Fabrication of Tri-Directional Poly(2,5-benzimidazole) Membrane Using Direct Casting for Vanadium Redox Flow Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
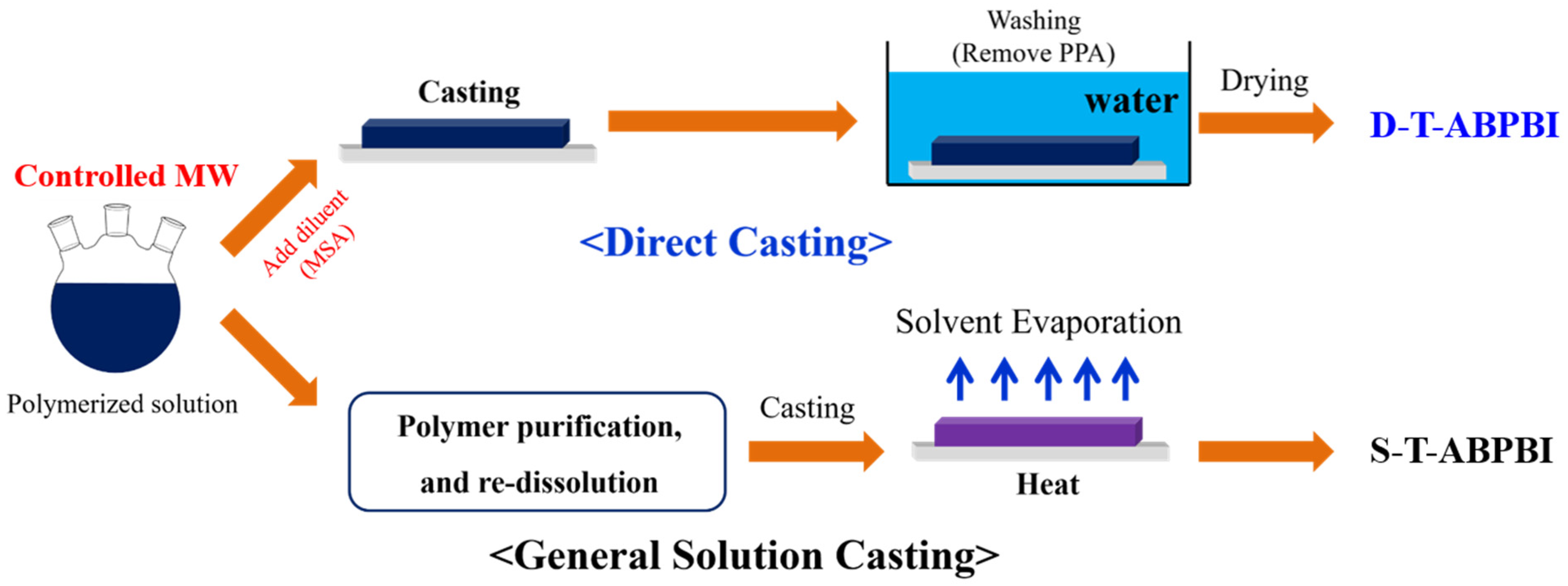
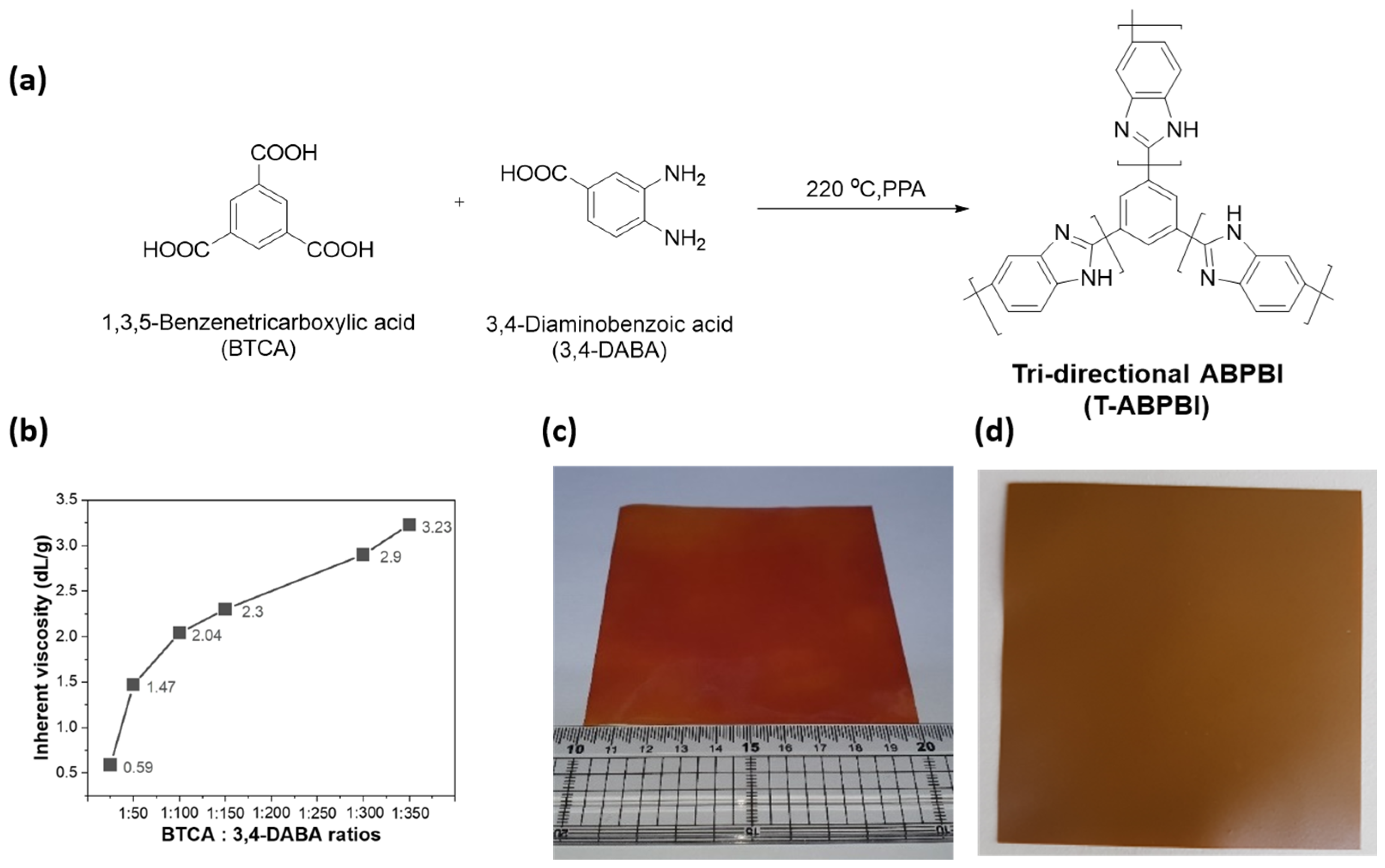
2.2. Polymer Synthesis and Membrane Preparation
2.3. Polymer Characterization
2.4. Membrane Characterization
2.5. VRFB Single-Cell Test
3. Results
3.1. Polymer Synthesis and Membrane Preparation
3.2. Wide-Angle X-ray Diffraction and Dielectric Constant
3.3. Absorption and Ion-Conduction Properties of PBI Membranes
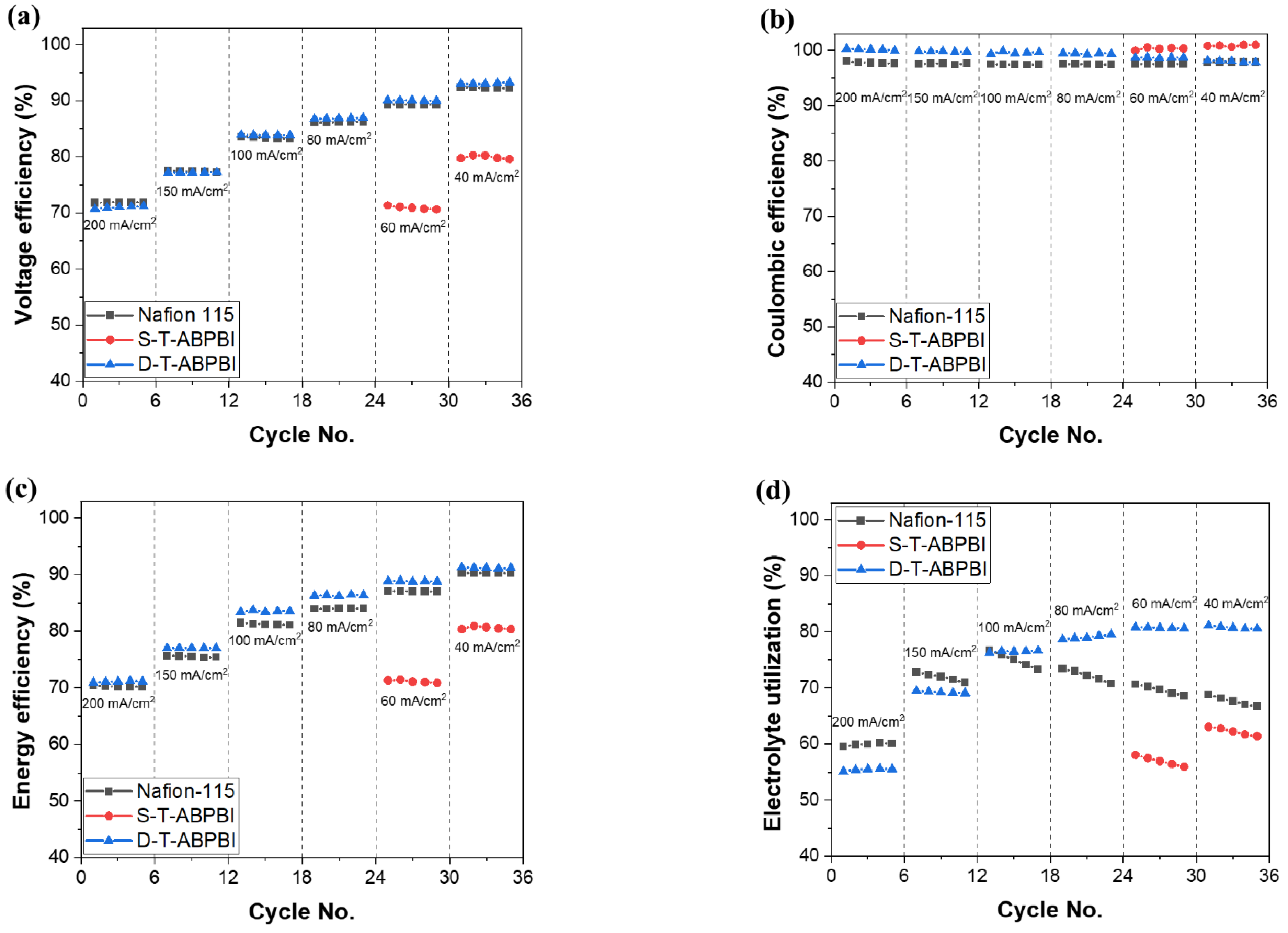
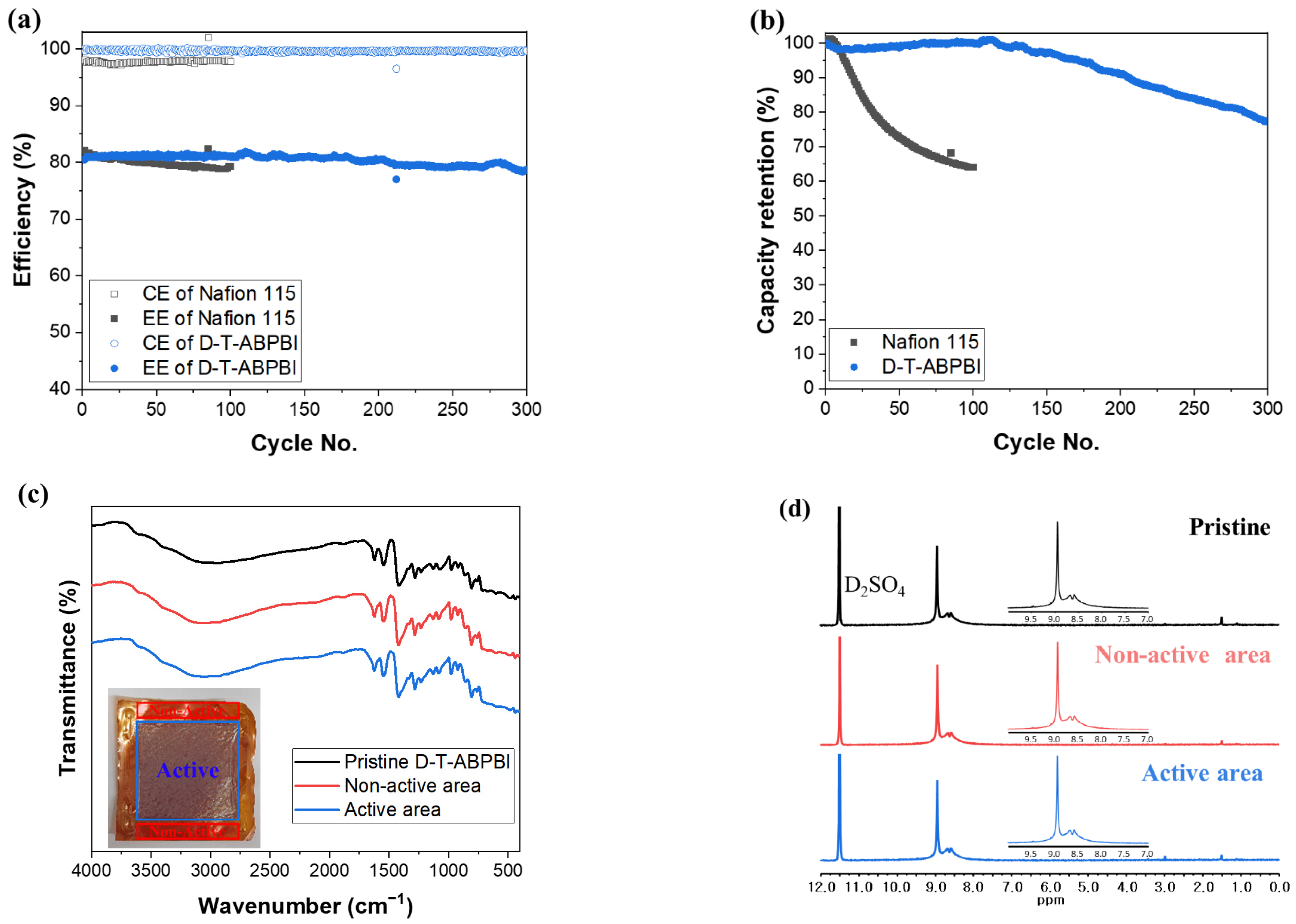
3.4. VRFB Single-Cell Test and Long-Term Cycling Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Hannan, M.A.; Wali, S.B.; Ker, P.J.; Rahman, M.S.A.; Mansor, M.; Ramachandaramurthy, V.K.; Muttaqi, K.M.; Mahlia, T.M.I.; Dong, Z.Y. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.-y.; Yang, J.H.; Kim, H.-T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sust. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.; Hu, J.; Xu, W.; Cao, J.; Zhang, H. Degradation mechanism of sulfonated poly(ether ether ketone) (SPEEK) ion exchange membranes under vanadium flow battery medium. Phys. Chem. Chem. Phys. 2014, 16, 19841–19847. [Google Scholar] [CrossRef]
- Hao, X.; Chen, N.; Chen, Y.; Chen, D. Accelerated degradation of quaternary ammonium functionalized anion exchange membrane in catholyte of vanadium redox flow battery. Polym. Degrad. Stab. 2022, 197, 109864. [Google Scholar] [CrossRef]
