Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors
Abstract
1. Introduction
2. Materials and Methods
3. Results
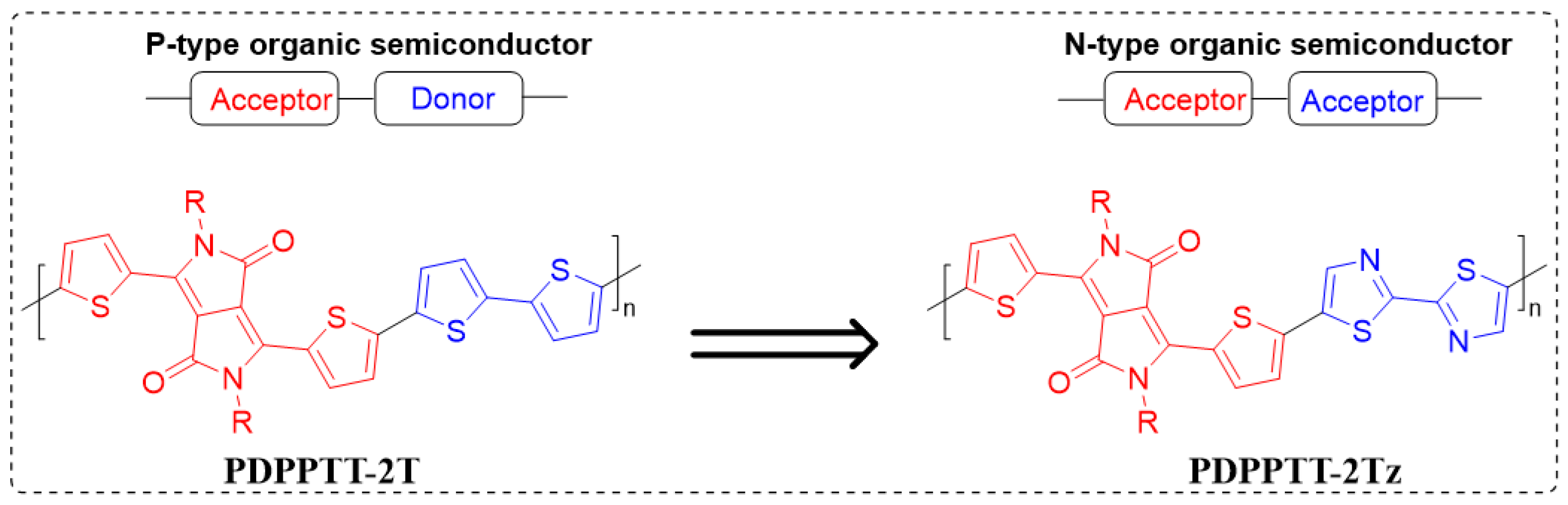
3.1. Synthesis
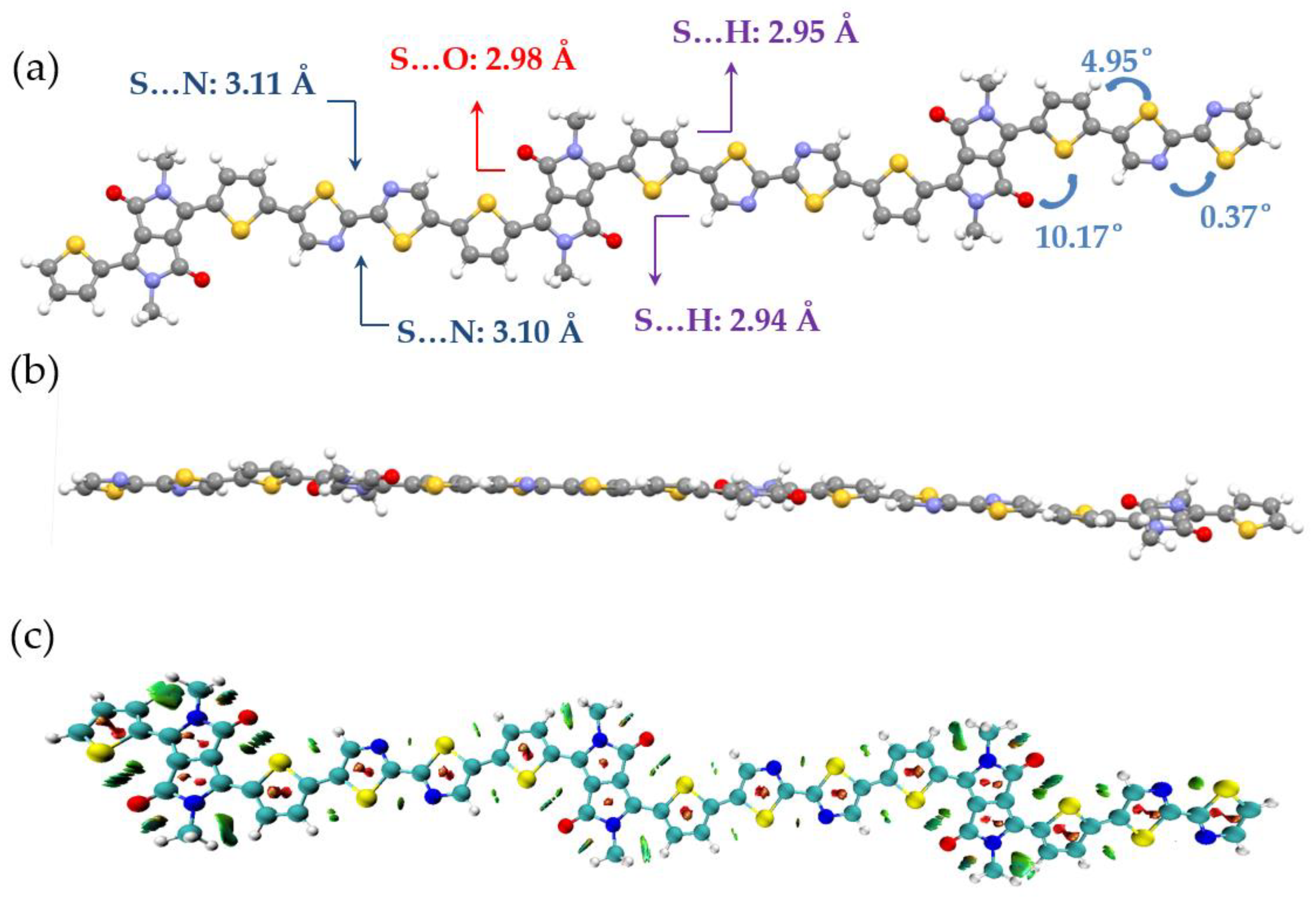
3.2. Density Functional Theory Calculations
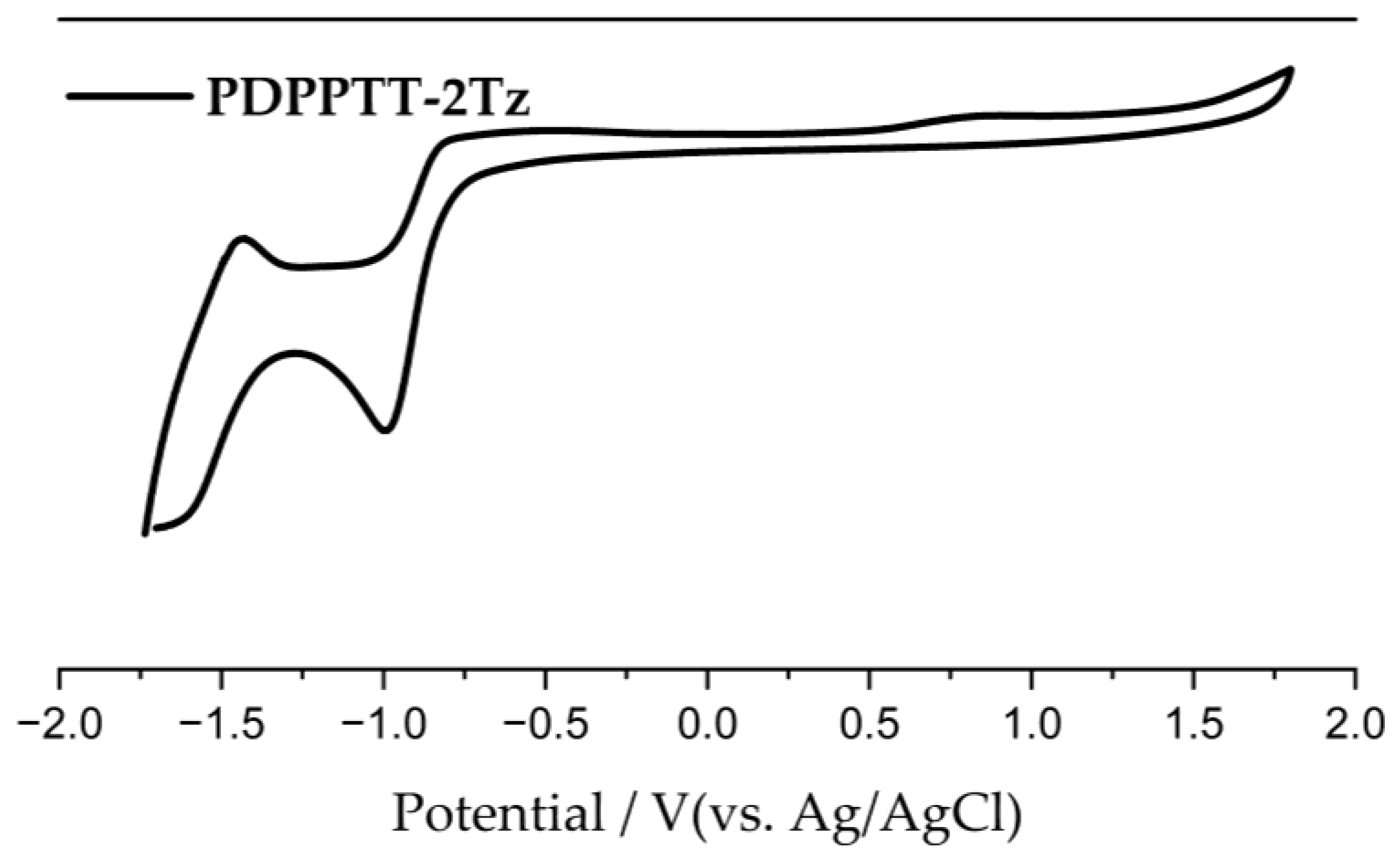
3.3. Photophysical Properties and Electrochemical Properties
3.4. OFET Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331–1381. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type organic semiconductors in organic electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bauerle, P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. Engl. 2012, 51, 2020–2067. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.; Wang, L.; Yu, G. Recent Research Progress of Organic Small-Molecule Semiconductors with High Electron Mobilities. Adv. Mater. 2023, 35, e2210772. [Google Scholar] [CrossRef]
- Lee, W.; Park, Y. Organic Semiconductor/Insulator Polymer Blends for High-Performance Organic Transistors. Polymers 2014, 6, 1057–1073. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Liu, Y. High-Mobility Organic Light-Emitting Semiconductors and Its Optoelectronic Devices. Small Struct. 2020, 2, 2000083. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Ren, S.; Yassar, A. Recent Research Progress in Indophenine-Based-Functional Materials: Design, Synthesis, and Optoelectronic Applications. Materials 2023, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhu, G.; Chen, J.; Zhang, C.; Zhu, X.; Liao, H.; Li, Z.; Hu, H.; McCulloch, I.; Nielsen, C.B.; et al. Highly Efficient Mixed Conduction in a Fused Oligomer n-Type Organic Semiconductor Enabled by 3D Transport Pathways. Adv. Mater. 2023, 35, e2300252. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Wang, Y.; Yassar, A. Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials. Electronics 2023, 12, 3313. [Google Scholar] [CrossRef]
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in flexible organic field-effect transistors and their applications for flexible electronics. NPJ Flex. Electron. 2022, 6, 1. [Google Scholar] [CrossRef]
