Excellent Characteristics of Environmentally Friendly 3D-Printed Nasopharyngeal Swabs for Medical Sample Collection
Abstract
1. Introduction
2. Materials and Methods
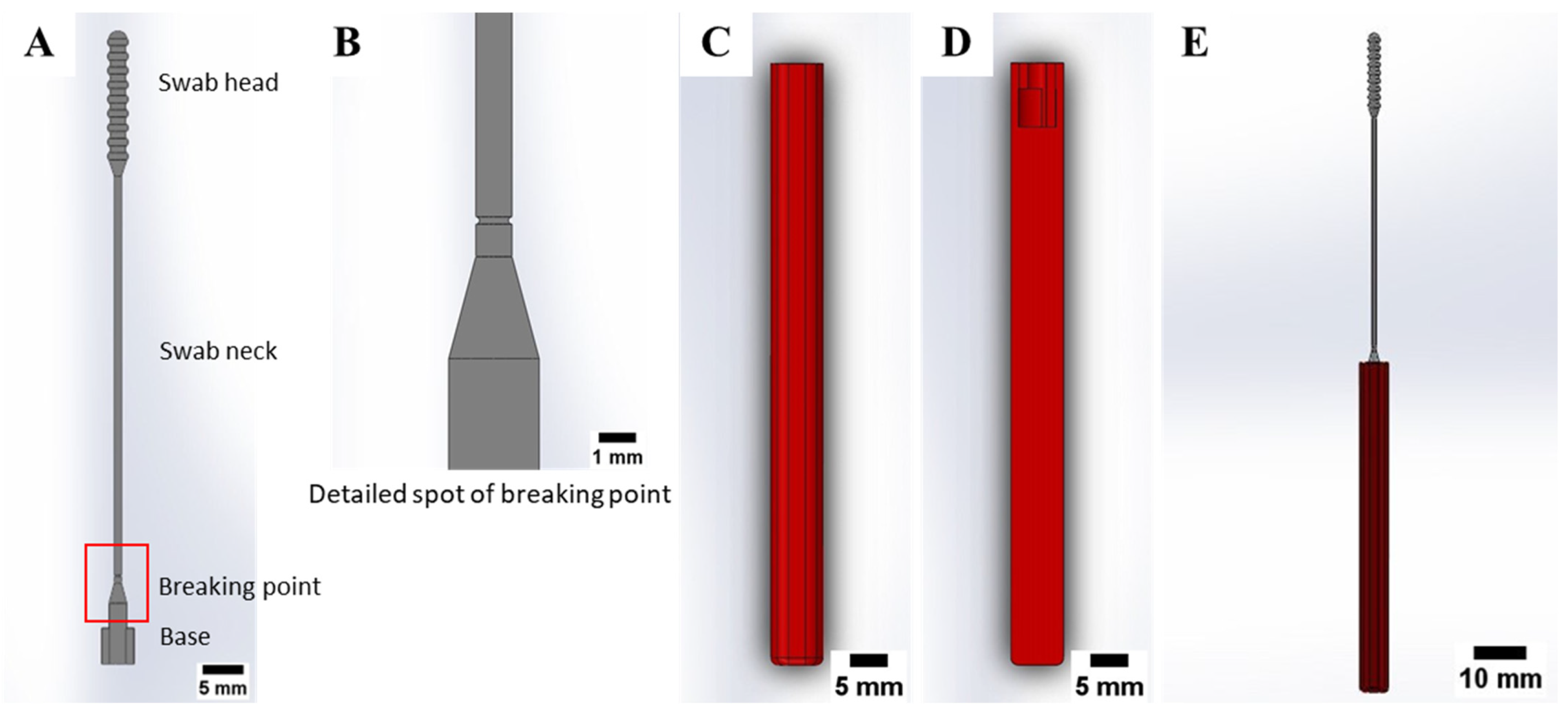
2.1. Nasopharyngeal Swab Design
2.2. Materials
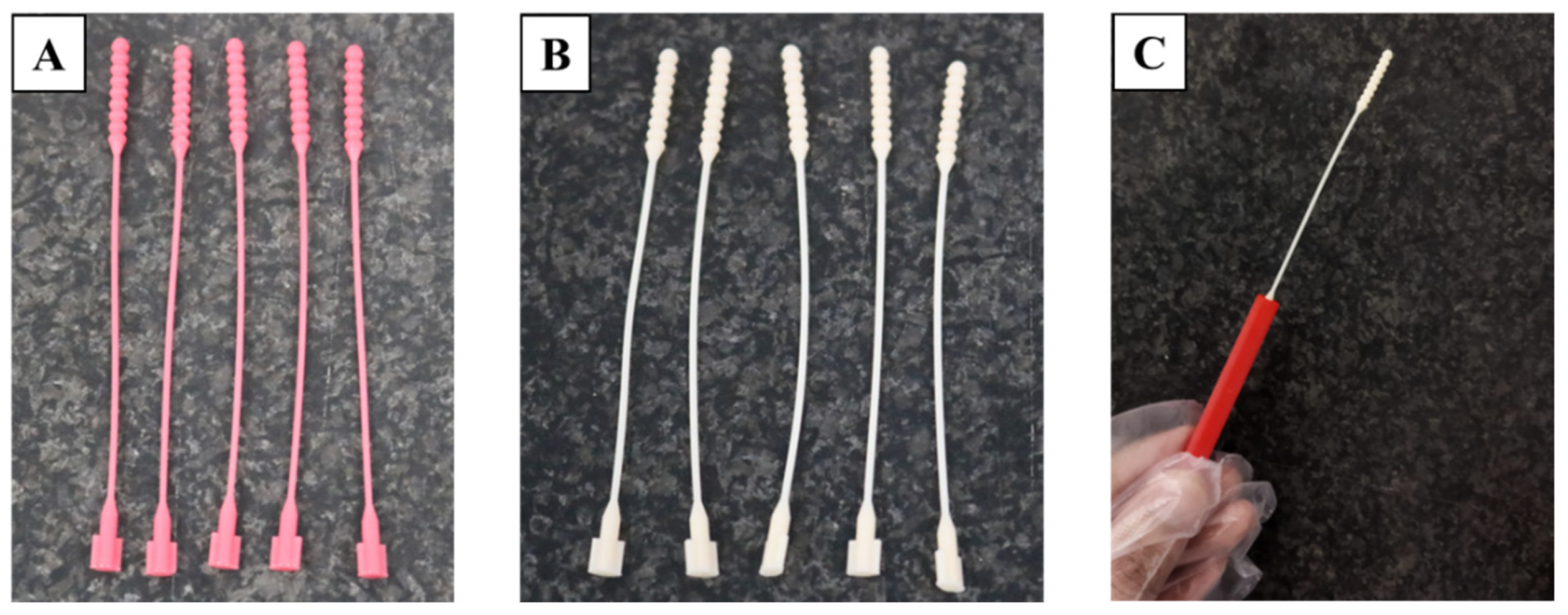
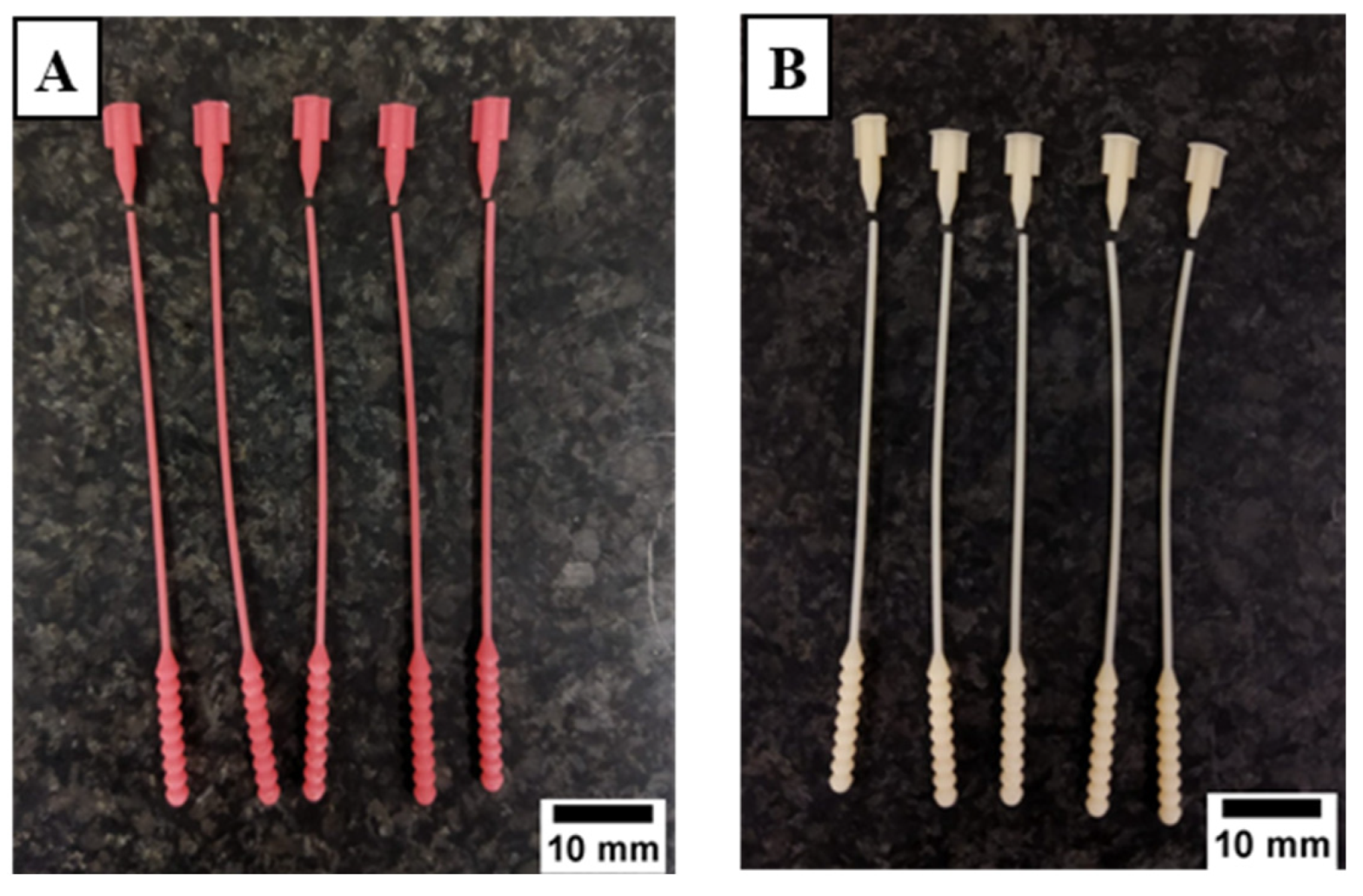
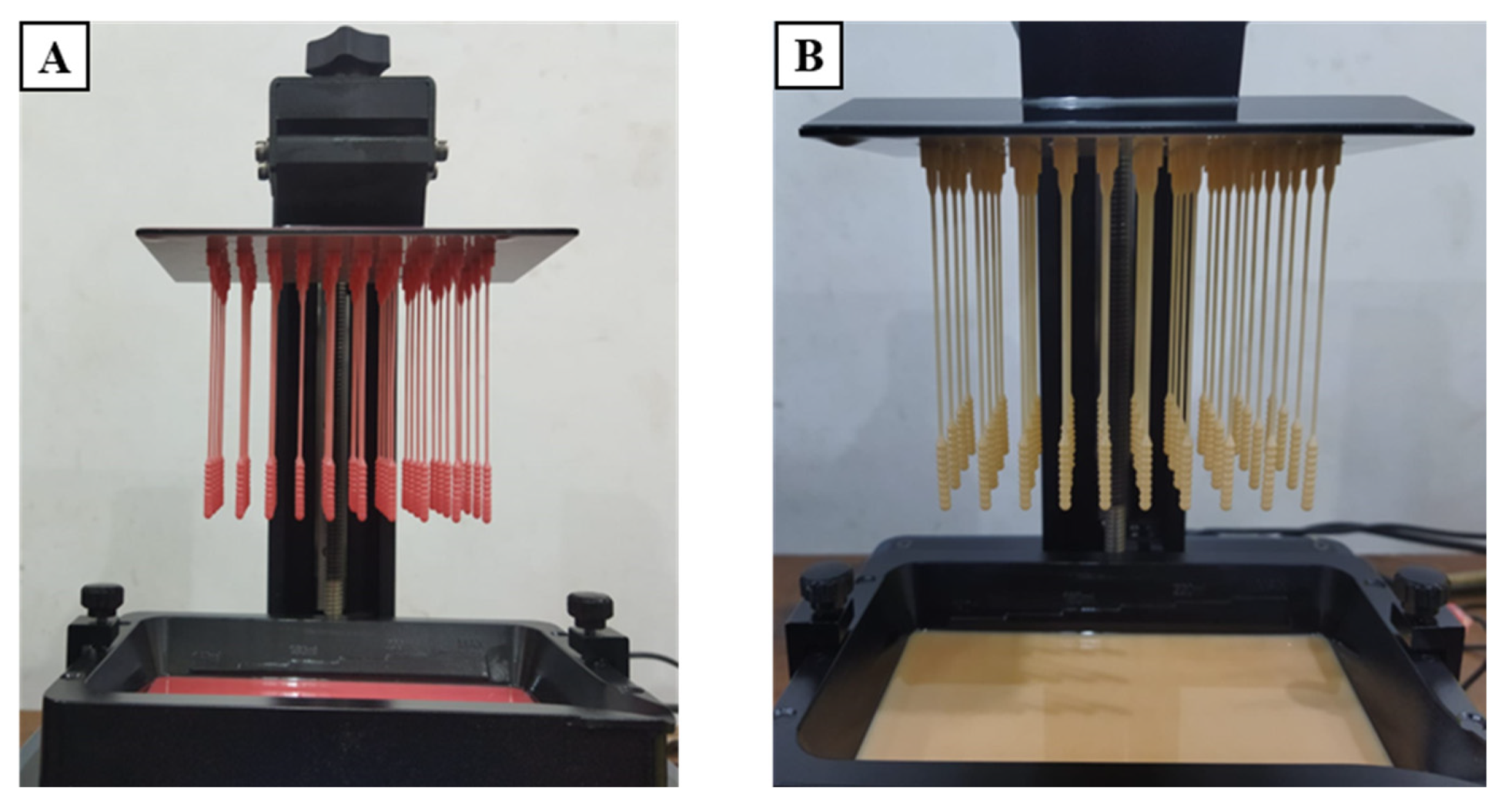
2.3. 3D Printing and Specimen Preparation
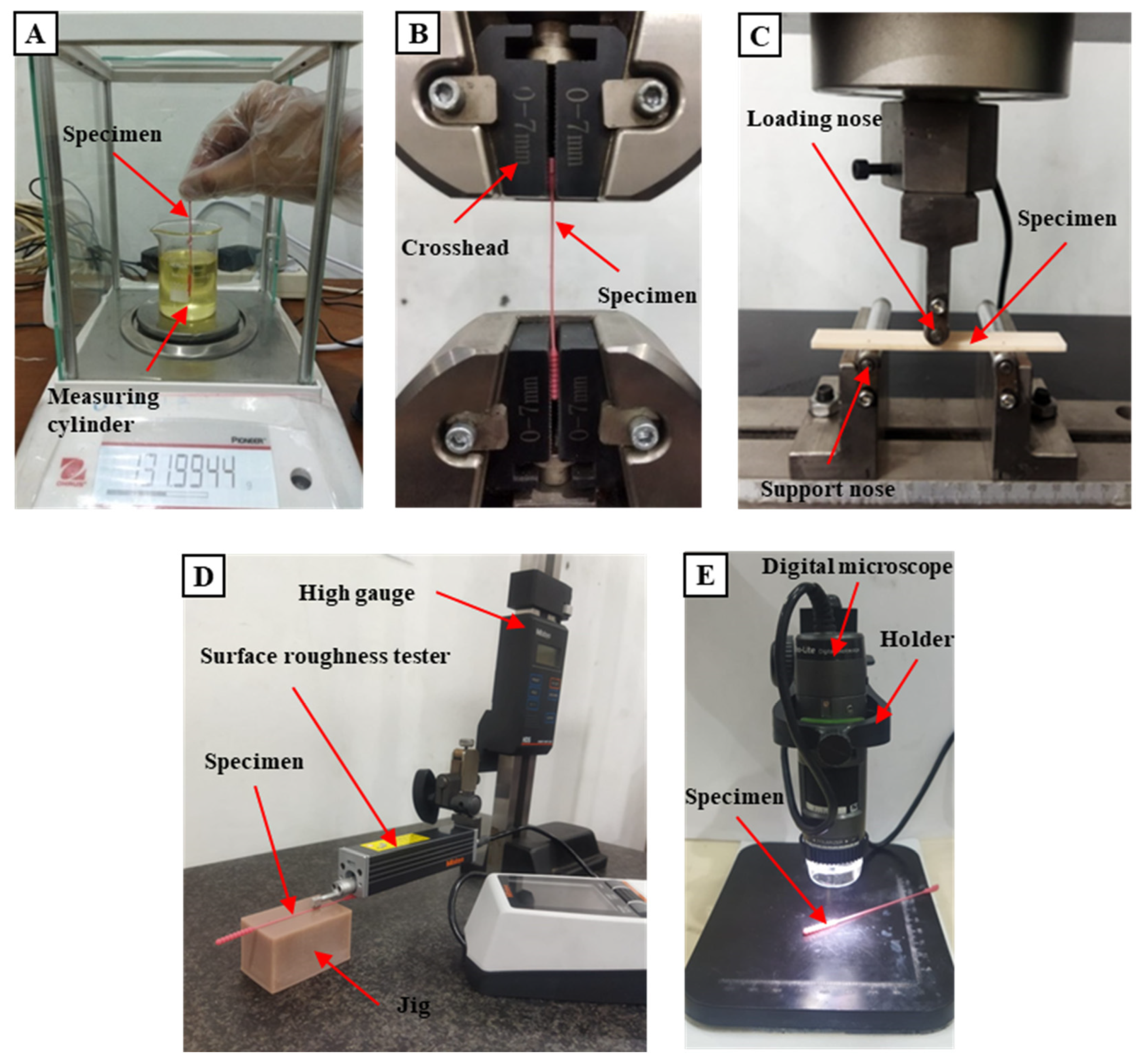
2.4. Testing Methods
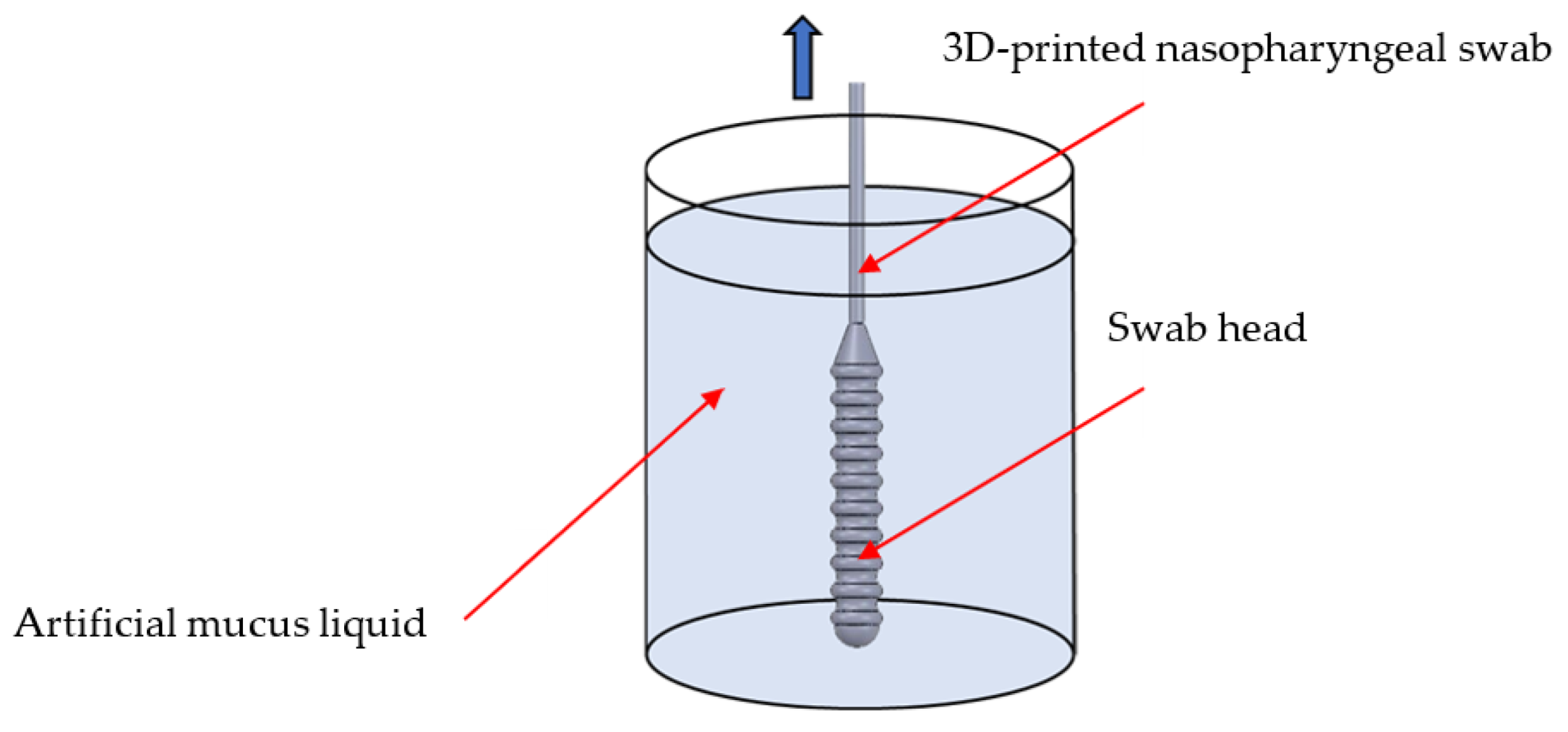
2.4.1. Sample Collection Test
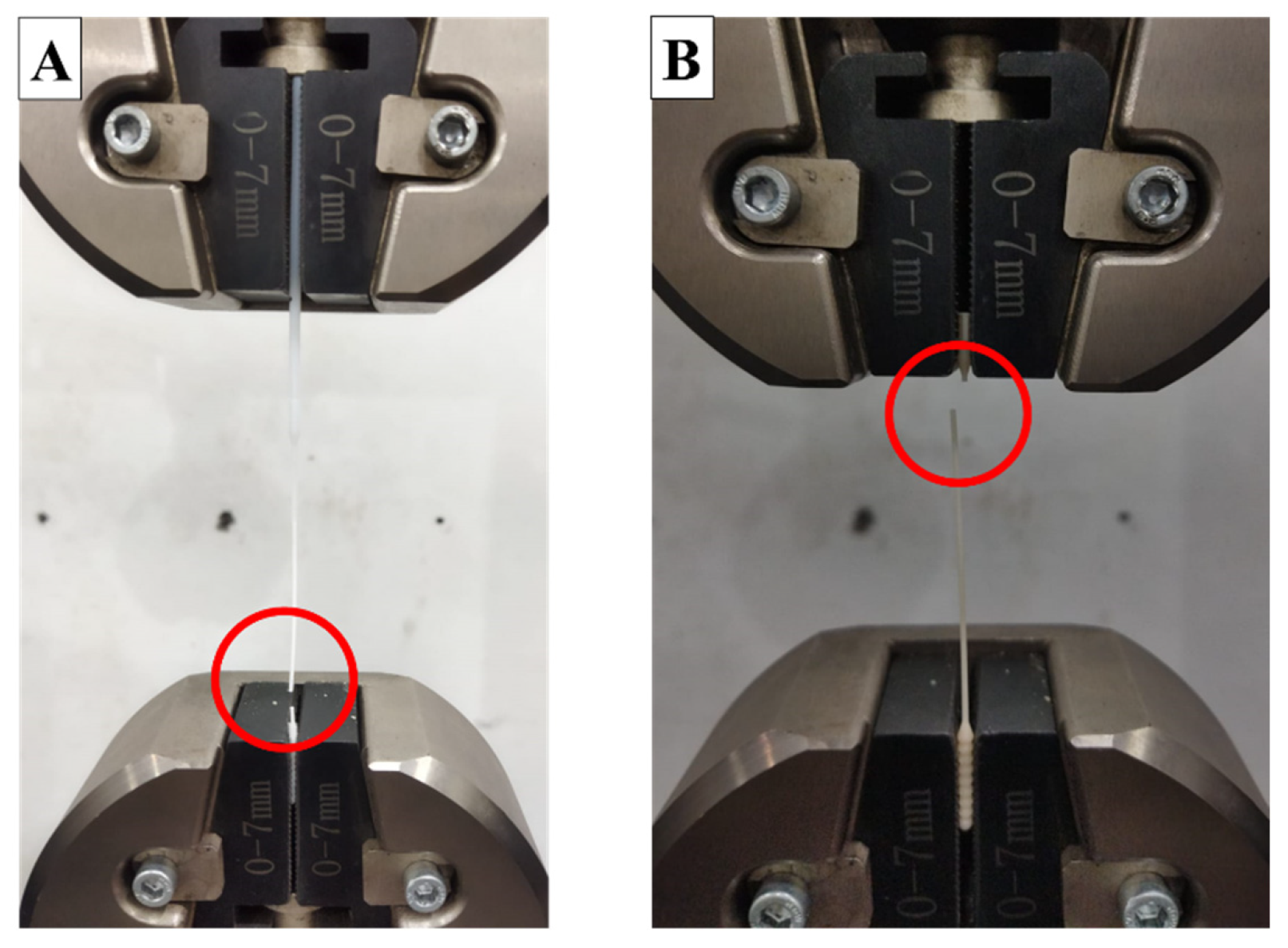
2.4.2. Tensile Test
2.4.3. Flexural Test
2.4.4. Surface Roughness Test
2.4.5. Dimensional Accuracy Test
3. Result and Discussion
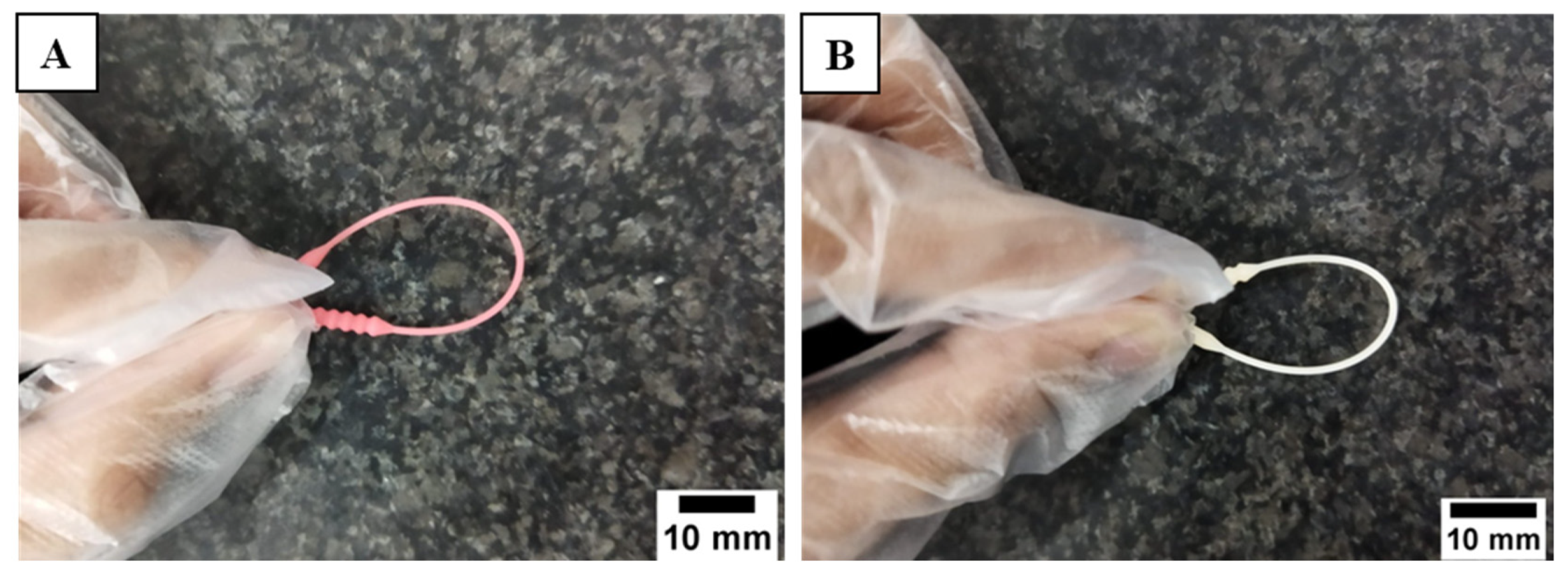
3.1. Defectiveness in the Manufacturing Process of 3D-Printed Nasopharyngeal Swab
3.2. Tensile Test
3.3. Flexural Test
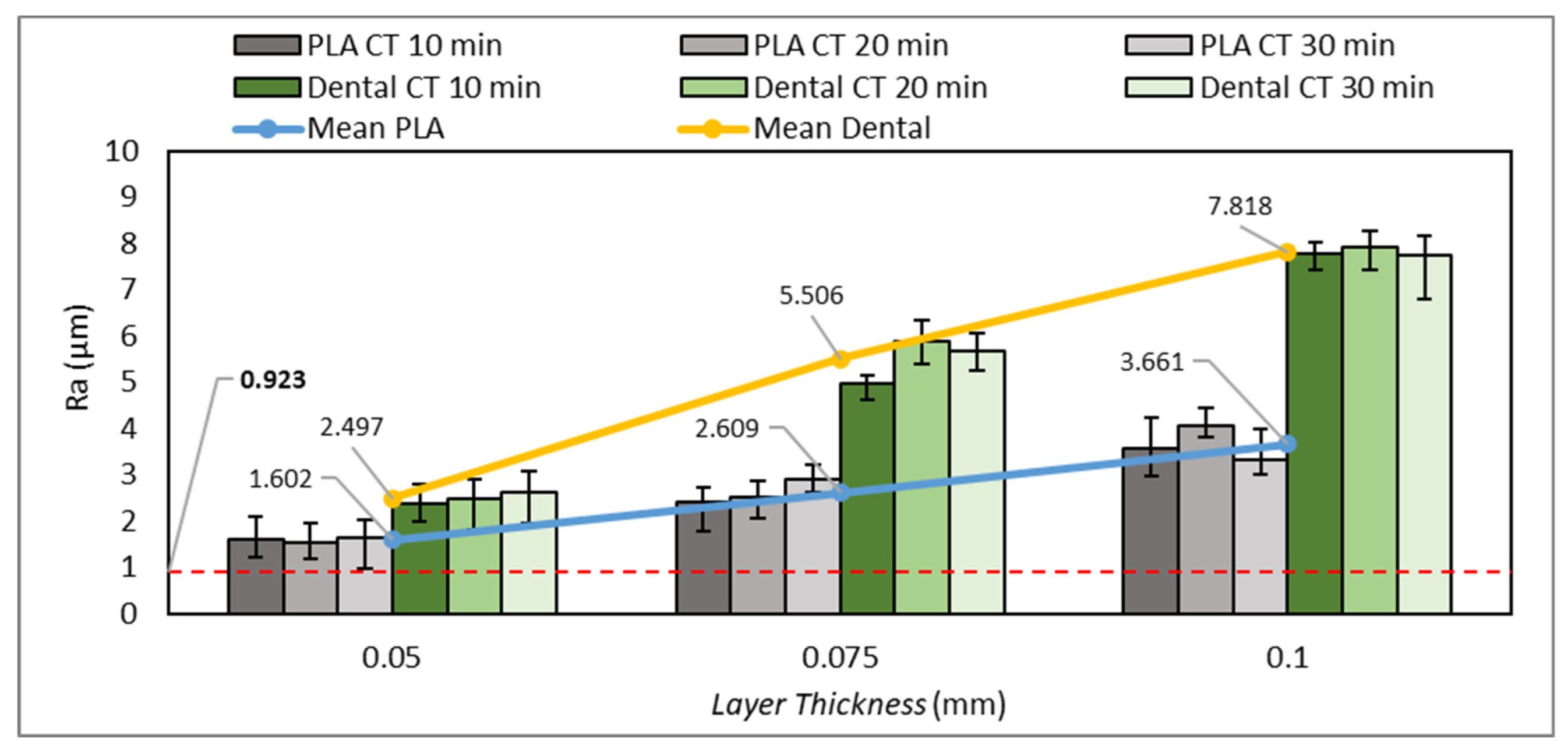
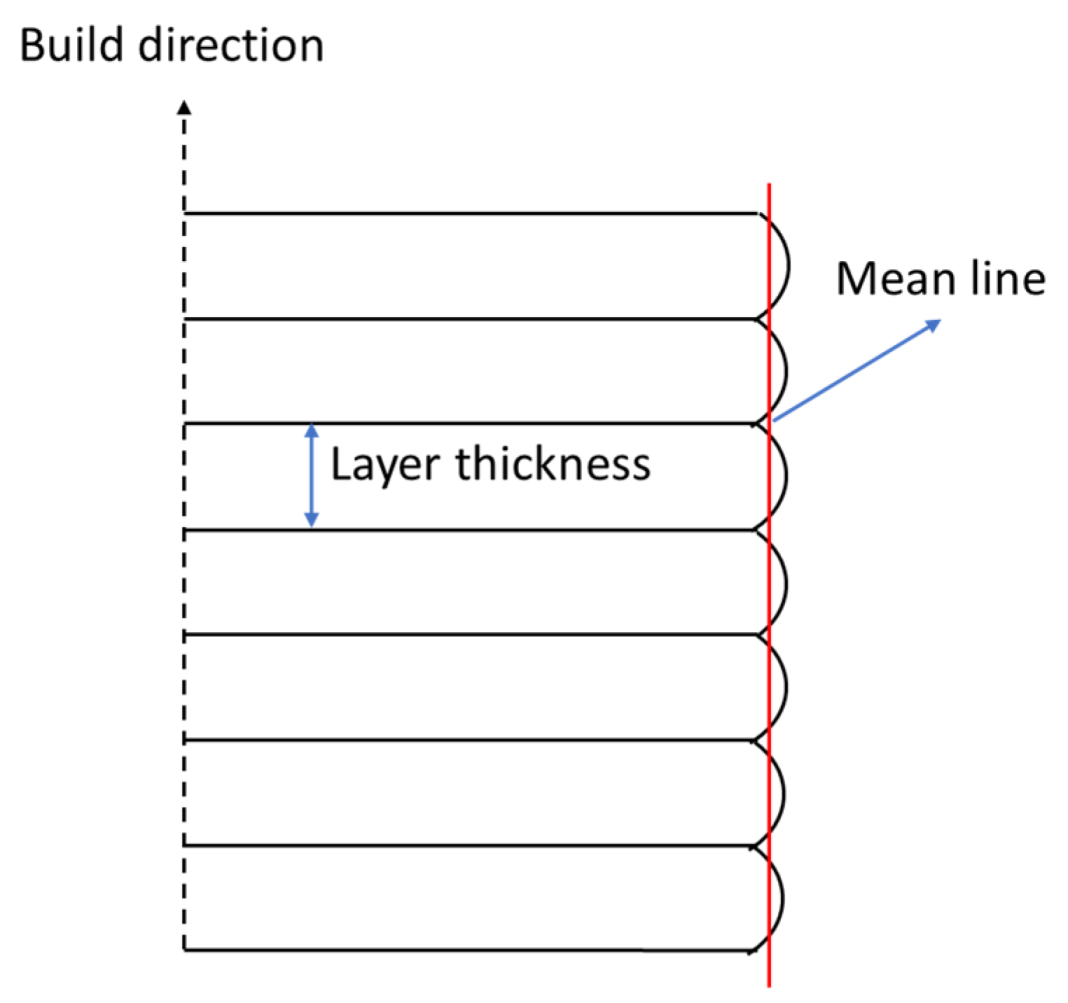
3.4. Surface Roughness Test
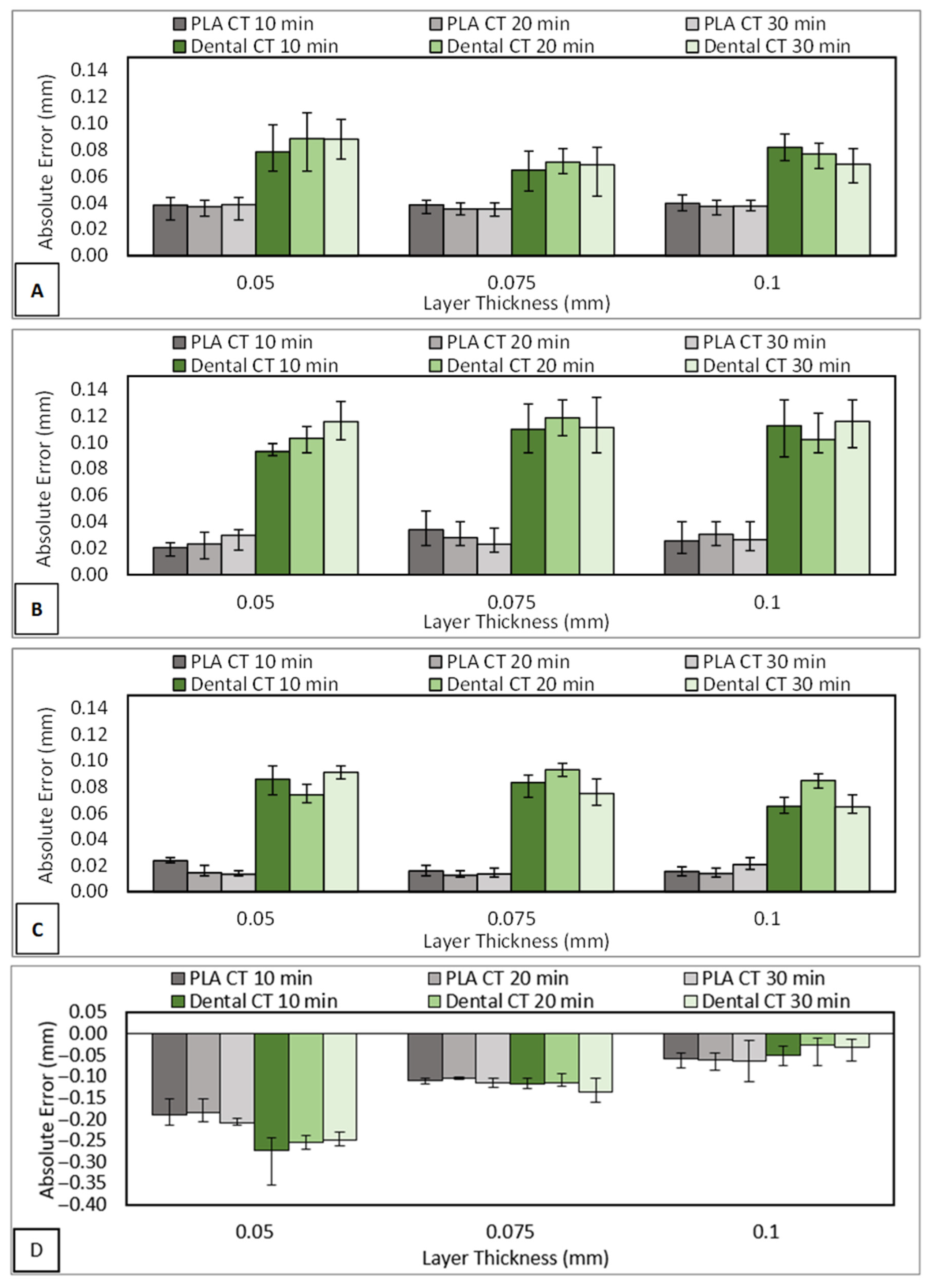
3.5. Dimensional Accuracy Test
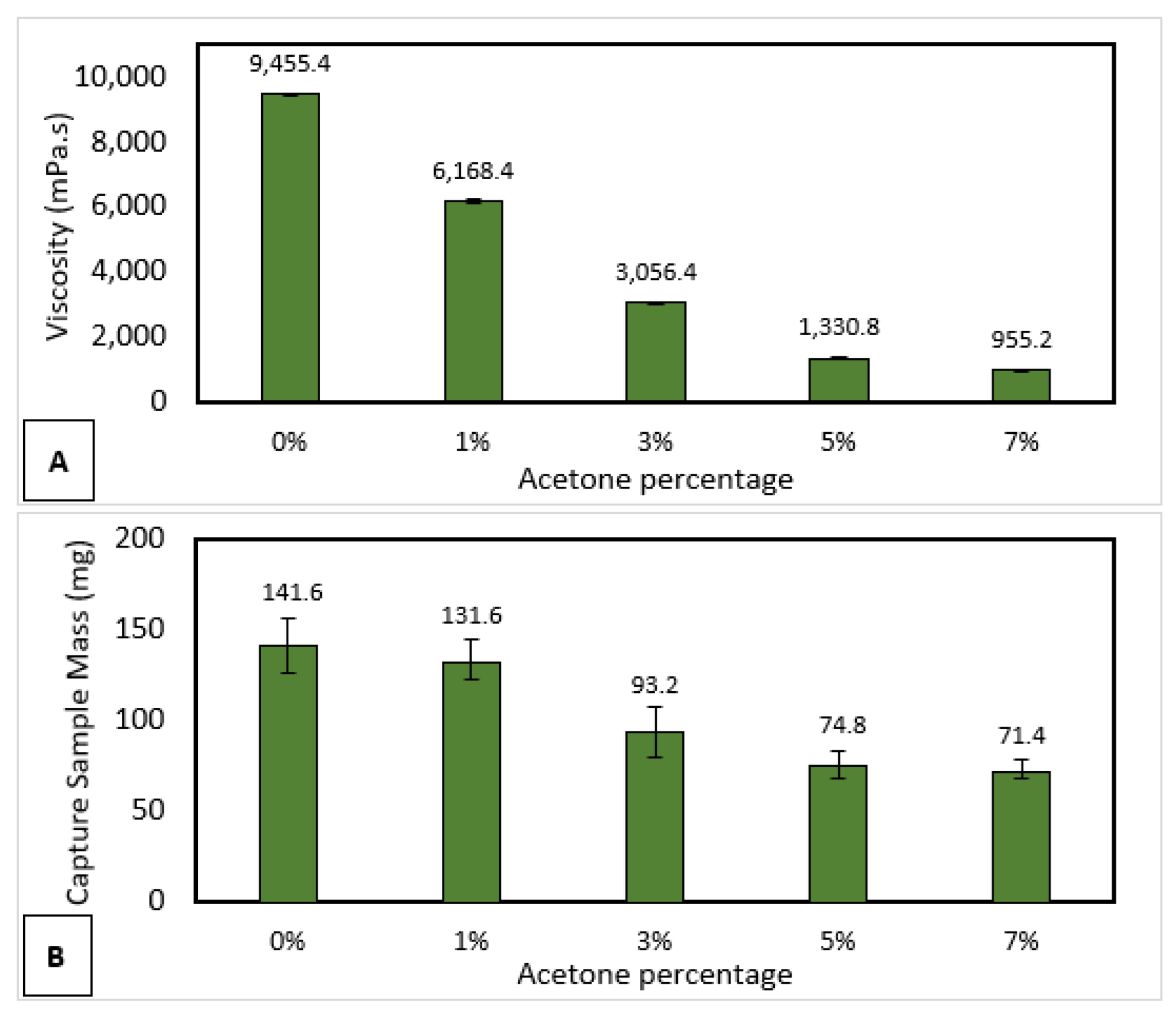
3.6. Sample Collection
3.7. Manufacturing Process and Cost Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, M.; Murphy, D.; Shoai, M.; George-William, J.N.; Al-ebini, Y. Geographical Distribution of Host’s Specific SARS-CoV-2 Mutations in the Early Phase of the COVID-19 Pandemic. Gene 2023, 851, 147020. [Google Scholar] [CrossRef]
- Yang, S.L.; Ripen, A.M.; Lee, J.V.; Koh, K.; Yen, C.H.; Chand, A.K.; Aisyah, N.; Abdul, B.; Gokilavanan, V.; Nur, N.; et al. Time from Last Immunity Event against Infection during Omicron-Dominant Period in Malaysia. Int. J. Infect. Dis. 2023, 128, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Wohlfahrt, J.; Bhatt, S.; Stegger, M.; Legarth, R.; Møller, C.H.; Skov, R.L.; Valentiner-Branth, P.; Voldstedlund, M.; Fischer, T.K.; et al. Risk of Hospitalisation Associated with Infection with SARS-CoV-2 Omicron Variant versus Delta Variant in Denmark: An Observational Cohort Study. Lancet Infect. Dis. 2022, 22, 967–976. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. 3D Printing Applications for Healthcare Research and Development. Glob. Health J. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Agarwal, R. The Personal Protective Equipment Fabricated via 3D Printing Technology during COVID-19. Ann. 3d Print. Med. 2022, 5, 100042. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlögl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Rajabi, M.; Sudhir Sali, S. Additive Manufacturing Potential for Medical Devices and Technology. Curr. Opin. Chem. Eng. 2020, 28, 127–133. [Google Scholar] [CrossRef]