- Sharma, J.; Kulshrestha, V. Advancements in polyelectrolyte membrane designs for vanadium redox flow battery (VRFB). Results Chem. 2023, 5, 100892. [Google Scholar] [CrossRef]
- Dai, Q.; Xing, F.; Liu, X.; Shi, D.; Deng, C.; Zhao, Z.; Li, X. High-performance PBI membranes for flow batteries: From the transport mechanism to the pilot plant. Energy Environ. Sci. 2022, 15, 1594–1600. [Google Scholar] [CrossRef]
- Yuan, Z.; Duan, Y.; Zhang, H.; Li, X.; Zhang, H.; Vankelecom, I. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries. Energy Environ. Sci. 2016, 9, 441–447. [Google Scholar] [CrossRef]
- Mara Ikhsan, M.; Abbas, S.; Do, X.H.; Choi, S.-Y.; Azizi, K.; Hjuler, H.A.; Jang, J.H.; Ha, H.Y.; Henkensmeier, D. Polybenzimidazole membranes for vanadium redox flow batteries: Effect of sulfuric acid doping conditions. Chem. Eng. J. 2022, 435, 134902. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Qiao, L.; Zhang, H.; Li, X. Advanced porous PBI membranes with tunable performance induced by the polymer-solvent interaction for flow battery application. Energy Storage Mater. 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Wang, L.; Han, X. Microstructure regulation of porous polybenzimidazole proton conductive membranes for high-performance vanadium redox flow battery. J. Membr. Sci. 2022, 642, 119934. [Google Scholar] [CrossRef]
- Jang, J.-K.; Kim, T.-H.; Yoon, S.J.; Lee, J.Y.; Lee, J.-C.; Hong, Y.T. Highly proton conductive, dense polybenzimidazole membranes with low permeability to vanadium and enhanced H2SO4 absorption capability for use in vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 14342–14355. [Google Scholar] [CrossRef]