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C Mater. 2021, 9, 8099–8128. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Wang, S.; Liu, Y. Design of High-Mobility Diketopyrrolopyrrole-Based pi-Conjugated Copolymers for Organic Thin-Film Transistors. Adv. Mater. 2015, 27, 3589–3606. [Google Scholar] [CrossRef]
- Sun, Y.; Di, C.A.; Xu, W.; Zhu, D. Advances in n-Type Organic Thermoelectric Materials and Devices. Adv. Electron. Mater. 2019, 5, 1800825. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, W.; Yu, G. Semiconducting Polymers Based on Isoindigo and Its Derivatives: Synthetic Tactics, Structural Modifications, and Applications. Adv. Funct. Mater. 2021, 31, 2010979. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, e1903882. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dyes Pigments 2023, 213, 111136. [Google Scholar] [CrossRef]
- Ye, T.; Jin, S.; Singh, R.; Kumar, M.; Chen, W.; Wang, D.; Zhang, X.; Li, W.; He, D. Effects of solvent additives on the morphology and transport property of a perylene diimide dimer film in perovskite solar cells for improved performance. Sol. Energy 2020, 201, 927–934. [Google Scholar] [CrossRef]
- Lei, T.; Wang, J.Y.; Pei, J. Design, synthesis, and structure-property relationships of isoindigo-based conjugated polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, C.H.; Tsai, B.S.; Hsueh, T.F.; Tsao, C.S.; Tan, S.; Chang, B.; Chang, Y.N.; Chu, T.Y.; Tsai, C.E.; et al. Alkoxy- and Alkyl-Side-Chain-Functionalized Terpolymer Acceptors for All-Polymer Photovoltaics Delivering High Open-Circuit Voltages and Efficiencies. Adv. Funct. Mater. 2023, 33, 2215095. [Google Scholar] [CrossRef]
- Wang, J.; Feng, K.; Jeong, S.Y.; Liu, B.; Wang, Y.; Wu, W.; Hou, Y.; Woo, H.Y.; Guo, X. Acceptor-acceptor type polymers based on cyano-substituted benzochalcogenadiazole and diketopyrrolopyrrole for high-efficiency n-type organic thermoelectrics. Polym. J. 2022, 55, 507–515. [Google Scholar] [CrossRef]
