Dial-In Synthesis of ‘Polymer Opal’ Core–Interlayer–Shell Composite Nanoparticles
Abstract
:1. Introduction
Synthesis Background
2. Materials and Methods
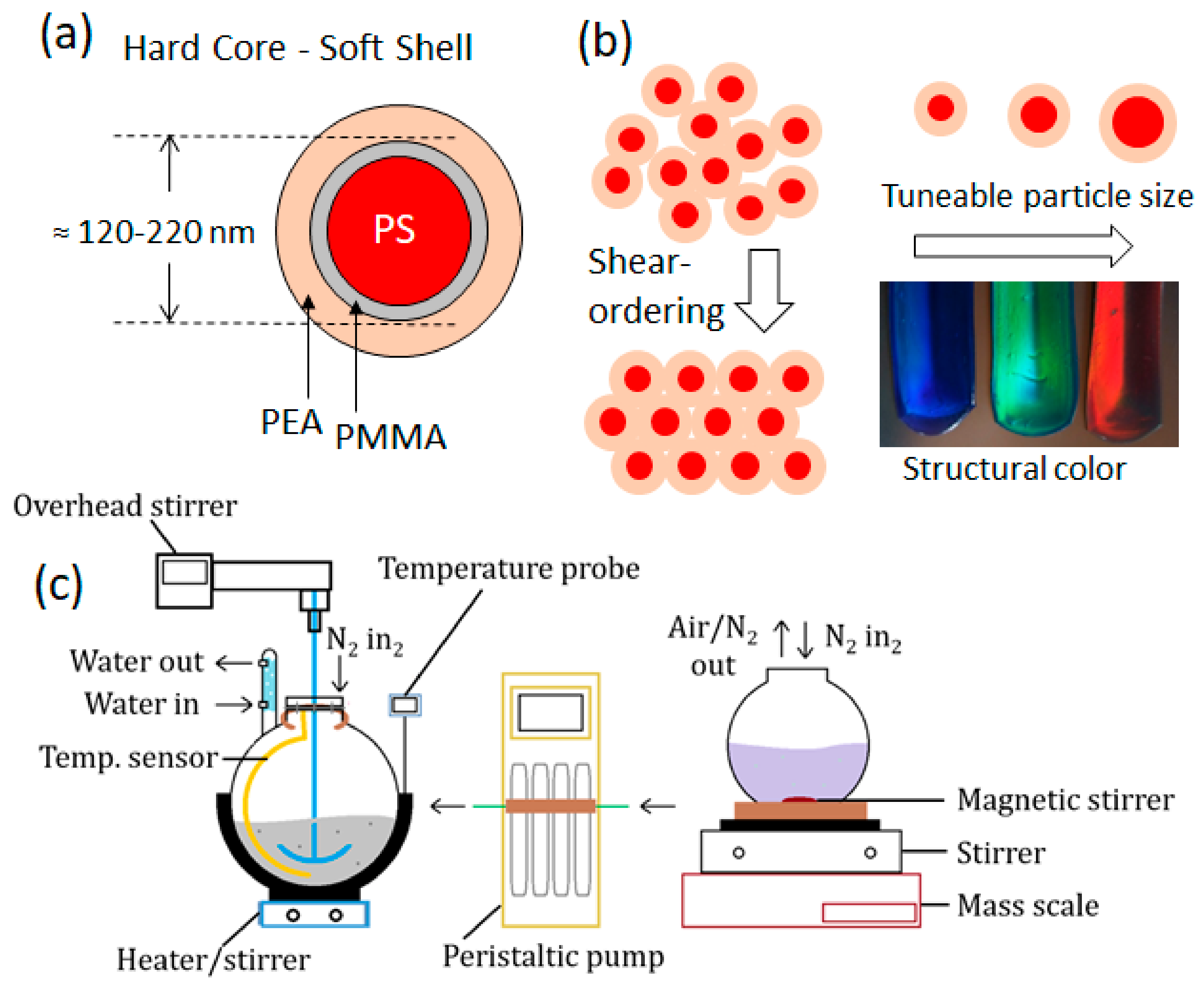
2.1. Synthesis Procedure
2.1.1. Washing
2.1.2. Polystyrene Cores
2.1.3. Interlayer, Shell Addition, and Particle Isolation
2.1.4. Control and Optimization of Reaction Parameters; Methodology
2.1.5. Synthesis Note
2.2. Particle Size Determination
2.2.1. Dynamic Light Scattering
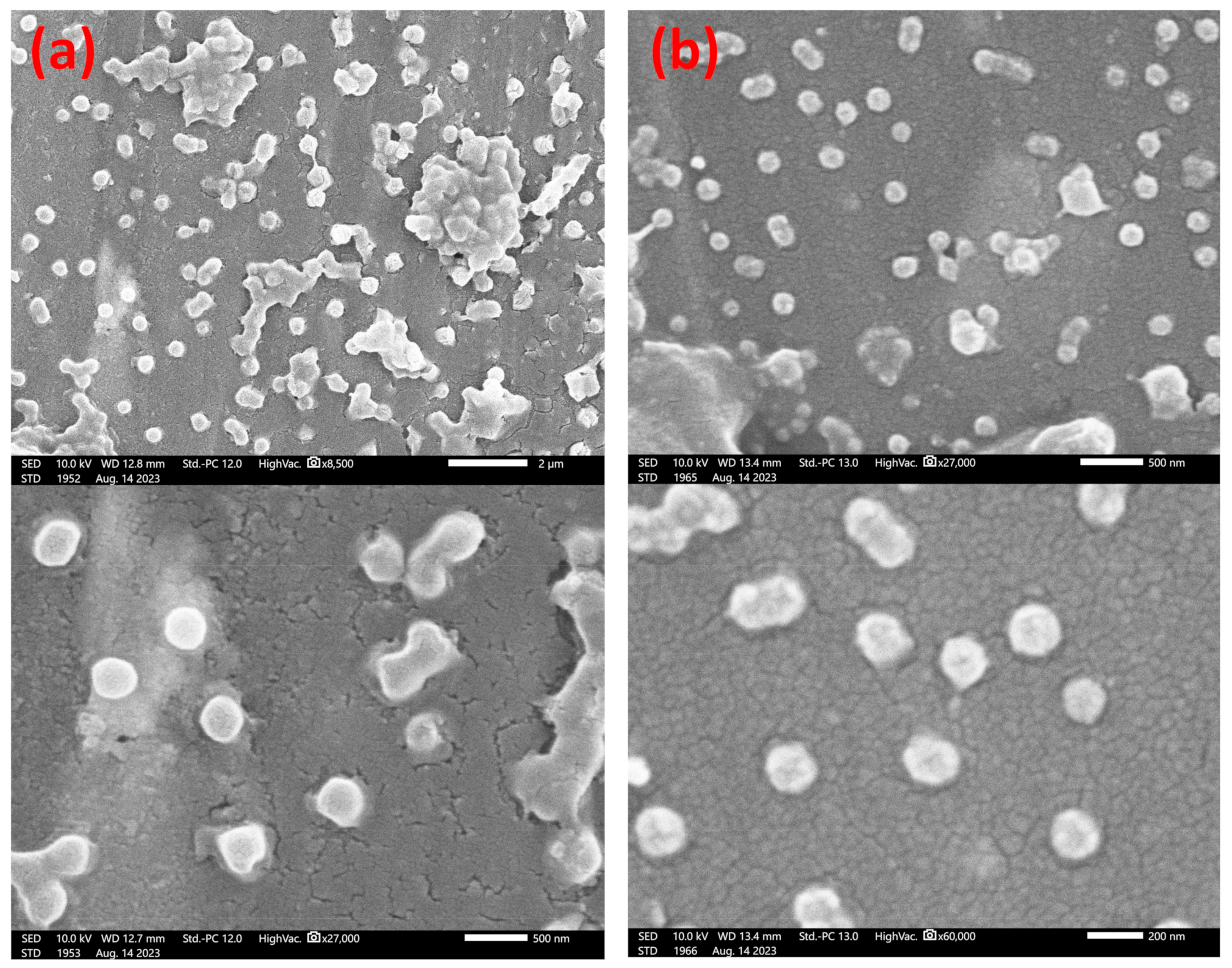
2.2.2. Electron Microscopy
3. Results and Discussion
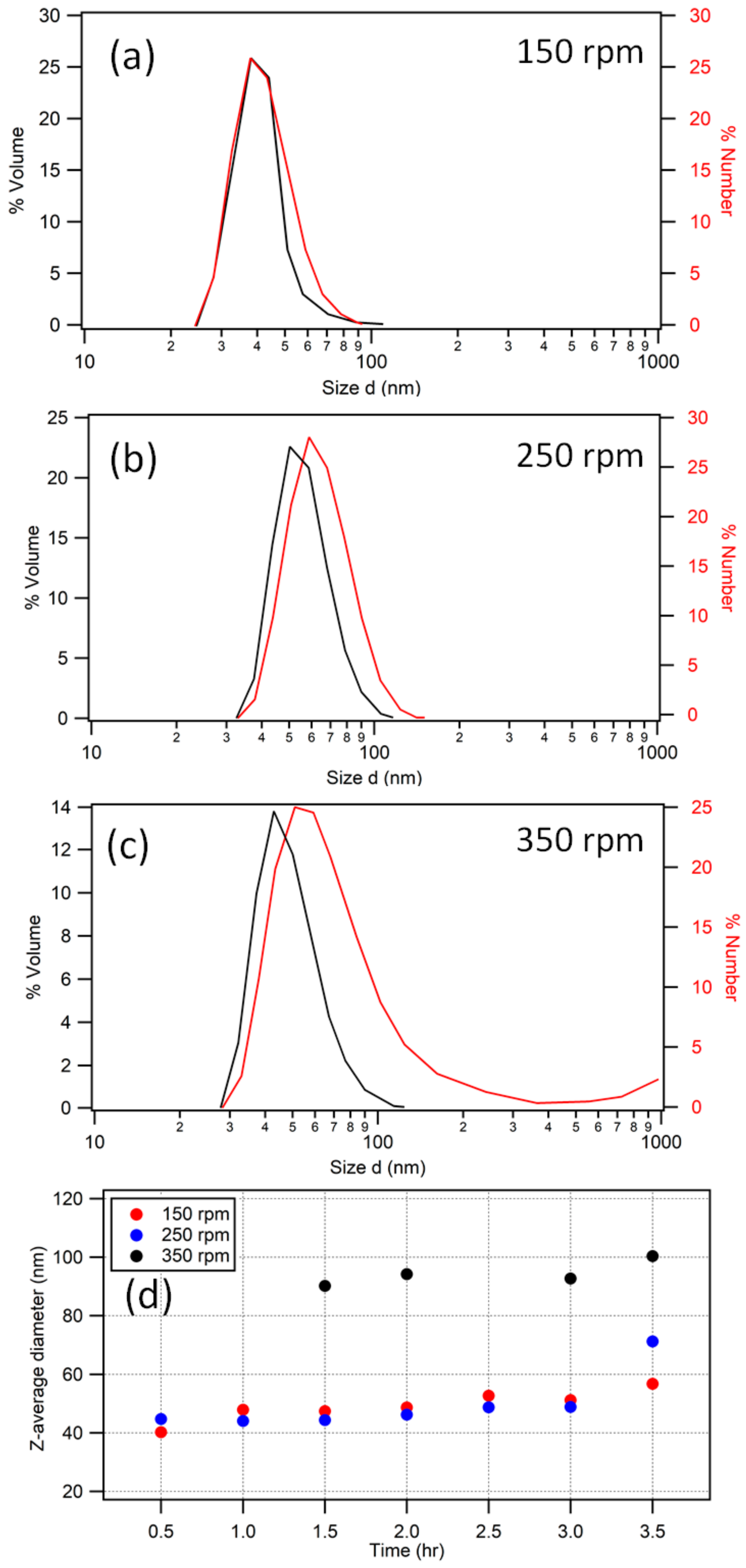
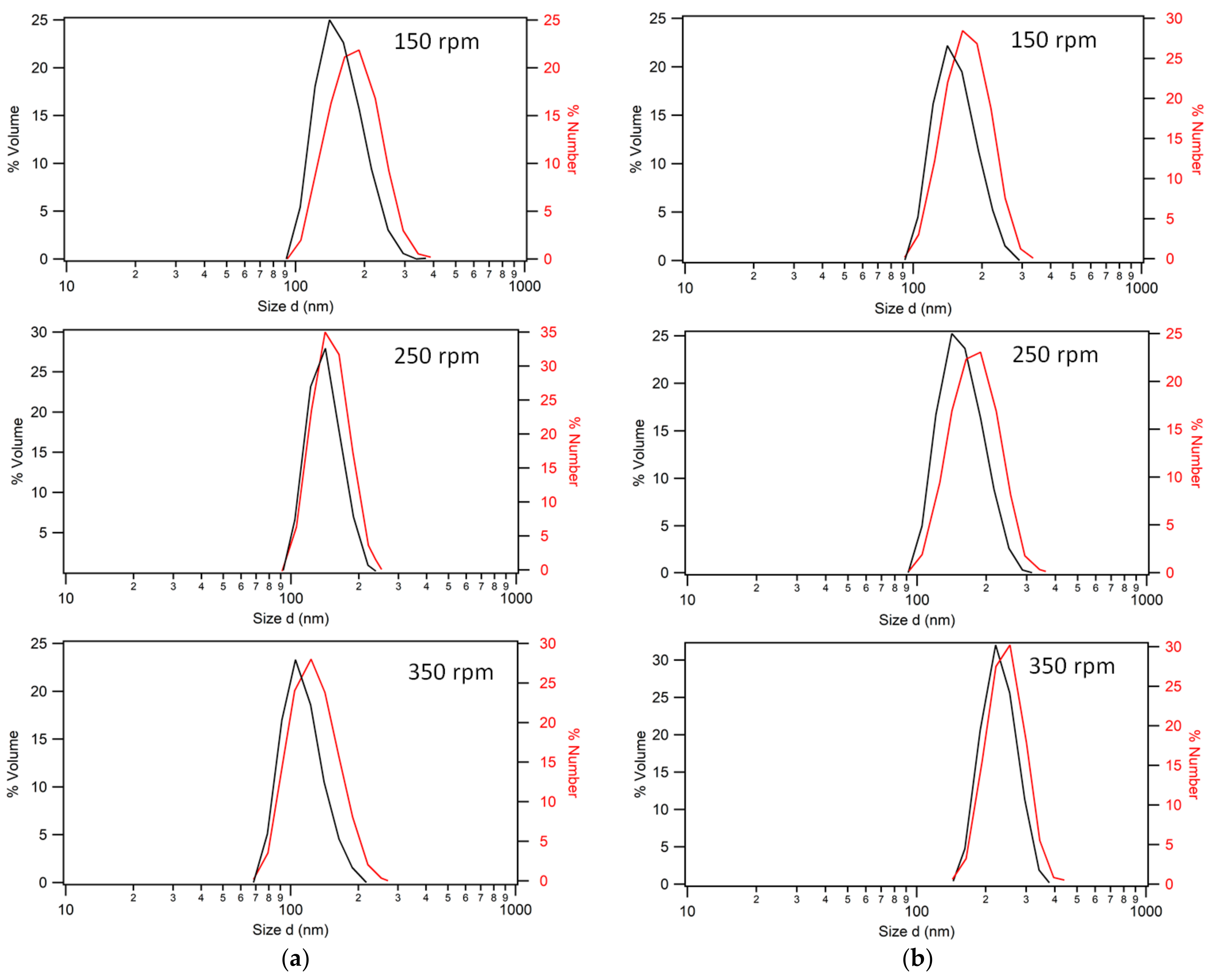
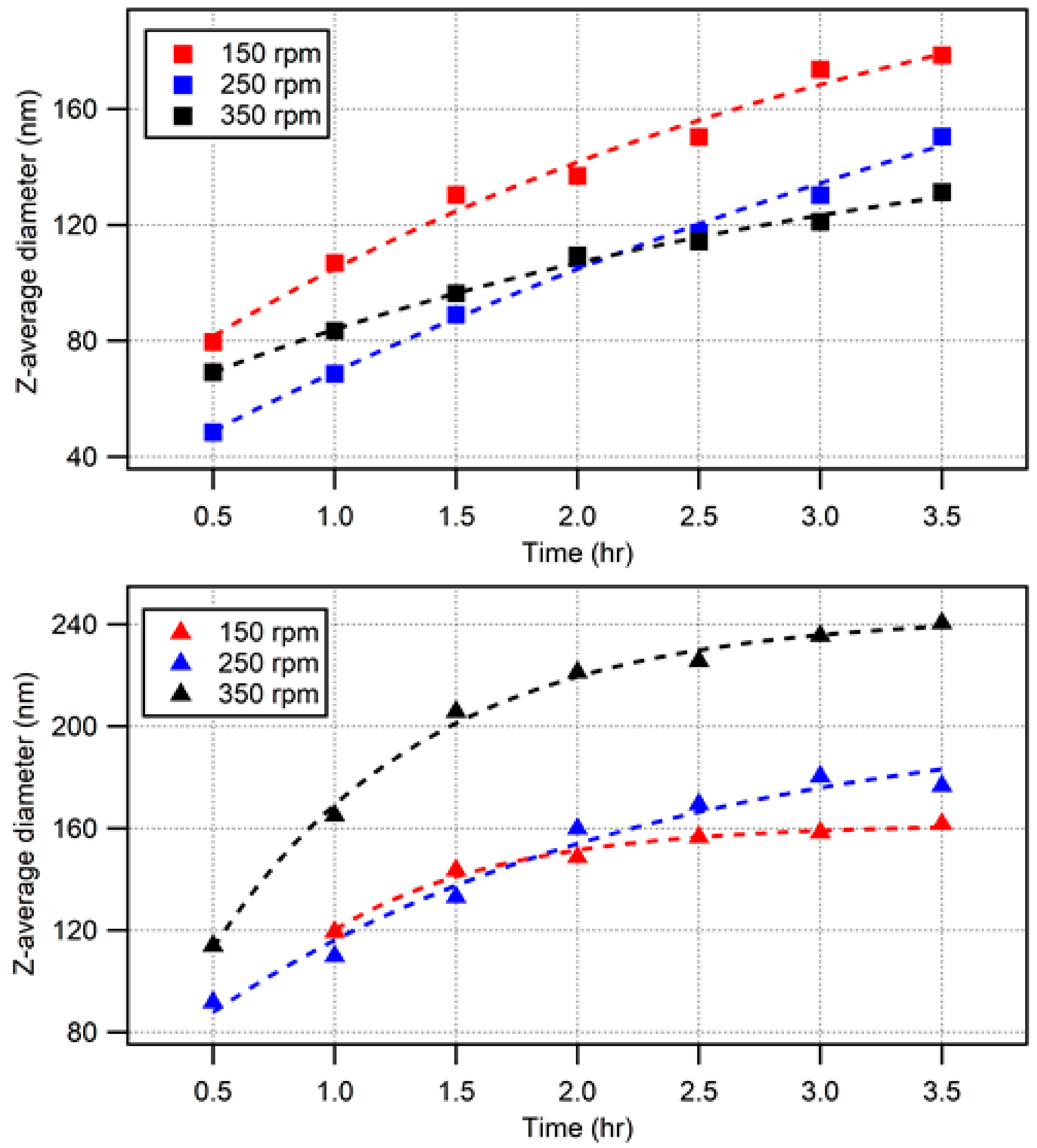
3.1. Low-Temperature Synthesis
Comparisons with the Literature
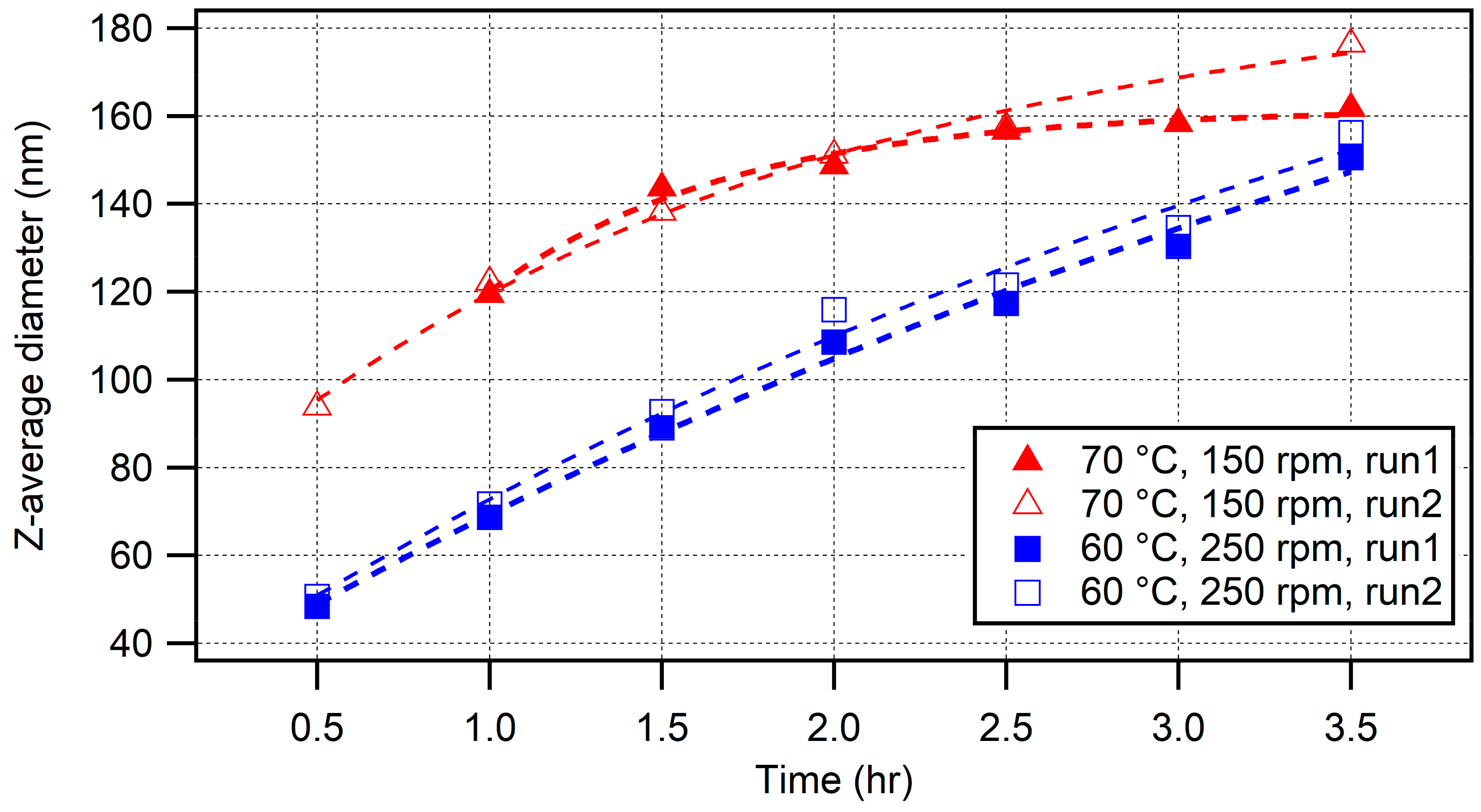
3.2. Higher-Temperature Synthesis
Comparisons with Lower-Temperature (50 °C) Syntheses
3.3. Reaction Dynamics and Deviation from Emulsion Polymerization Conditions
| Time (h) | 60 °C | 70 °C | ||||
|---|---|---|---|---|---|---|
| 150 rpm | 250 rpm | 350 rpm | 150 rpm | 250 rpm | 350 rpm | |
| 0.5 | 0.003 | 0.031 | 0.022 | 0.02 † | 0.010 | 0.018 |
| 1.0 | 0.016 | 0.010 | 0.011 | 0.017 | 0.008 | 0.017 |
| 1.5 | 0.015 | 0.015 | 0.020 | 0.015 | 0.019 | 0.041 |
| 2.0 | 0.013 | 0.009 | 0.019 | 0.019 | 0.017 | 0.041 |
| 2.5 | 0.037 | 0.018 | 0.008 | 0.009 | 0.010 | 0.029 |
| 3.0 | 0.024 | 0.016 | 0.020 | 0.021 | 0.033 | 0.017 |
| End | 0.013 | 0.010 | 0.012 | 0.019 | 0.026 | 0.029 |
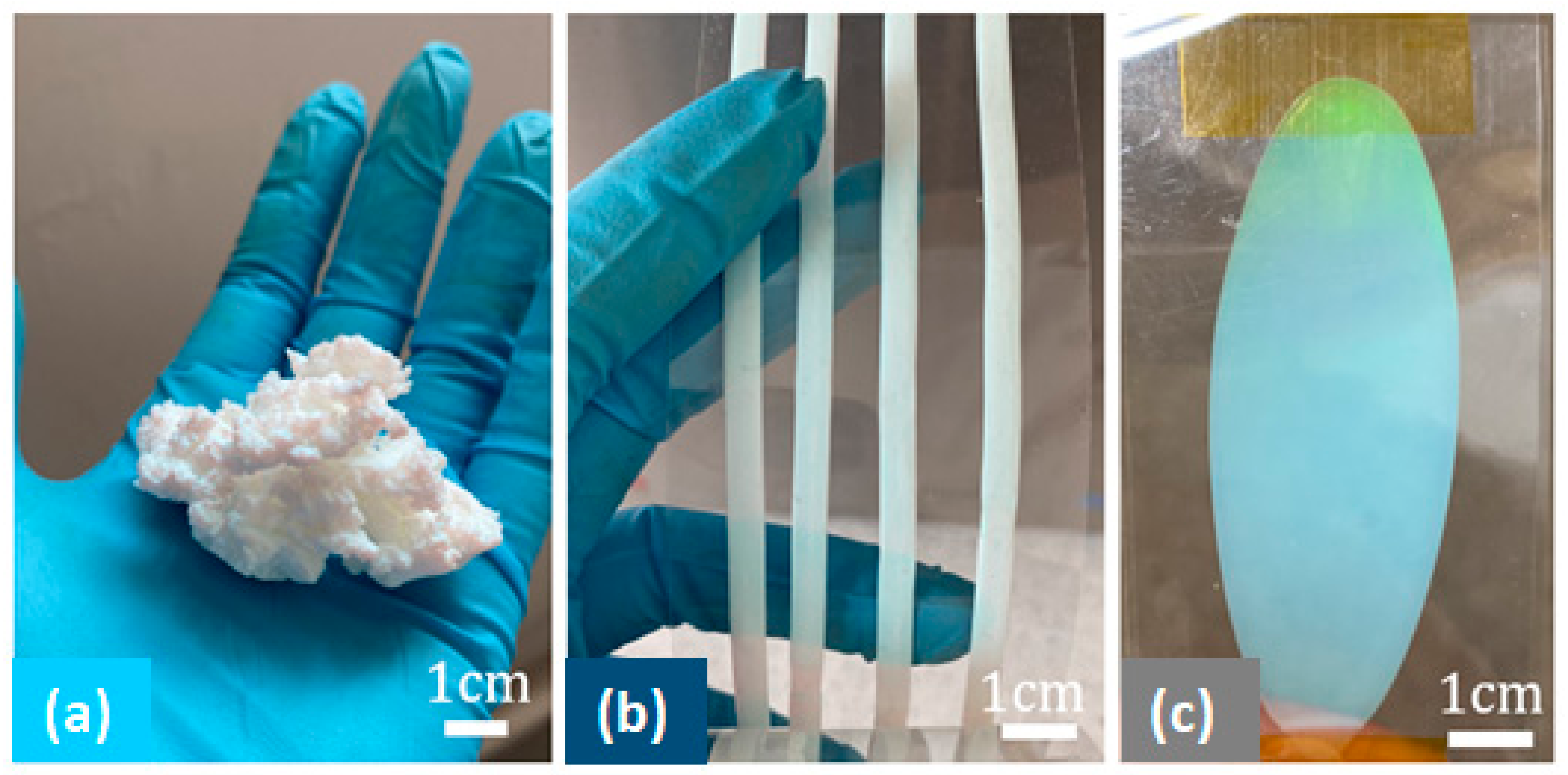
3.4. Final Particle Shape Integrity and Sizing
3.5. Toward Polymer Opal Synthesis as a ‘Dial-In’ Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Styrene Purification Method | C–I–S Particle PDI (±0.0005) |
|---|---|
| None | 0.074 |
| Water only | 0.054 |
| Caustic soda + MgSO4 | 0.033 |
| Time (h) | 150 rpm | 250 rpm | 350 rpm | |||
|---|---|---|---|---|---|---|
| 0.5 | 43.0 | 100% | 46.9 | 100% | 1674 | 67.1% |
| - | - | - | - | 61.5 | 30.1% | |
| - | - | - | - | 1804 | 2.8% | |
| 1.0 | 49.1 | 100% | 47.2 | 100% | 84.8 | 81.4% |
| - | - | 77.6 | 17.2% | |||
| - | - | 1795 | 1.4% | |||
| 1.5 | 49.8 | 100% | 47.3 | 100% | 101.1 | 86.8% |
| - | - | 3763 | 11.9% | |||
| - | - | 1795 | 1.3% | |||
