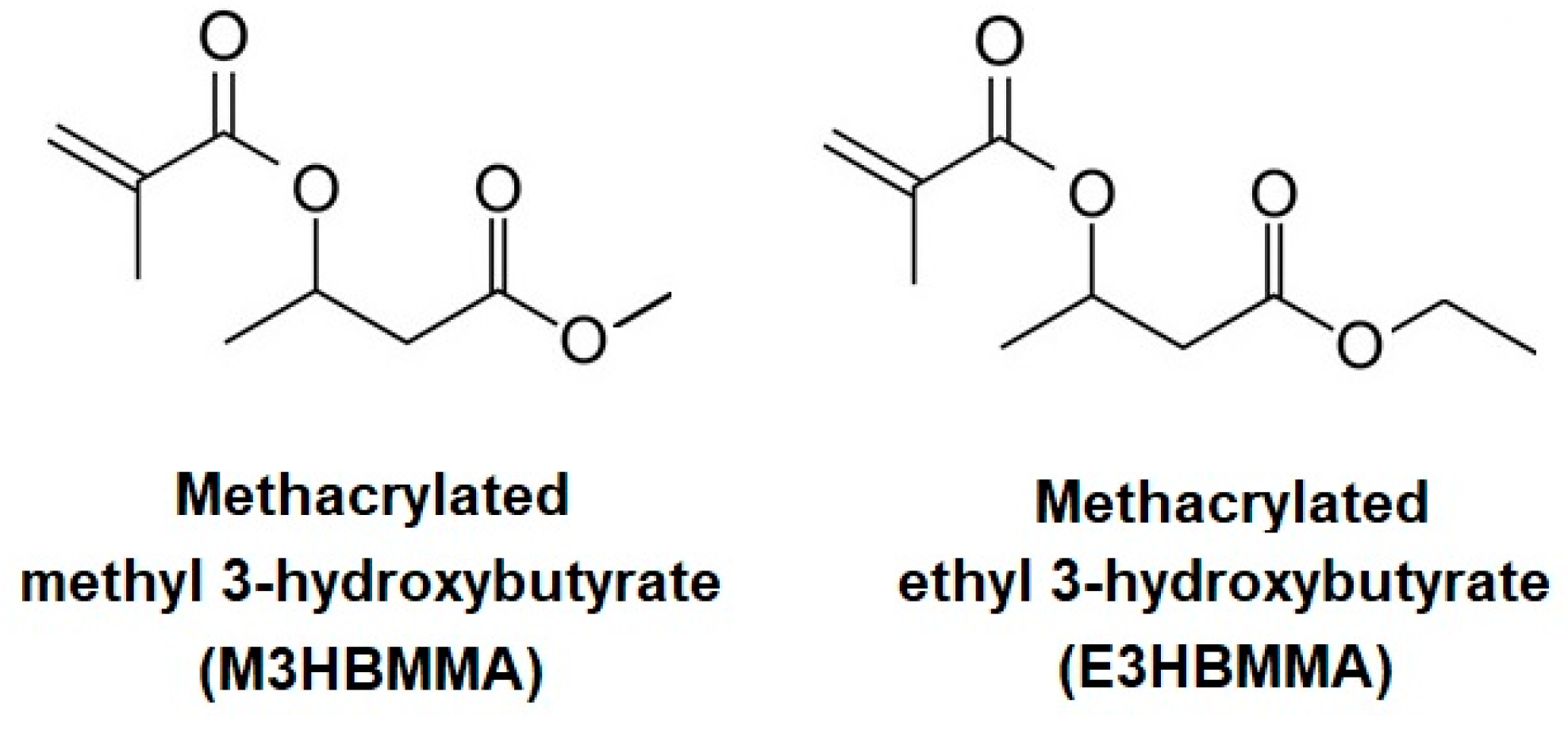
3.4. Ability of Isosorbide Dimethacrylate to Enhance Thermo-Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ternel, J.; Lopes, A.; Sauthier, M.; Buffe, C.; Wiatz, V.; Bricout, H.; Tilloy, S.; Monflier, E. Reductive Hydroformylation of Isosorbide Diallyl Ether. Molecules 2021, 26, 7322. [Google Scholar] [CrossRef] [PubMed]
- Marie, B.; Clark, R.; Gillece, T.; Ozkan, S.; Jaffe, M.; Ravindra, N.M. Hydrophobically Modified Isosorbide Dimethacrylates as a Bisphenol-A (Bpa)-Free Dental Filling Material. Materials 2021, 14, 2139. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing Conditions, Thermal and Mechanical Responses of Stretchable Poly (Lactic Acid)/Poly (Butylene Succinate) Films. Materials 2017, 10, 809. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, Y.; Yu, L.; Xie, F. Chitosan–Gelatin Films: Plasticizers/Nanofillers Affect Chain Interactions and Material Properties in Different Ways. Polymers 2022, 14, 3797. [Google Scholar] [CrossRef]
- Gómez-López, R.A.; Montilla-Buitrago, C.E.; Villada-Castillo, H.S.; Sáenz-Galindo, A.; Avalos-Belmontes, F.; Serna-Cock, L. Co-Plasticization of Starch with Glycerol and Isosorbide: Effect on Retrogradation in Thermo-Plastic Cassava Starch Films. Polymers 2023, 15, 2104. [Google Scholar] [CrossRef]
- Song, Z.; Xu, F.; Wang, H.; Zhang, Z.; Feng, M.; Zhang, Y.; Yang, Z.; Xie, J.; Su, D.; Li, T. Design and Synthesis of Isosorbide-Based Copolycarbonates with High Transparency and Low Hygroscopicity for Optical Applications. J. Appl. Polym. Sci. 2023, 140, e54009. [Google Scholar] [CrossRef]
- Chen, M.; Pan, X.; Tian, Y.; Li, J.; Tan, Q.; Jin, M.; Ren, J. Synthesis of Renewable Isosorbide-Based Polyurethane Acrylate Resins for Uv-Cured Coating with Adjustable Properties. Prog. Org. Coat. 2023, 182, 107695. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcille, S.; Fontaine, G. Synthesis of Isosorbide Based Flame Retardants: Application for Polybutylene Succinate. Polym. Degrad. Stab. 2019, 164, 9–17. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Zammarano, M.; Gilman, J.W.; Shields, J.R.; Boday, D.J. Synthesis and Characterization of Isosorbide-Based Polyphosphonates as Biobased Flame-Retardants. Polym. Chem. 2014, 5, 5139–5146. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame Retardant Properties of Isosorbide Bis-Phosphorus Esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Polaert, I.; Felix, M.C.; Fornasero, M.; Marcotte, S.; Buvat, J.-C.; Estel, L. A Greener Process for Isosorbide Production: Kinetic Study of the Catalytic Dehydration of Pure Sorbitol under Microwave. Chem. Eng. J. 2013, 222, 228–239. [Google Scholar] [CrossRef]
- Redina, E.; Tkachenko, O.; Salmi, T. Recent Advances in C5 And C6 Sugar Alcohol Synthesis By Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules 2022, 27, 1353. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jeon, K.-J.; Park, Y.-K.; Kim, B.-J.; Chung, K.-H.; Jung, S.-C. Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration. Catalysts 2020, 10, 148. [Google Scholar] [CrossRef]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol Over Ni-Hzsm-5/Sba-15 Catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef]
- Patiño, Y.; Faba, L.; Peláez, R.; Cueto, J.; Marín, P.; Díaz, E.; Ordóñez, S. The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges. Catalysts 2023, 13, 999. [Google Scholar] [CrossRef]
- Brandi, F.; Al-Naji, M. Sustainable Sorbitol Dehydration to Isosorbide Using Solid Acid Catalysts: Transition from Batch Reactor to Continuous-Flow System. ChemSusChem 2022, 15, e202102525. [Google Scholar] [CrossRef]
- Ginés-Molina, M.J.; Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P. Dehydration of Sorbitol to Isosorbide over Sulfonic Acid Resins under Solvent-Free Conditions. Appl. Catal. A Gen. 2017, 537, 66–73. [Google Scholar] [CrossRef]
- Durand, M.; Zhu, Y.; Molinier, V.; Féron, T.; Aubry, J.-M. Solubilizing and Hydrotropic Properties of Isosorbide Monoalkyl- and Dimethyl-Ethers. J. Surfactants Deterg. 2009, 12, 371–378. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Lorenzo-Ibarreta, L.; Diñeiro-García, C.; Gómez-Jiménez-Aberasturi, O. Isosorbide Bis(Methyl Carbonate) Synthesis from Isosorbide and Dimethyl Carbonate: The Key Role of Dual Basic–Nucleophilic Catalysts. RSC Adv. 2020, 10, 18728–18739. [Google Scholar] [CrossRef]
- Sadler, J.M.; Toulan, F.R.; Nguyen, A.-P.T.; Kayea, R.V.; Ziaee, S.; Palmese, G.R.; La Scala, J.J. Isosorbide as the Structural Component of Bio-Based Unsaturated Polyesters for Use as Thermosetting Resins. Carbohydr. Polym. 2014, 100, 97–106. [Google Scholar] [CrossRef]