- Mueller, C.J.; Singh, C.R.; Fried, M.; Huettner, S.; Thelakkat, M. High Bulk Electron Mobility Diketopyrrolopyrrole Copolymers with Perfluorothiophene. Adv. Funct. Mater. 2015, 25, 2725–2736. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Chen, J.; Huang, J.; Jiang, Y.; Zhang, J.; Shi, L.; Sun, Y.; Wei, Z.; Yu, G.; et al. Bis-Diketopyrrolopyrrole Moiety as a Promising Building Block to Enable Balanced Ambipolar Polymers for Flexible Transistors. Adv. Mater. 2017, 29, 1606162–1606169. [Google Scholar] [CrossRef] [PubMed]
- Su, H.L.; Sredojevic, D.N.; Bronstein, H.; Marks, T.J.; Schroeder, B.C.; Al-Hashimi, M. Bithiazole: An Intriguing Electron-Deficient Building for Plastic Electronic Applications. Macromol. Rapid Commun. 2017, 38, 1600610–1600634. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Wu, Y.; Li, C.; Ji, Y.; Li, L.; Hu, W.; Wang, Z.; Ma, W.; Li, W. Effect of Fluorination on Molecular Orientation of Conjugated Polymers in High Performance Field-Effect Transistors. Macromolecules 2016, 49, 6431–6438. [Google Scholar] [CrossRef]
- Sun, B.; Hong, W.; Aziz, H.; Abukhdeir, N.M.; Li, Y. Dramatically enhanced molecular ordering and charge transport of a DPP-based polymer assisted by oligomers through antiplasticization. J. Mater. Chem. C 2013, 1, 4423–4426. [Google Scholar] [CrossRef]
- Di Pietro, R.; Erdmann, T.; Carpenter, J.H.; Wang, N.; Shivhare, R.R.; Formanek, P.; Heintze, C.; Voit, B.; Neher, D.; Ade, H.; et al. Synthesis of High-Crystallinity DPP Polymers with Balanced Electron and Hole Mobility. Chem. Mater. 2017, 29, 10220–10232. [Google Scholar] [CrossRef]
- Peña-Alcántara, A.; Nikzad, S.; Michalek, L.; Prine, N.; Wang, Y.; Gong, H.; Ponte, E.; Schneider, S.; Wu, Y.; Root, S.E.; et al. Effect of Molecular Weight on the Morphology of a Polymer Semiconductor–Thermoplastic Elastomer Blend. Adv. Electron. Mater. 2023, 2201055–2201068. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.-R.; Zhou, J.; Najari, A.; Leclerc, M.; Tao, Y. Effects of the Molecular Weight and the Side-Chain Length on the Photovoltaic Performance of Dithienosilole/Thienopyrrolodione Copolymers. Adv. Funct. Mater. 2012, 22, 2345–2351. [Google Scholar] [CrossRef]