| 2.0 | 47.7 | 100% | 49.1 | 99.6% | ||
| 1781 | 0.4% | |||||
| - | - | |||||
| 2.5 | 52.8 | 98.2% | 48.0 | 96.9% | ||
| 3485 | 1.8% | 5030 | 3.1% | |||
| 3.0 | 54.5 | 100% | ||||
| End | 56.9 | 100% | ||||
| Reaction Parameters | Unreacted Styrene Content (%) (±0.0005%) |
|---|---|
| 60 °C, 150 rpm | 0.501 |
| 60 °C, 250 rpm | 0.655 |
| 60 °C, 350 rpm | 0.104 |
| 70 °C, 150 rpm | 1.412 |
| 70 °C, 350 rpm | 0.481 |
References
- Wijnhoven, J.E.; Vos, W.L. Preparation of photonic crystals made of air spheres in titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S. Photonic crystals: Putting a new twist on light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- Lindley, D. Landmarks—The birth of photonic crystals. Physics 2013, 6, 94. [Google Scholar] [CrossRef]
- Finlayson, C.E.; Baumberg, J.J. Polymer opals as novel photonic materials. Polym. Int. 2013, 62, 1403–1407. [Google Scholar] [CrossRef]
- Spahn, P.; Finlayson, C.; Etah, W.M.; Snoswell, D.; Baumberg, J.; Hellmann, G. Modification of the refractive-index contrast in polymer opal films. J. Mater. Chem. 2011, 21, 8893–8897. [Google Scholar] [CrossRef]
- Ruhl, T.; Spahn, P.; Hellmann, G. Artificial opals prepared by melt compression. Polymer 2003, 44, 7625–7634. [Google Scholar] [CrossRef]
- Ruhl, T.; Hellmann, G.P. Colloidal crystals in latex films: Rubbery opals. Macromol. Chem. Phys. 2001, 202, 3502–3505. [Google Scholar] [CrossRef]
- Viel, B.; Ruhl, T.; Hellmann, G.P. Reversible deformation of opal elastomers. Chem. Mater. 2007, 19, 5673–5679. [Google Scholar] [CrossRef]
- Gallei, M. Functional Polymer Opals and Porous Materials by Shear-Induced Assembly of Tailor-Made Particles. Macromol. Rapid Commun. 2018, 39, 16. [Google Scholar] [CrossRef]
- Snoswell, D.; Kontogeorgos, A.; Baumberg, J.; Lord, T.; Mackley, M.; Spahn, P.; Hellmann, G. Shear ordering in polymer photonic crystals. Phys. Rev. E 2010, 81, 020401. [Google Scholar] [CrossRef]
- Pursiainen, O.; Baumberg, J.; Winkler, H.; Viel, B.; Spahn, P.; Ruhl, T. Shear-induced organization in flexible polymer opals. Adv. Mater. 2008, 20, 1484–1487. [Google Scholar] [CrossRef]