- Peng, S.; Wu, X.; Yan, X.; Gao, L.; Zhu, Y.; Zhang, D.; Li, J.; Wang, Q.; He, G. Polybenzimidazole membranes with nanophase-separated structure induced by non-ionic hydrophilic side chains for vanadium flow batteries. J. Mater. Chem. A 2018, 6, 3895–3905. [Google Scholar] [CrossRef]
- Yan, X.; Dong, Z.; Di, M.; Hu, L.; Zhang, C.; Pan, Y.; Zhang, N.; Jiang, X.; Wu, X.; Wang, J.; et al. A highly proton-conductive and vanadium-rejected long-side-chain sulfonated polybenzimidazole membrane for redox flow battery. J. Membr. Sci. 2020, 596, 117616. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, P.; Xiao, S.; Zhu, Y.; Peng, S.; He, G. Ion conductive mechanisms and redox flow battery applications of polybenzimidazole-based membranes. Energy Storage Mater. 2022, 45, 595–617. [Google Scholar] [CrossRef]
- Rath, R.; Kumar, P.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Current scenario of poly(2,5-benzimidazole) (ABPBI) as prospective PEM for application in HT-PEMFC. Polym. Rev. 2020, 60, 267–317. [Google Scholar] [CrossRef]
- Jang, J.-K.; Jo, S.-W.; Jeon, J.W.; Kim, B.G.; Yoon, S.J.; Yu, D.M.; Hong, Y.T.; Kim, H.-T.; Kim, T.-H. Alkyl spacer grafted ABPBI membranes with enhanced acid-absorption capabilities for use in vanadium redox flow batteries. ACS Appl. Energy Mater. 2021, 4, 4672–4685. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Kharul, U.K. New N-substituted ABPBI: Synthesis and evaluation of gas permeation properties. J. Membr. Sci. 2010, 360, 418–425. [Google Scholar] [CrossRef]