- Zen, A.; Pflaum, J.; Hirschmann, S.; Zhuang, W.; Jaiser, F.; Asawapirom, U.; Rabe, J.P.; Scherf, U.; Neher, D. Effect of Molecular Weight and Annealing of Poly(3-hexylthiophene)s on the Performance of Organic Field-Effect Transistors. Adv. Funct. Mater. 2004, 14, 757–764. [Google Scholar] [CrossRef]
- Tripathi, A.S.M.; Sadakata, S.; Gupta, R.K.; Nagamatsu, S.; Ando, Y.; Pandey, S.S. Implication of Molecular Weight on Optical and Charge Transport Anisotropy in PQT-C12 Films Fabricated by Dynamic FTM. ACS Appl. Mater. Interfaces 2019, 11, 28088–28095. [Google Scholar] [CrossRef]
- Kanimozhi, C.; Yaacobi-Gross, N.; Chou, K.W.; Amassian, A.; Anthopoulos, T.D.; Patil, S. Diketopyrrolopyrrole-diketopyrrolopyrrole-based conjugated copolymer for high-mobility organic field-effect transistors. J. Am. Chem. Soc. 2012, 134, 16532–16535. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, K.; Lai, J.; Zhou, Y.; Wei, X.; Che, Q.; Wei, J.; Wang, L.; Yu, G. Record-High Electron Mobility Exceeding 16 cm(2) V(-) (1) s(-) (1) in Bisisoindigo-Based Polymer Semiconductor with a Fully Locked Conjugated Backbone. Adv. Mater. 2023, 35, e2300145. [Google Scholar] [CrossRef]
- Goerigk, L. A Comprehensive Overview of the DFT-D3 London-Dispersion Correction. In Non-Covalent Interactions in Quantum Chemistry and Physics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–219. [Google Scholar]
- Tunega, D.; Bucko, T.; Zaoui, A. Assessment of ten DFT methods in predicting structures of sheet silicates: Importance of dispersion corrections. J. Chem. Phys. 2012, 137, 114105. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent advances in the development of semiconducting DPP-containing polymers for transistor applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Saragi, T.P.I.; Fuhrmann-Lieker, T.; Salbeck, J. High ON/OFF ratio and stability of amorphous organic field-effect transistors based on spiro-linked compounds. Synth. Metals 2005, 148, 267–270. [Google Scholar] [CrossRef]