- Tom, T.; Sreenilayam, S.P.; Brabazon, D.; Jose, J.P.; Joseph, B.; Madanan, K.; Thomas, S. Additive Manufacturing in the Biomedical Field-Recent Research Developments. Results Eng. 2022, 16, 100661. [Google Scholar] [CrossRef]
- Nugraha, A.D.; Ruli; Supriyanto, E.; Rasgianti; Prawara, B.; Martides, E.; Junianto, E.; Wibowo, A.; Sentanuhady, J.; Muflikhun, M.A. First-Rate Manufacturing Process of Primary Air Fan (PAF) Coal Power Plant in Indonesia Using Laser Powder Bed Fusion (LPBF) Technology. J. Mater. Res. Technol. 2022, 18, 4075–4088. [Google Scholar] [CrossRef]
- Nugraha, A.D.; Syahril, M.; Muflikhun, M.A. Excellent Performance of Hybrid Model Manufactured via Additive Manufacturing Process Reinforced with GFRP for Sport Climbing Equipment. Heliyon 2023, 9, e14706. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Sentanu, D.A. Characteristics and Performance of Carabiner Remodeling Using 3D Printing with Graded Filler and Different Orientation Methods. Eng. Fail. Anal. 2021, 130, 105795. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Syahril, M.; Mamba’udin, A.; Santos, G.N. A Novel of Hybrid Laminates Additively Manufactured via Material Extrusion—Vat Photopolymerization. J. Eng. Res. 2023, 100146. [Google Scholar] [CrossRef]
- Alfarisi, N.A.S.; Santos, G.N.C.; Norcahyo, R.; Sentanuhady, J.; Azizah, N.; Muflikhun, M.A. Model Optimization and Performance Evaluation of Hand Cranked Music Box Base Structure Manufactured via 3D Printing. Heliyon 2021, 7, E08432. [Google Scholar] [CrossRef]
- Ware, H.O.T.; Ding, Y.; Collins, C.; Akar, B.; Akbari, N.; Wang, H.; Duan, C.; Ameer, G.; Sun, C. In Situ Formation of Micro/Nano Phase Composite for 3D Printing Clinically Relevant Bioresorbable Stents. Mater. Today Chem. 2022, 26, 101231. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Özdilli, Ö. Comparison of the Surface Quality of the Products Manufactured by the Plastic Injection Molding and SLA and FDM Method. Uluslararası Muhendis. Arast. Ve Gelistirme Derg. 2021, 13, 428–437. [Google Scholar] [CrossRef]
- Naeem, O.A.; Bencharit, S.; Yang, I.H.; Stilianoudakis, S.C.; Carrico, C.; Tüfekçi, E. Comparison of 3-Dimensional Printing Technologies on the Precision, Trueness, and Accuracy of Printed Retainers. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 582–591. [Google Scholar] [CrossRef]