- Snoswell, D.R.E.; Finlayson, C.E.; Zhao, Q.; Baumberg, J.J. Real-time measurements of crystallization processes in viscoelastic polymeric photonic crystals. Phys. Rev. E 2015, 92, 052315. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, C.E.; Rosetta, G.; Baumberg, J.J. An Experimental and Theoretical Determination of Oscillatory Shear-Induced Crystallization Processes in Viscoelastic Photonic Crystal Media. Materials 2021, 14, 5298. [Google Scholar] [CrossRef] [PubMed]
- Pursiainen, O.; Baumberg, J.; Ryan, K.; Bauer, J.; Winkler, H.; Viel, B.; Ruhl, T. Compact strain-sensitive flexible photonic crystals for sensors. Appl. Phys. Lett. 2005, 87, 101902. [Google Scholar] [CrossRef]
- Astratov, V.N.; Adawi, A.M.; Fricker, S.; Skolnick, M.S.; Whittaker, D.M.; Pusey, P.N. Interplay of order and disorder in the optical properties of opal photonic crystals. Phys. Rev. B 2002, 66, 165215. [Google Scholar] [CrossRef]
- Rogach, O.; Kornowski, A.; Kapitonov, A.; Gaponenko, N.; Gaponenko, S.; Eychmüller, A.; Rogach, A. Self-organization of uniform silica globules into the three-dimensional superlattice of artificial opals. Mater. Sci. Eng. B 1999, 64, 64–67. [Google Scholar] [CrossRef]
- De La Rue, R. Photonic crystals: Microassembly in 3D. Nat. Mater. 2003, 2, 74–76. [Google Scholar] [CrossRef]
- Parchine, M.; McGrath, J.; Bardosova, M.; Pemble, M.E. Large area 2D and 3D colloidal photonic crystals fabricated by a roll-to-roll Langmuir–Blodgett method. Langmuir 2016, 32, 5862–5869. [Google Scholar] [CrossRef]
- Dou, B.; Whitaker, J.B.; Bruening, K.; Moore, D.T.; Wheeler, L.M.; Ryter, J.; Breslin, N.J.; Berry, J.J.; Garner, S.M.; Barnes, F.S.; et al. Roll-to-Roll Printing of Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2558–2565. [Google Scholar] [CrossRef]
- Krebs, F.; Gevorgyan, S.; Gholamkhass, B.; Holdcroft, S.; Schlenker, C.; Thompson, M.; Thompson, B.; Olson, D.; Ginley, D.; Shaheen, S.; et al. A round robin study of flexible large-area roll-to-roll processed polymer solar cell modules. Sol. Energy Mater. Sol. Cells 2009, 93, 1968–1977. [Google Scholar] [CrossRef]