- Dussenne, C.; Delaunay, T.; Wiatz, V.; Wyart, H.; Suisse, I.; Sauthier, M. Synthesis of Isosorbide: An Overview of Challenging Reactions. Green Chem. 2017, 19, 5332–5344. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, S.; Zhang, J.; Wu, J.; Shao, Y.; Wang, Z.; Yang, M. The Preparation of Sorbitol and Its Application in Polyurethane: A Review. Polym. Bull. 2022, 79, 2667–2684. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A Guide Through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fei, M.; Qiu, R.; Liu, W.; Qiu, J. A Review on Styrene Substitutes in Thermosets and Their Composites. Polymers 2019, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kim, B.; Kim, Y.-J.; Lee, S.-H.; Shin, S.; Cho, J.K. Synthesis of the Bio-Based Alternative to Bis-Gma and Its Application to Photo-Polymerizable Adhesives. Int. J. Adhes. Adhes. 2018, 80, 60–65. [Google Scholar] [CrossRef]
- Kang, E.C.; Ogura, A.; Kataoka, S.; Iwata, T. Diol (Meth) Acrylate Compoundhaving Urethane Bond, Method Forproducing the Same, and Polymer Thereof. U.S. Patent No. 7,649,105, 19 January 2010. [Google Scholar]
- Sadler, J.M.; Nguyen, A.-P.T.; Toulan, F.R.; Szabo, J.P.; Palmese, G.R.; Scheck, C.; Lutgen, S.; La Scala, J.J. Isosorbide-Methacrylate as a Bio-Based Low Viscosity Resin for High Performance Thermosetting Applications. J. Mater. Chem. A 2013, 1, 12579–12586. [Google Scholar] [CrossRef]
- Xu, Y.; Hua, G.; Hakkarainen, M.; Odelius, K. Isosorbide as Core Component for Tailoring Biobased Unsaturated Polyester Thermosets for a Wide Structure–Property Window. Biomacromolecules 2018, 19, 3077–3085. [Google Scholar] [CrossRef]
- Shin, S.; Kim, B.-C.; Chang, E.; Cho, J.K.; Suh, D.H. A Biobased Photocurable Binder for Composites with Transparency and Thermal Stability from Biomass-Derived Isosorbide. RSC Adv. 2014, 4, 6226–6231. [Google Scholar] [CrossRef]
- Fertier, L.; Ibert, M.; Buffe, C.; Saint-Loup, R.; Joly-Duhamel, C.; Robin, J.-J.; Giani, O. New BiosourcedUv Curable Coatings Based On Isosorbide. Prog. Org. Coat. 2016, 99, 393–399. [Google Scholar] [CrossRef]