- Vieira Magaldi, B.; de Oliveira da Costa Maia Pinto, M.; Thiré, R.; Araujo, A.C. Comparison of the Porosity of Scaffolds Manufactured by Two Additive Manufacturing Technologies: SLA and FDM. In Proceedings of the 24th International Congress of Mechanical Engineering, Curitiba, Brazil, 3–8 December 2017. [Google Scholar] [CrossRef]
- Medellin, A.; Du, W.; Miao, G.; Zou, J.; Pei, Z.; Ma, C. Vat Photopolymerization 3d Printing of Nanocomposites: A Literature Review. J. Micro Nano Manuf. 2019, 7, 031006. [Google Scholar] [CrossRef]
- Andjela, L.; Abdurahmanovich, V.M.; Vladimirovna, S.N.; Mikhailovna, G.I.; Yurievich, D.D.; Alekseevna, M.Y. A Review on Vat Photopolymerization 3D-Printing Processes for Dental Application. Dent. Mater. 2022, 38, e284–e296. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Millat-Martinez, P.; Ouchi, D.; Roberts, C.H.; Alemany, A.; Corbacho-Monné, M.; Ubals, M.; Tobias, A.; Tebé, C.; Ballana, E.; et al. Transmission of COVID-19 in 282 Clusters in Catalonia, Spain: A Cohort Study. Lancet Infect. Dis. 2021, 21, 629–636. [Google Scholar] [CrossRef]
- Patel, R.; Babady, E.; Theel, E.; Storch, G.; Pinsky, B.; George, K.; Smith, T.; Bertuzzi, S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS-CoV-2/COVID-19. mBio 2020, 11, e00722-20. [Google Scholar] [CrossRef]
- Callahan, C.J.; Lee, R.; Zulauf, K.E.; Tamburello, L.; Smith, K.P.; Previtera, J.; Cheng, A.; Green, A.; Azim, A.A.; Yano, A.; et al. Open Development and Clinical Validation of Multiple 3d-Printed Nasopharyngeal Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck. J. Clin. Microbiol. 2020, 58, e00876-20. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K. The Latest Obstacle to Getting Tested? A Shortage of Face Masks and Swabs. New York Times, 18 March 2020. [Google Scholar]
- Arjunan, A.; Zahid, S.; Baroutaji, A.; Robinson, J. 3D Printed Auxetic Nasopharyngeal Swabs for COVID-19 Sample Collection. J. Mech. Behav. Biomed. Mater. 2021, 114, 104175. [Google Scholar] [CrossRef]
- Alghounaim, M.; Almazeedi, S.; Al Youha, S.; Papenburg, J.; Alowaish, O.; AbdulHussain, G.; Al-Shemali, R.; Albuloushi, A.; Alzabin, S.; Al-Wogayan, K.; et al. Low-Cost Polyester-Tipped Three-Dimensionally Printed Nasopharyngeal Swab for the Detection of Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2). J. Clin. Microbiol. 2020, 58, 1–9. [Google Scholar] [CrossRef]