- Li, H.T.; Wu, P.; Zhao, G.W.; Guo, J.; Wang, C.C. Fabrication of industrial-level polymer photonic crystal films at ambient temperature Based on uniform core/shell colloidal particles. J. Colloid Interface Sci. 2021, 584, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zakhidov, A.A.; Baughman, R.H.; Iqbal, Z.; Cui, C.; Khayrullin, I.; Dantas, S.O.; Marti, J.; Ralchenko, V.G. Carbon structures with three-dimensional periodicity at optical wavelengths. Science 1998, 282, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, C.E.; Goddard, C.; Papachristodoulou, E.; Snoswell, D.R.E.; Kontogeorgos, A.; Spahn, P.; Hellmann, G.P.; Hess, O.; Baumberg, J.J. Ordering in stretch-tunable polymeric opal fibers. Opt. Express 2011, 19, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Spahn, P.; Winkler, H.; Hellmann, G.P. Mesophases, Polymers, and Particles; Lagaly, G., Richtering, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 129. [Google Scholar]
- Ruhl, T.; Spahn, P.; Winkler, H.; Hellmann, G.P. Large area monodomain order in colloidal crystals. Macromol. Chem. Phys. 2004, 205, 1385–1393. [Google Scholar] [CrossRef]
- Finlayson, C.E.; Spahn, P.; Snoswell, D.R.E.; Yates, G.; Kontogeorgos, A.; Haines, A.I.; Hellmann, G.P.; Baumberg, J.J. 3D Bulk Ordering in Macroscopic Solid Opaline Films by Edge-Induced Rotational Shearing. Adv. Mater. 2011, 23, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Finlayson, C.; Snoswell, D.; Haines, A.; Schafer, C.; Spahn, P.; Hellmann, G.; Petukhov, A.; Herrmann, L.; Burdet, P.; et al. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 11661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Finlayson, C.; Schaefer, C.; Spahn, P.; Gallei, M.; Herrmann, L.; Petukhov, A.; Baumberg, J. Nanoassembly of Polydisperse Photonic Crystals Based on Binary and Ternary Polymer Opal Alloys. Adv. Opt. Mater. 2016, 4, 1494–1500. [Google Scholar] [CrossRef]
- Wong, H.S.; Mackley, M.; Butler, S.; Baumberg, J.; Snoswell, D.; Finlayson, C.E.; Zhao, Q.B. The rheology and processing of “edge sheared” colloidal polymer opals. J. Rheol. 2014, 58, 397–409. [Google Scholar] [CrossRef]
- Anselmann, R.; Winkler, H.; Hellmann, G.P.; Ruhl, T.; Vulpius, G.; Dörr, H. Moulded Bodies Consisting of Core-Shell Particles, WO2003025035A; E P Office (EU: Merck GmbH): Darmstadt, Germany, 2002. [Google Scholar]
- Rosetta, G. Towards the Realisation of Polymer Opals as Next Generation Functional Materials. Ph.D. Thesis, Aberystwyth University, Aberystwyth, UK, 2023. [Google Scholar]