- Ding, L.; Song, X.; Wang, L.; Zhao, Z.; He, G. Preparation of dense polybenzimidazole proton exchange membranes with different basicity and flexibility for vanadium redox flow battery applications. Electrochim. Acta 2018, 292, 10–19. [Google Scholar] [CrossRef]
- Asensio, J. Proton-conducting membranes based on poly(2,5-benzimidazole) (ABPBI) and phosphoric acid prepared by direct acid casting. J. Membr. Sci. 2004, 241, 89–93. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, H.; Scanlon, E.; Ramanathan, L.S.; Choe, E.-W.; Rogers, D.; Apple, T.; Benicewicz, B.C. High-temperature polybenzimidazole fuel cell membranes via a sol−gel process. Chem. Mater. 2005, 17, 5328–5333. [Google Scholar] [CrossRef]
- Chen, J.-C.; Hsiao, Y.-R.; Liu, Y.-C.; Chen, P.-Y.; Chen, K.-H. Polybenzimidazoles containing heterocyclic benzo[c]cinnoline structure prepared by sol-gel process and acid doping level adjustment for high temperature PEMFC application. Polymer 2019, 182, 121814. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lim, T.-W.; Park, Y.-S.; Shin, K.; Lee, J.-C. Proton-conducting zirconium pyrophosphate/poly(2,5-benzimidazole) composite membranes prepared by a PPA direct casting method. Macromol. Chem. Phys. 2007, 208, 2293–2302. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lim, T.-W.; Lee, J.-C. High-temperature fuel cell membranes based on mechanically stable para-ordered polybenzimidazole prepared by direct casting. J. Power Sources 2007, 172, 172–179. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, T.-H.; Ko, T.; Lee, J.