- Williams, E.; Bond, K.; Isles, N.; Chong, B.; Johnson, D.; Druce, J. Pandemic Printing: A Novel 3D-printed Swab for Detecting SARS-CoV-2. Med. J. Aust. 2020, 213, 276–279. [Google Scholar] [CrossRef]
- Ford, J.; Goldstein, T.; Trahan, S.; Neuwirth, A.; Tatoris, K.; Decker, S. A 3D-Printed Nasopharyngeal Swab for COVID-19 Diagnostic Testing. 3d Print. Med. 2020, 6, 21. [Google Scholar] [CrossRef]
- Tay, J.K.; Cross, G.B.; Lee, C.K.; Yan, B.; Loh, J.; Lim, Z.Y.; Ngiam, N.; Chee, J.; Gan, S.W.; Saraf, A.; et al. Design and Clinical Validation of a 3D-Printed Nasopharyngeal Swab for COVID-19 Testing. medRxiv 2020. [Google Scholar] [CrossRef]
- Starosolski, Z.; Admane, P.; Dunn, J.; Kaziny, B.; Huisman, T.A.G.M.; Annapragada, A. Design of 3D-Printed Nasopharyngeal Swabs for Children Is Enabled by Radiologic Imaging. Am. J. Neuroradiol. 2020, 41, 2345–2347. [Google Scholar] [CrossRef]
- Alazemi, A.; AbdulHussain, G.; Alawwam, A.; Al-Shatti, A.; Alghounaim, M.; Almazeedi, S.; Al Youha, S.; Al-Sabah, S. Innovative Design of 3D-Printed Nasopharyngeal Pediatric Swab for COVID-19 Detection. 3d Print. Med. 2021, 7, 3–8. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Mamba’udin, A. Swab Nasofaring Dengan Komponen Terpisah Yang Dilengkapi Bagian Titik Patah. Indonesia Patent P00202304192, 2023. pp. 1–13. [Google Scholar]
- ASP STERRAD® 100S Sterilizer. Available online: https://www.asp.com/en-us (accessed on 25 July 2023).
- Ambrosio, D.; Gabrion, X.; Malécot, P.; Amiot, F.; Thibaud, S. Influence of Manufacturing Parameters on the Mechanical Properties of Projection Stereolithography–Manufactured Specimens. Int. J. Adv. Manuf. Technol. 2020, 106, 265–277. [Google Scholar] [CrossRef]
- Wang, S.; D’hooge, D.R.; Daelemans, L.; Xia, H.; De Clerck, K.; Cardon, L. The Transferability and Design of Commercial Printer Settings in Pla/Pbat Fused Filament Fabrication. Polymers 2020, 12, 2573. [Google Scholar] [CrossRef]
- Kurimoto, M.; Manabe, Y.; Mitsumoto, S.; Suzuoki, Y. Layer Interface Effects on Dielectric Breakdown Strength of 3D Printed Rubber Insulator Using Stereolithography. Addit. Manuf. 2021, 46, 102069. [Google Scholar] [CrossRef]
- Riccio, C.; Civera, M.; Grimaldo Ruiz, O.; Pedullà, P.; Rodriguez Reinoso, M.; Tommasi, G.; Vollaro, M.; Burgio, V.; Surace, C. Effects of Curing on Photosensitive Resins in SLA Additive Manufacturing. Appl. Mech. 2021, 2, 942–955. [Google Scholar] [CrossRef]