- Dheepika, R.; Mohamed Imran, P.; Bhuvanesh, N.S.P.; Nagarajan, S. Solution-Processable Unsymmetrical Triarylamines: Towards High Mobility and ON/OFF Ratio in Bottom-Gated OFETs. Chemistry 2019, 25, 15155–15163. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Duan, J.; Peng, P.; Zhang, J.; Zhou, M. Progress in the synthesis of imide-based N-type polymer semiconductor materials. RSC Adv. 2020, 10, 41764–41779. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; An, T.K.; Kim, Y.-H. Diketopyrrolopyrrole (DPP)-Based Polymers and Their Organic Field-Effect Transistor Applications: A Review. Macromol. Res. 2022, 30, 71–84. [Google Scholar] [CrossRef]




| HOMO (eV) | LUMO (eV) | ΔEg (eV) | |
|---|---|---|---|
| PDPPTT-2Tz | −5.16 | −3.79 | 1.81 |
| λmax solution (nm) | λmax film (nm) | Eg opt (eV) 1 | Ered (eV) | HOMO (eV) 2 | LUMO (eV) 3 | Eg cv (eV) 4 | |
|---|---|---|---|---|---|---|---|
| PDPPTT-2Tz | 765 | 775 | 1.31 | −0.99 | −5.08 | −3.72 | 1.36 |
| Coating Speed (mm/s) | Annealing (°C) | Mobilities 1 (cm2/(V s)) | Max Mobilities (cm2/(V s) | Threshold Voltages (V) | ION/OFF | |
|---|---|---|---|---|---|---|
| PDPPTT-2Tz | 2000 | 220 | 0.81 | 1.29 | 72 | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Zhang, W.; Wang, Z.; Yassar, A.; Liao, Z.; Yi, Z. Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors. Polymers 2023, 15, 3392. https://doi.org/10.3390/polym15163392
Ren S, Zhang W, Wang Z, Yassar A, Liao Z, Yi Z. Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors. Polymers. 2023; 15(16):3392. https://doi.org/10.3390/polym15163392
Chicago/Turabian StyleRen, Shiwei, Wenqing Zhang, Zhuoer Wang, Abderrahim Yassar, Zhiting Liao, and Zhengran Yi. 2023. "Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors" Polymers 15, no. 16: 3392. https://doi.org/10.3390/polym15163392
APA StyleRen, S., Zhang, W., Wang, Z., Yassar, A., Liao, Z., & Yi, Z. (2023). Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors. Polymers, 15(16), 3392. https://doi.org/10.3390/polym15163392