- Baumberg, J.J.; Snoswell, D.R.E.; Finlayson, C.E.; Zhao, Q.; Hellmann, G.P.; Spahn, P.; Schafer, C.G. Manufacture of Composite Optical Materials, WO2012095634A2; W (PCT) Cambridge Enterprise Ltd.: Cambridge, UK; Deutsches Kunstsoff-Institut: Darmstadt, Germany, 2012. [Google Scholar]
- Hellmann, G.P.; Spahn, P. Composite Optical Materials Exhibiting Structural Colour, WO 2012/172084 A2; W (PCT) Deutsches Kunststoff-Institut: Darmstadt, Germany, 2012. [Google Scholar]
- Odian, G. Principles of Polymerization; Wiley Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]
- Smith, W.V.; Ewart, R.H. Kinetics of emulsion polymerization. J. Chem. Phys. 1948, 16, 592–599. [Google Scholar] [CrossRef]
- Moad, G.; Chiefari, J.; Chong, Y.K.; Krstina, J.; Mayadunne, R.T.A.; Postma, A.; Rizzardo, E.; Thang, S.H. Living free radical polymerization with reversible addition—Fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49, 993–1001. [Google Scholar] [CrossRef]
- Irfan, M.H. Chemistry and Technology of Thermosetting Polymers in Construction Applications; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Hancock, S.L.; Mahon, M.F.; Jones, M.D. Aluminium salalen complexes based on 1, 2-diaminocyclohexane and their exploitation for the polymerisation of rac-lactide. Dalton Trans. 2013, 42, 9279–9285. [Google Scholar] [CrossRef] [PubMed]
- Adumitrăchioaie, A.; Tertis, M.; Cernat, A.; Sandulescu, R.; Cristea, C. Electrochemical Methods Based on Molecularly Imprinted Polymers for Drug Detection. A Review. Int. J. Electrochem. Sci. 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Painter, P.C.; Coleman, M.M. Essentials of Polymer Science and Engineering; DEStech Publications, Incorporated: Lancaster, PA, USA, 2008. [Google Scholar]
- Chern, C.S. Principles and Applications of Emulsion Polymerization; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Anderson, C.D.; Daniels, E.S. Emulsion Polymerisation and Latex Applications; Rapra Technology Ltd.: Shropshire, UK, 2003; Volume 14. [Google Scholar]