- Dzadz, Ł.; Pszczółkowski, B. Analysis of the Influence of UV Light Exposure Time on Hardness and Density Properties of SLA Models. Tech. Sci. 2020, 23, 175–184. [Google Scholar] [CrossRef]
- Soh, M.S.; Yap, A.U.J. Influence of Curing Modes on Crosslink Density in Polymer Structures. J. Dent. 2004, 32, 321–326. [Google Scholar] [CrossRef]
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased Rates of Photopolymerisation by Ternary Type II Photoinitiator Systems in Dental Resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, K.; Jawahar, N.; Chandrasekhar, U. Influence of Layer Thickness on Mechanical Properties in Stereolithography. Rapid Prototyp. J. 2006, 12, 106–113. [Google Scholar] [CrossRef]
- Nowacki, B.; Kowol, P.; Kozioł, M.; Olesik, P.; Wieczorek, J.; Wacławiak, K. Effect of Post-Process Curing and Washing Time on Mechanical Properties of Mslaprintouts. Materials 2021, 14, 4856. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Decker, C. Photocrosslinking of Acrylated Natural Rubber. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 769–780. [Google Scholar] [CrossRef]
- Pfaffinger, M. Hot Lithography—New Possibilities in Polymer 3D Printing. Opt. Photonik 2018, 13, 99–101. [Google Scholar] [CrossRef]
- Ocaña, I.R.; Molina, S.I. Cork Photocurable Resin Composite for Stereolithography (SLA): Influence of Cork Particle Size on Mechanical and Thermal Properties. Addit. Manuf. 2022, 51, 102586. [Google Scholar] [CrossRef]
- Chambers, A.R.; Earl, J.S.; Squires, C.A.; Suhot, M.A. The Effect of Voids on the Flexural Fatigue Performance of Unidirectional Carbon Fibre Composites Developed for Wind Turbine Applications. Int. J. Fatigue 2006, 28, 1389–1398. [Google Scholar] [CrossRef]
- Arnold, C.; Monsees, D.; Hey, J.; Schweyen, R. Surface Quality of 3D-Printed Models as a Function of Various Printing Parameters. Materials 2019, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, K.; Nobes, D.; Qureshi, A. Investigation of Light-Induced Surface Roughness in Projection Micro-Stereolithography Additive Manufacturing (PμSLA). Procedia CIRP 2020, 92, 187–193. [Google Scholar] [CrossRef]
- Shah, J.; Snider, B.; Clarke, T.; Kozutsky, S.; Lacki, M.; Hosseini, A. Large-Scale 3D Printers for Additive Manufacturing: Design Considerations and Challenges. Int. J. Adv. Manuf. Technol. 2019, 104, 3679–3693. [Google Scholar] [CrossRef]