-C. Cross-linked poly(2,5-benzimidazole) consisting of wholly aromatic groups for high-temperature PEM fuel cell applications. J. Membr. Sci. 2011, 373, 80–88. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Sun, Y.-M. Relationship between free volume properties and structure of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes via various crystallization conditions. Polymer 2009, 50, 5298–5307. [Google Scholar]
- Singha, S.; Jana, T. Effect of composition on the properties of PEM based on polybenzimidazole and poly(vinylidene fluoride) blends. Polymer 2014, 55, 594–601. [Google Scholar]
- Eren, B.; Aydın, R.; Eren, E. Synthesis, characterization, and performance of a benzimidazole-based membrane in removal of phosphate: First lab-scale experimental results. J. Mater. Sci. 2013, 48, 7456–7465. [Google Scholar] [CrossRef]
- Ramani, R.; Das, V.; Singh, A.; Ramachandran, R.; Amarendra, G.; Alam, S. Free volume study on the origin of dielectric constant in a fluorine-containing polyimide blend: Poly(vinylidene fluoride-co-hexafluoro propylene)/poly(ether imide). J. Phys. Chem. B 2014, 118, 12282–12296. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Water uptake and acid doping of polybenzimidazoles as electrolyte membranes for fuel cells. Solid State Ion. 2004, 168, 177–185. [Google Scholar] [CrossRef]
- Glipa, X.; Bonnet, B.; Mula, B.; Jones, D.J.; Rozière, J. Investigation of the conduction properties of phosphoric and sulfuric acid doped polybenzimidazole. J. Mater. Chem. 1999, 9, 3045–3049. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, L.; Wu, L.; Mu, D.; Liu, L.; Xi, J.; Qiu, X. Insights into the impact of the Nafion membrane pretreatment process on vanadium flow battery performance. ACS Appl. Mater. Interfaces 2016, 8, 12228–12238. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Wei, L.; Zhang, C. The use of polybenzimidazole membranes in vanadium redox flow batteries leading to increased coulombic efficiency and cycling performance. Electrochim. Acta 2015, 153, 492–498. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Ion conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 2014, 26, 361–380. [Google Scholar] [CrossRef]
- Subianto, S. Recent advances in polybenzimidazole/phosphoric acid membranes for high-temperature fuel cells. Polym. Int. 2014, 63, 1134–1144. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-K.; Kim, T.-H. Fabrication of Tri-Directional Poly(2,5-benzimidazole) Membrane Using Direct Casting for Vanadium Redox Flow Battery. Polymers 2023, 15, 3577. https://doi.org/10.3390/polym15173577
Jang J-K, Kim T-H. Fabrication of Tri-Directional Poly(2,5-benzimidazole) Membrane Using Direct Casting for Vanadium Redox Flow Battery. Polymers. 2023; 15(17):3577. https://doi.org/10.3390/polym15173577
Chicago/Turabian StyleJang, Jung-Kyu, and Tae-Ho Kim. 2023. "Fabrication of Tri-Directional Poly(2,5-benzimidazole) Membrane Using Direct Casting for Vanadium Redox Flow Battery" Polymers 15, no. 17: 3577. https://doi.org/10.3390/polym15173577
APA StyleJang, J.-K., & Kim, T.-H. (2023). Fabrication of Tri-Directional Poly(2,5-benzimidazole) Membrane Using Direct Casting for Vanadium Redox Flow Battery. Polymers, 15(17), 3577. https://doi.org/10.3390/polym15173577