- Nicholson, J. The Chemistry of Polymers; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Ottewill, R.H.; Shaw, J.N. Studies on the preparation and characterisation of monodisperse polystyrene latices. Kolloid-Z. Und Z. Für Polym. 1967, 218, 34–40. [Google Scholar] [CrossRef]
- Ottewill, R.H.; Shaw, J.N. Studies on the preparation and characterization of monodisperse polystyrene latices. Kolloid-Z. Und Z. Für Polym. 1967, 215, 161. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Zhu, M.; Guo, J.; Wang, C. Robust Large-Sized Photochromic Photonic Crystal Film for Smart Decoration and Anti-Counterfeiting. ACS Appl. Mater. Interfaces 2022, 14, 14618–14629. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.C.; Herrera-Ordonez, J.; Maldonado-Textle, H. Kinetics of the styrene emulsion polymerization above cmc. II. Agitation effect on molecular weight. Polym. Bull. 2005, 53, 333–337. [Google Scholar] [CrossRef]
- Capek, I.; Lin, S.-Y.; Hsu, T.-J.; Chern, C.-S. Effect of temperature on styrene emulsion polymerization in the presence of sodium dodecyl sulfate. II. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1477–1486. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chern, C.-S.; Hsu, T.-J.; Hsu, C.-T.; Capek, I. Emulsion polymerization of styrene: Double emulsion effect. Polymer 2001, 42, 1481–1491. [Google Scholar] [CrossRef]
- Nomura, M.; Harada, M.; Eguchi, W.; Nagata, S. Effect of stirring on the emulsion polymerization of styrene. J. Appl. Polym. Sci. 1972, 16, 835–847. [Google Scholar] [CrossRef]
- Roudsari, S.F.; Dhib, R.; Ein-Mozaffari, F. Mixing effect on emulsion polymerization in a batch reactor. Polym. Eng. Sci. 2015, 55, 945–956. [Google Scholar] [CrossRef]
- Kiparissides, C.; MacGregor, J.; Hamielec, A. Continuous emulsion polymerization of vinyl acetate. Part I: Experimental studies. Can. J. Chem. Eng. 1980, 58, 48–55. [Google Scholar] [CrossRef]
- Sun, F.; Ruckenstein, E. Preparation of high molecular weight monodisperse polystyrene latexes by concentrated emulsion polymerization. J. Appl. Polym. Sci. 1993, 48, 1279–1288. [Google Scholar] [CrossRef]
- Lane, W.H. Determination of Solubility of Styrene in Water and of Water in Styrene. Ind. Eng. Chem. Anal. Ed. 1946, 18, 295–296. [Google Scholar] [CrossRef]
- Roudsari, S.F.; Turcotte, G.; Dhib, R.; Ein-Mozaffari, F. CFD modeling of the mixing of water in oil emulsions. Comput. Chem. Eng. 2012, 45, 124–136. [Google Scholar] [CrossRef]