- Sabbah, A.; Romanos, G.; Delgado-Ruiz, R. Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin. Prosthesis 2022, 4, 80–90. [Google Scholar] [CrossRef]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer Formulation to Minimize Feature Size, Surface Roughness, and Stair-Stepping in Digital Light Processing-Based Three-Dimensional Printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Favero, C.S.; English, J.D.; Cozad, B.E.; Wirthlin, J.O.; Short, M.M.; Kasper, F.K. Effect of Print Layer Height and Printer Type on the Accuracy of 3-Dimensional Printed Orthodontic Models. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 557–565. [Google Scholar] [CrossRef]
- McCarty, M.C.; Chen, S.J.; English, J.D.; Kasper, F. Effect of Print Orientation and Duration of Ultraviolet Curing on the Dimensional Accuracy of a 3-Dimensionally Printed Orthodontic Clear Aligner Design. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 889–897. [Google Scholar] [CrossRef]
- Boca, M.A.; Sover, A.; Slătineanu, L. The Printing Parameters Effects on the Dimensional Accuracy of the Parts Made of Photosensitive Resin. Macromol. Symp. 2021, 396, 2000287. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and Macrorheology of Mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Girod, S.; Zahm, J.M.; Plotkowski, C.; Beck, G.; Puchelle, E. Role of the Physiochemical Properties of Mucus in the Protection of the Respiratory Epithelium. Eur. Respir. J. 1992, 5, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Pandzic, A. Influence of Layer Height, Build Orientation and Post Curing on Tensile Mechanical Properties of Sla 3D Printed Material. In Proceedings of the 32nd International DAAAM Symposium, Vienna, Austria, 28–29 October 2021; Volume 32, pp. 200–208. [Google Scholar] [CrossRef]













| eSun PLA PRO | ||
|---|---|---|
| Chemical Name | CAS No. | % by Weight |
| Acrylates aliphatic urethane | 68987-79-1 | 40–50% |
| Monomer | 13048-33-4 | 20–40% |
| Photoinitiators | 75980-60-8 | 3–5% |
| Color pigment | 2–5% | |
| Anycubic dental non-castable | ||
| Chemical Name | CAS No. | % by weight |
| Epoxy acrylate resin | 61788-97-4 | 40–50% |
| Monomer | 13048-33-4 | 20–40% |
| Photoinitiators | 947-19-3 | 3–5% |
| Color pigment | 2–5% | |
| eSun standard | ||
| Chemical Name | CAS No. | % by weight |
| Urethane acrylate | 68987-79-1 | 35% |
| Trimethylolpropane triacrylate | 15625-89-5 | 25% |
| Tripropyleneglycol diacrylate | 42978-66-5 | 19.5% |
| 2-Phenoxyethyl acrylate | 48145-04-6 | 15% |