- Vazifehasl, Z.; Hemmati, S.; Zamanloo, M.; Dizaj, S.M. New series of dimethacrylate-based monomers on isosorbide as a dental material: Synthesis and characterization. Int. J. Compos. Mater. 2013, 3, 100–107. [Google Scholar]
- Alli, Y.A.; Anuar, H.; Manshor, M.R.; Bankole, O.M.; Rahman, N.A.A.; Olatunde, S.K.; Omotola, E.O.; Oladoye, P.O.; Ejeromedoghene, O.; Suhr, J.; et al. Influence of Nanocomposites in Extrusion-Based 3D Printing: A Review. Hybrid Adv. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Kim, S.; Cho, J.K.; Shin, S.; Kim, B.-J. Photo-Curing Behaviors of Bio-Based Isosorbide Dimethacrylate by Irradiation of Light-Emitting Diodes and the Physical Properties of Its Photo-Cured Materials. J. Appl. Polym. Sci. 2015, 132, 42726. [Google Scholar] [CrossRef]
- Yadav, S.K.; Schmalbach, K.M.; Kinaci, E.; Stanzione, J.F.; Palmese, G.R. Recent Advances In Plant-Based Vinyl Ester Resins and Reactive Diluents. Eur. Polym. J. 2018, 98, 199–215. [Google Scholar] [CrossRef]
- Wei, G.; Xu, H.; Chen, L.; Li, Z.; Liu, R. Isosorbide-Based High Performance Uv-Curable Reactive Diluents. Prog. Org. Coat. 2019, 126, 162–167. [Google Scholar] [CrossRef]
- Badía, A.; Barandiaran, M.J.; Leiza, J.R. Biobased Alkali Soluble Resins Promoting Supramolecular Interactions in Sustainable Waterborne Pressure-Sensitive Adhesives: High Performance and Removability. Eur. Polym. J. 2021, 144, 110244. [Google Scholar] [CrossRef]
- Lastovickova, D.N.; Toulan, F.R.; Mitchell, J.R.; VanOosten, D.; Clay, A.M.; Stanzione, J.F.; Palmese, G.R.; La Scala, J.J. Resin, Cure, and Polymer Properties of Photopolymerizable Resins Containing Bio-Derived Isosorbide. J. Appl. Polym. Sci. 2021, 138, app50574. [Google Scholar] [CrossRef]
- George, J.; Prasana, J.C.; Muthu, S.; Kuruvilla, T.K. Spectroscopic (FT-IR, FT Raman) and quantum mechanical study on isosorbide mononitrate by density functional theory. Int. J. Mater. Sci. 2017, 12, 268–278. [Google Scholar]
- Battegazzore, D.; Bocchini, S.; Nicola, G.; Martini, E.; Frache, A. Isosorbide, a Green Plasticizer for Thermoplastic Starch That Does Not Retrogradate. Carbohydr. Polym. 2015, 119, 78–84. [Google Scholar] [CrossRef]