- Song, Z.; Poehlein, G.W. Kinetics of emulsifier-free emulsion polymerization of styrene. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 2359–2392. [Google Scholar] [CrossRef]
- Tanrisever, T.; Okay, O.; Sönmezoğlu, I.Ç. Kinetics of emulsifier–free emulsion polymerization of methyl methacrylate. J. Appl. Polym. Sci. 1996, 61, 485–493. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Tan, Z.; Zhang, H. Effect of temperature on emulsion polymerization of n-butyl acrylate. Procedia Eng. 2011, 18, 353–357. [Google Scholar] [CrossRef]
- Chern, C.-S.; Lin, S.-Y.; Hsu, T.J. Effects of temperature on styrene emulsion polymerization kinetics. Polym. J. 1999, 31, 516–523. [Google Scholar] [CrossRef]
- Shen, S.; Sudol, E.; El-Aasser, M. Control of particle size in dispersion polymerization of methyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1393–1402. [Google Scholar] [CrossRef]
- Lok, K.P.; Ober, C.K. Particle size control in dispersion polymerization of polystyrene. 100 Years CSC Pages CJC 2017, 1, 209–216. [Google Scholar] [CrossRef]
- Barrett, K.E. Dispersion polymerisation in organic media. Br. Polym. J. 1973, 5, 259–271. [Google Scholar] [CrossRef]
- Morgen, T.O.; Krumova, M.; Luttikhedde, H.; Mecking, S. Free-Radical Dispersion Polymerization of Ethylene with Laponite to Polyethylene–Clay Nanocomposite Particles. Macromolecules 2018, 51, 4118–4128. [Google Scholar] [CrossRef]
- Li, K.; Stöver, H.D. Highly crosslinked micron-range polymer microspheres by dispersion polymerization of divinylbenzene. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2473–2479. [Google Scholar] [CrossRef]
- Rosetta, G.; An, T.; Zhao, Q.B.; Baumberg, J.J.; Tomes, J.J.; Gunn, M.D.; Finlayson, C.E. Chromaticity of structural color in polymer thin film photonic crystals. Opt. Express 2020, 28, 36219–36228. [Google Scholar] [CrossRef] [PubMed]
- Rosetta, G.; Gunn, M.; Tomes, J.J.; Butters, M.; Pieschel, J.; Hartmann, F.; Gallei, M.; Finlayson, C.E. Transparent Polymer Opal Thin Films with Intense UV Structural Color. Molecules 2022, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Snoswell, D.R.E.; Baumberg, J.J. Stretching the Imagination. Textiles 2009, 4, 8–10. [Google Scholar]