| Trimethylbenzoyldiphenylphosphine oxide | 75980-60-8 | 3% |
| Phenylbis phosphine oxide | 162881-26-7 | 1% |
| BYK 1790 | 128-37-0 | 1% |
| UV Color pigment | 133-86-4/482-89-3 | 0.5% |
| eSun PLA PRO | ||
|---|---|---|
| Properties | Value | Unit |
| Density | 1.09–1.10 | g/cm3 |
| Elongation at break | 25–28 | % |
| Flexural strength | 36–49 | MPa |
| Hardness | 78–80 | Shore D |
| IZOD impact strength | 32–36 | J/m |
| Tensile strength | 37–48 | MPa |
| Viscosity | 200–300 | mPa.s |
| Anycubic dental non-castable | ||
| Properties | Value | Unit |
| Density | 1.05–1.13 | g/cm3 |
| Elongation at break | 11–20 | % |
| Hardness | 88 | Shore D |
| Shrinkage | 1.86–2.12 | % |
| Tensile strength | 42–62 | Mpa |
| Viscosity | 100–150 | mPa.s |
| eSun standard | ||
| Properties | Value | Unit |
| Density | 1.08–1.13 | g/cm3 |
| Elongation at break | 28–36 | % |
| Flexural strength | 46–72 | Mpa |
| Hardness | 78–82 | Shore D |
| IZOD impact strength | 14–42 | J/m |
| Tensile strength | 46–67 | Mpa |
| Viscosity | 170–200 | mPa.s |
| Slicing Setting | |
|---|---|
| Parameter | Value |
| Layer Thickness (mm) | 0.05, 0.075, 0.1 |
| Normal Exposure Time (s) | 4 |
| Off Time (s) | 1 |
| Bottom Exposure Time (s) | 40 |
| Bottom Layers | 4 |
| Z Lift Distance (mm) | 4, 5 |
| Z Lift Speed (mm/s) | 1, 2 |
| Z Retract Speed (mm/s) | 1, 2 |
| Anti-alias | 4 |
| Build orientation (deg) | 90 |
| Post-processing | |
| Washing time (minute) | 5 |
| Curing time (minute) | 10, 20, 30 |
| Printing | |
|---|---|
| System | Anycubic Photon Ultra |
| Software | Anycubic photon workshop |
| Operation | 2.8-inch color TFT screen |
| Connectivity | USB memory stick |
| Specifications | |
| Technique | Digital Light Projection |
| Light source | UV-LED (wavelength 405 nm) |
| XY resolution | 0.08 mm 1280 × 720 (720 p) |
| Z axis accuracy | 0.01 mm |
| Suggested layer thickness | 0.01–0.15 mm |
| Suggested print speed | Max 50 mm/h |
| Rated power | 15 W |
| Physical Dimensions | |
| Dimensions | 222 × 227 × 383 mm (L × W × H) |
| Build volume | 102.4 × 57.6 × 165 mm (L × W × H) |
| Materials | 405 nm UV resin |
| Net weight | ~4 kg |
| Specification | |
|---|---|
| Control method | Digital displays + LED dual light, knob |
| Rated power | 25 W |
| Input voltage | AC 110/220 V 50/60 Hz |
| UV LED | 405 nm |
| Time mode | 1~60 min |
| Physical Dimensions | |
| Machine size | 225 × 235 × 365 mm (L × W × H) |
| Maximum wash volume | 120 × 74 × 165 mm (L × W × H) |