- Erol, I.; Akbıyık, H. Kinetic Parameters, Thermal Stability, Biological Activity, and Dielectric Properties of New Methacrylate-Based Copolymers Functionalized with Methylparaben. J. Polym. Res. 2022, 29, 93. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Nouailhas, H.; Aouf, C.; Le Guerneve, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and Properties of Biobased Epoxy Resins. Part 1. Glycidylation of Flavonoids by Epichlorohydrin. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2261–2270. [Google Scholar] [CrossRef]
- Aouf, C.; Nouailhas, H.; Fache, M.; Caillol, S.; Boutevin, B.; Fulcrand, H. Multi-Functionalization of Gallic Acid. Synthesis of a Novel Bio-Based Epoxy Resin. Eur. Polym. J. 2013, 49, 1185–1195. [Google Scholar] [CrossRef]
- Badía, A.; Agirre, A.; Barandiaran, M.J.; Leiza, J.R. Removable Biobased Waterborne Pressure-Sensitive Adhesives Containing Mixtures of Isosorbide Methacrylate Monomers. Biomacromolecules 2020, 21, 4522–4531. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sui, B.; Cui, Y.; Liu, X.; Sun, J.; Wang, J. A Novel Dental Infiltration Resin Based on Isosorbide-Derived Dimethacrylate with High Biocompatibility, Hydrolysis Resistance, and Antibacterial Effect. Front. Bioeng. Biotechnol. 2022, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jašek, V.; Fučík, J.; Ivanová, L.; Veselý, D.; Figalla, S.; Mravcova, L.; Sedlacek, P.; Krajčovič, J.; Přikryl, R. High-Pressure Depolymerization of Poly(lactic acid) (PLA) and Poly(3-hydroxybutyrate) (PHB) Using Bio-Based Solvents: A Way to Produce Alkyl Esters Which Can Be Modified to Polymerizable Monomers. Polymers 2022, 14, 5236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased Polyurethanes Prepared from Different Vegetable Oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Çayli, G.; Gürbüz, D.; Çınarli, A. Characterization and Polymerization of Epoxidized Methacrylated Castor Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1700189. [Google Scholar] [CrossRef]
- Khan, M.A.; Masudul Hassan, M.; Drzal, L.T. Effect of 2-Hydroxyethyl Methacrylate (Hema) on the Mechanical and Thermal Properties of Jute-Polycarbonate Composite. Compos. Part A Appl. Sci. Manuf. 2005, 36, 71–81. [Google Scholar] [CrossRef]
- Karasz, F.E.; MacKnight, W.J. The Influence of Stereoregularity on the Glass Transition Temperatures of Vinyl Polymers. Macromolecules 1968, 1, 537–540. [Google Scholar] [CrossRef]