- Rosetta, G.; Butters, M.; Tomes, J.J.; Little, J.; Gunn, M.D.; Finlayson, C.E. Quantifying the saturation of structural color from thin film polymeric photonic crystals. In Proceedings of the Conference on Photonic and Phononic Properties of Engineered Nanostructures X, San Francisco, CA, USA, 3–6 February 2020; Spie-Int Soc Optical Engineering: San Francisco, CA, USA, 2020. [Google Scholar]

| Time (h) | 150 rpm | 250 rpm | 350 rpm |
|---|---|---|---|
| 0.5 | 0.047 | 0.025 | 1.000 |
| 1.0 | 0.122 | 0.139 | 0.688 |
| 1.5 | 0.032 | 0.147 | 0.380 |
| 2.0 | 0.131 | 0.149 | 0.348 |
| 2.5 | 0.206 | 0.215 | 0.3 † |
| 3.0 | 0.103 | 0.194 | 0.228 |
| End | 0.111 | 0.017 | 0.238 |
| Structural Colour, Core Size (nm) | 60 °C | 70 °C | ||||
|---|---|---|---|---|---|---|
| 150 rpm | 250 rpm | 350 rpm | 150 rpm | 250 rpm | 350 rpm | |
| UV, 113 | 1.1 | 2.1 | 2.1 | 0.8 * | 0.8 | - |
| Violet, 126 | 1.5 | 2.7 | 3.4 | 1.1 | 1.2 | 0.6 |
| Blue, 151 | 2.3 | 3.5 | - | 1.9 | 1.8 | 0.8 |
| Blue-green, 167 | 3.1 | - | - | - | 2.7 | 1.0 |
| Green, 175 | 3.4 | - | - | - | 3.1 | 1.1 |
| Yellow, 188 | - | - | - | - | - | 1.3 |
| Orange, 199 | - | - | - | - | - | 1.5 |
| Red, 207 | - | - | - | - | - | 1.7 |
| Infrared, 231 | - | - | - | - | - | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosetta, G.; Macaire, L.; Butters, M.; Finlayson, C.E. Dial-In Synthesis of ‘Polymer Opal’ Core–Interlayer–Shell Composite Nanoparticles. Polymers 2023, 15, 3507. https://doi.org/10.3390/polym15173507
Rosetta G, Macaire L, Butters M, Finlayson CE. Dial-In Synthesis of ‘Polymer Opal’ Core–Interlayer–Shell Composite Nanoparticles. Polymers. 2023; 15(17):3507. https://doi.org/10.3390/polym15173507
Chicago/Turabian StyleRosetta, Giselle, Line Macaire, Mike Butters, and Chris E. Finlayson. 2023. "Dial-In Synthesis of ‘Polymer Opal’ Core–Interlayer–Shell Composite Nanoparticles" Polymers 15, no. 17: 3507. https://doi.org/10.3390/polym15173507
APA StyleRosetta, G., Macaire, L., Butters, M., & Finlayson, C. E. (2023). Dial-In Synthesis of ‘Polymer Opal’ Core–Interlayer–Shell Composite Nanoparticles. Polymers, 15(17), 3507. https://doi.org/10.3390/polym15173507