| Maximum curing volume | 140 × 165 mm (D × H) |
| Net weight | ~3.7 kg |
| Material | No | Layer Thickness (mm) | Normal Exposure Time (s) | Z Speed (mm/s) | Printing Time | Post-Curing Time (Menit) | |
|---|---|---|---|---|---|---|---|
| 3D-Printed Nasopharyngeal Swab | Flexural Test Specimen | ||||||
| PLA PRO | 1 | 0.05 | 4 | 2 | 7 h 48 min | 1 h 8 min | 10 |
| 2 | 0.05 | 4 | 2 | 7 h 48 min | 1 h 8 min | 20 | |
| 3 | 0.05 | 4 | 2 | 7 h 48 min | 1 h 8 min | 30 | |
| 4 | 0.075 | 4 | 2 | 5 h 12 min | 46 min | 10 | |
| 5 | 0.075 | 4 | 2 | 5 h 12 min | 46 min | 20 | |
| 6 | 0.075 | 4 | 2 | 5 h 12 min | 46 min | 30 | |
| 7 | 0.1 | 4 | 2 | 3 h 54 min | 35 min | 10 | |
| 8 | 0.1 | 4 | 2 | 3 h 54 min | 35 min | 20 | |
| 9 | 0.1 | 4 | 2 | 3 h 54 min | 35 min | 30 | |
| Dental Non-castable | 10 | 0.05 | 4 | 1 | 10 h 1 min | 1 h 41 min | 10 |
| 11 | 0.05 | 4 | 1 | 10 h 1 min | 1 h 41 min | 20 | |
| 12 | 0.05 | 4 | 1 | 10 h 1 min | 1 h 41 min | 30 | |
| 13 | 0.075 | 4 | 1 | 6 h 40 min | 1 h 7 min | 10 | |
| 14 | 0.075 | 4 | 1 | 6 h 40 min | 1 h 7 min | 20 | |
| 15 | 0.075 | 4 | 1 | 6 h 40 min | 1 h 7 min | 30 | |
| 16 | 0.1 | 4 | 1 | 5 h 2 min | 51 min | 10 | |
| 17 | 0.1 | 4 | 1 | 5 h 2 min | 51 min | 20 | |
| 18 | 0.1 | 4 | 1 | 5 h 2 min | 51 min | 30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamba’udin, A.; Handayani, M.; Triawan, F.; Rahmayanti, Y.D.; Muflikhun, M.A. Excellent Characteristics of Environmentally Friendly 3D-Printed Nasopharyngeal Swabs for Medical Sample Collection. Polymers 2023, 15, 3363. https://doi.org/10.3390/polym15163363
Mamba’udin A, Handayani M, Triawan F, Rahmayanti YD, Muflikhun MA. Excellent Characteristics of Environmentally Friendly 3D-Printed Nasopharyngeal Swabs for Medical Sample Collection. Polymers. 2023; 15(16):3363. https://doi.org/10.3390/polym15163363
Chicago/Turabian StyleMamba’udin, Ahmad, Murni Handayani, Farid Triawan, Yosephin Dewiani Rahmayanti, and Muhammad Akhsin Muflikhun. 2023. "Excellent Characteristics of Environmentally Friendly 3D-Printed Nasopharyngeal Swabs for Medical Sample Collection" Polymers 15, no. 16: 3363. https://doi.org/10.3390/polym15163363
APA StyleMamba’udin, A., Handayani, M., Triawan, F., Rahmayanti, Y. D., & Muflikhun, M. A. (2023). Excellent Characteristics of Environmentally Friendly 3D-Printed Nasopharyngeal Swabs for Medical Sample Collection. Polymers, 15(16), 3363. https://doi.org/10.3390/polym15163363