- Schneider, H.A. Polymer Class Specificity of the Glass Temperature. Polymer 2005, 46, 2230–2237. [Google Scholar] [CrossRef]







| Differential Scanning Calorimetry (DSC) | ||||||||
|---|---|---|---|---|---|---|---|---|
| System | Tp (°C) | E (kJ/mol) | lnA | R2 | ||||
| 5 °C/min | 10 °C/min | 15 °C/min | 20 °C/min | 25 °C/min | ||||
| ISDMMA | 398.6 | 403.89 | 407.22 | 410.81 | 412.84 | 146.2 | 43.57 | 0.992 |
| Thermogravimetric Analysis (TGA) | ||||
|---|---|---|---|---|
| Thermoset | T5 (°C) | T30 (°C) | Tmax (°C) | Ts (−) |
| ISDMMA | 299.9 | 374.7 | 411.0 | 168.9 |
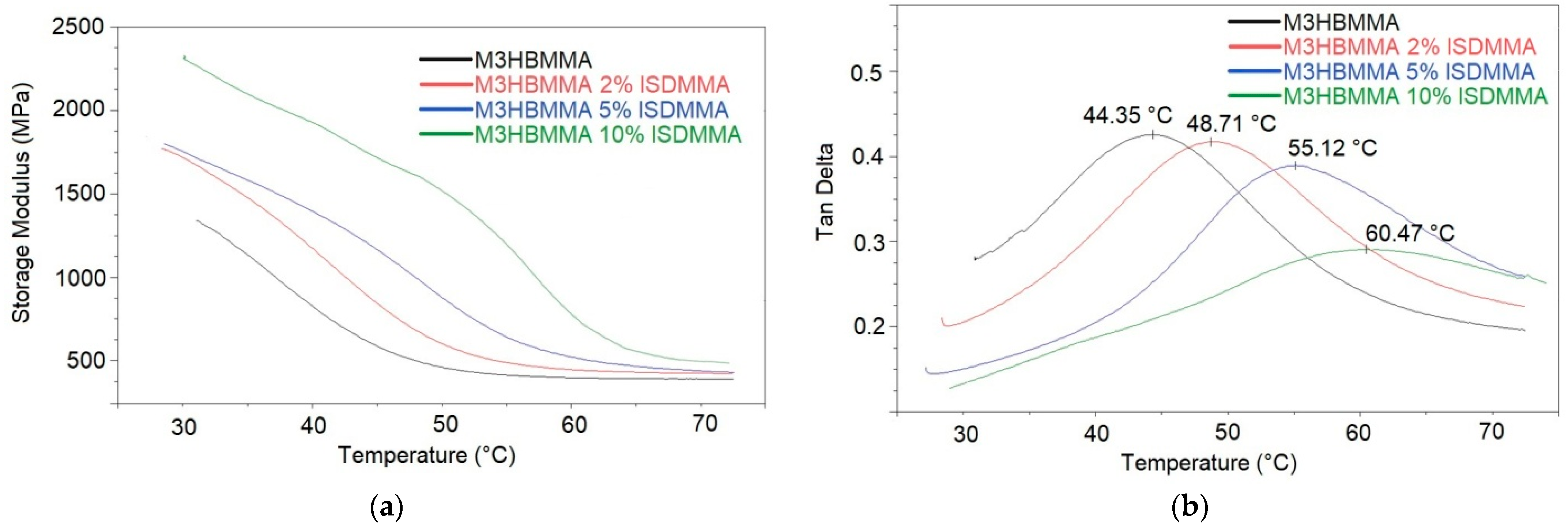
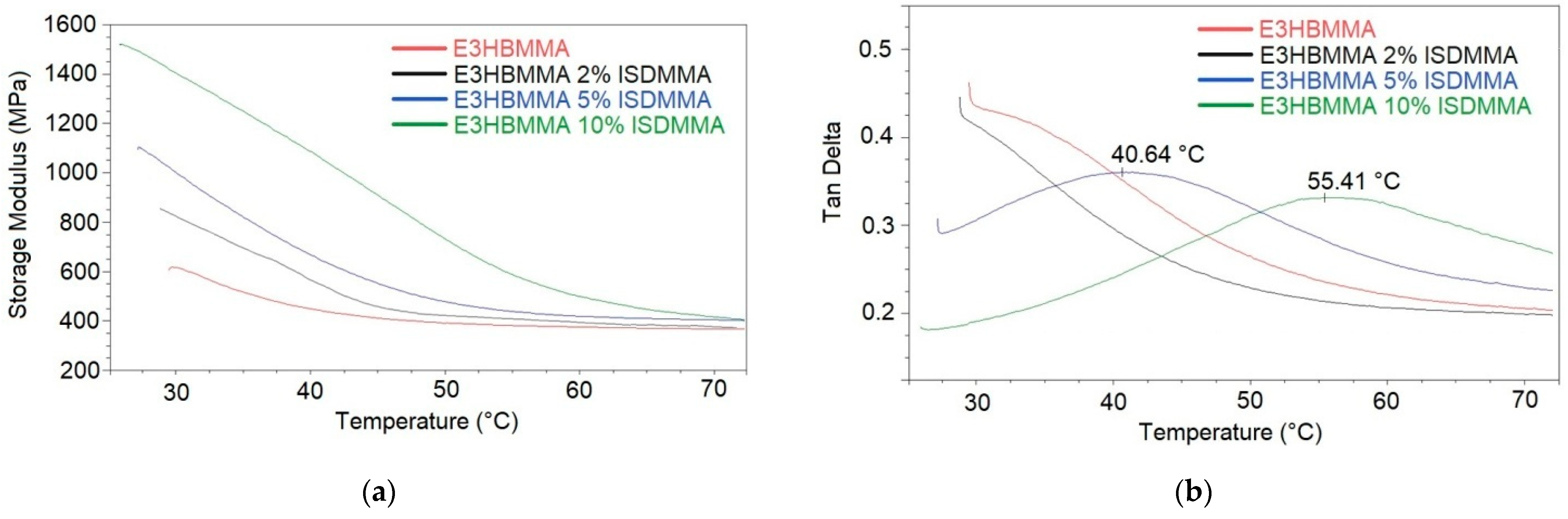
| Dynamic Mechanical Analysis (DMA) | |||
|---|---|---|---|
| Thermoset | E’30°C (MPa) | Tg (°C) | νe (kmol/m3) |
| M3HBMMA | 1370 | 44.4 | 40.4 |
| M3HBMMA 2% ISDMMA | 1750 | 48.7 | 41.3 |
| M3HBMMA 5% ISDMMA | 1860 | 55.1 | 41.9 |
| M3HBMMA 10% ISDMMA | 2310 | 60.5 | 46.2 |
| E3HBMMA | 630 | <25.0 | – |
| E3HBMMA 2% ISDMMA | 870 | <25.0 | – |
| E3HBMMA 5% ISDMMA | 1020 | 40.6 | 41.3 |
| E3HBMMA 10% ISDMMA | 1410 | 55.4 | 44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jašek, V.; Fučík, J.; Krhut, J.; Mravcova, L.; Figalla, S.; Přikryl, R. A Study of Isosorbide Synthesis from Sorbitol for Material Applications Using Isosorbide Dimethacrylate for Enhancement of Bio-Based Resins. Polymers 2023, 15, 3640. https://doi.org/10.3390/polym15173640
Jašek V, Fučík J, Krhut J, Mravcova L, Figalla S, Přikryl R. A Study of Isosorbide Synthesis from Sorbitol for Material Applications Using Isosorbide Dimethacrylate for Enhancement of Bio-Based Resins. Polymers. 2023; 15(17):3640. https://doi.org/10.3390/polym15173640
Chicago/Turabian StyleJašek, Vojtěch, Jan Fučík, Jiří Krhut, Ludmila Mravcova, Silvestr Figalla, and Radek Přikryl. 2023. "A Study of Isosorbide Synthesis from Sorbitol for Material Applications Using Isosorbide Dimethacrylate for Enhancement of Bio-Based Resins" Polymers 15, no. 17: 3640. https://doi.org/10.3390/polym15173640
APA StyleJašek, V., Fučík, J., Krhut, J., Mravcova, L., Figalla, S., & Přikryl, R. (2023). A Study of Isosorbide Synthesis from Sorbitol for Material Applications Using Isosorbide Dimethacrylate for Enhancement of Bio-Based Resins. Polymers, 15(17), 3640. https://doi.org/10.3390/polym15173640





