Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers
Abstract
1. Introduction
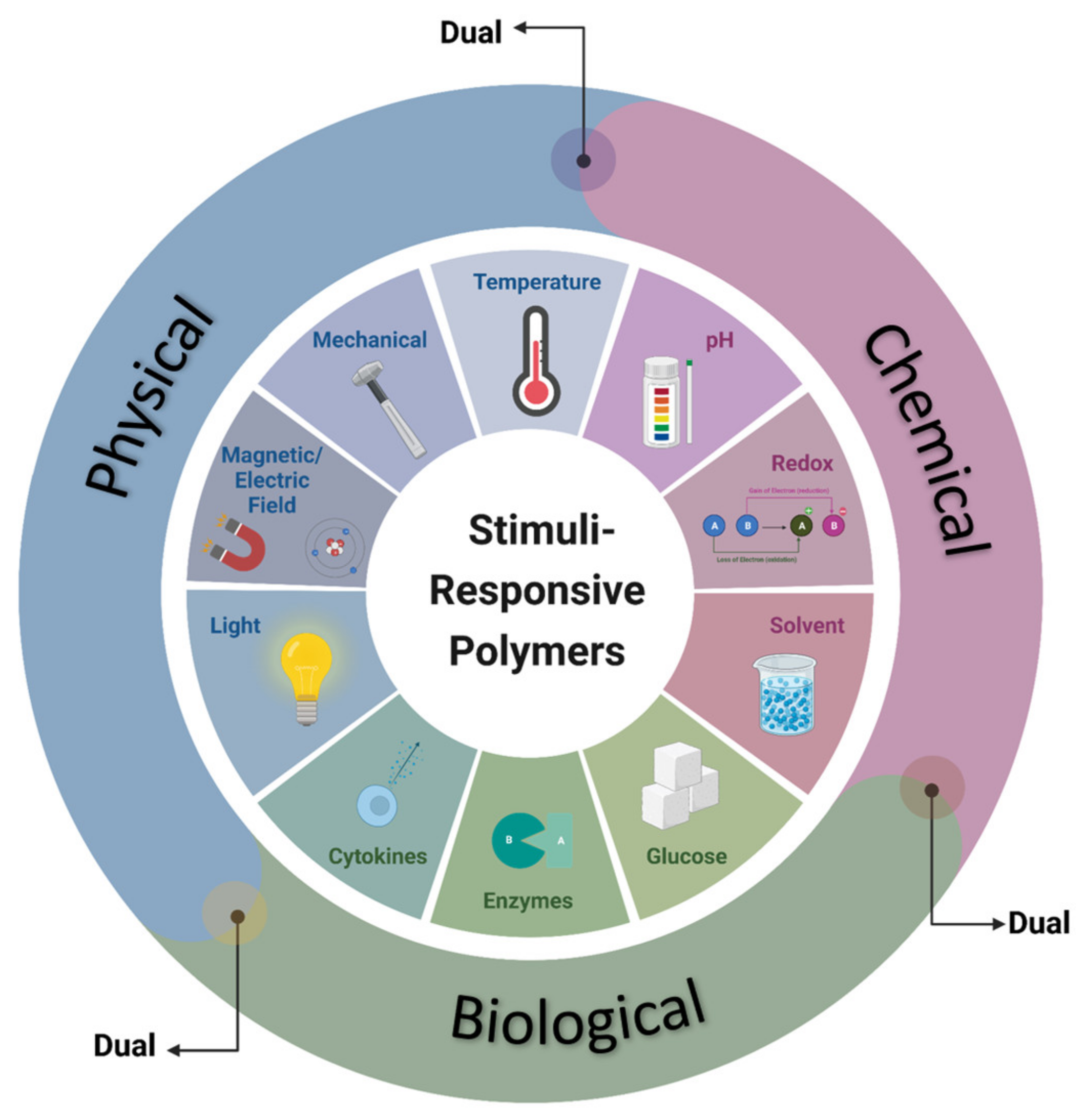
2. Stimuli-Responsive Polymers—Properties and Classification
2.1. Physical-Stimuli-Responsive Polymers
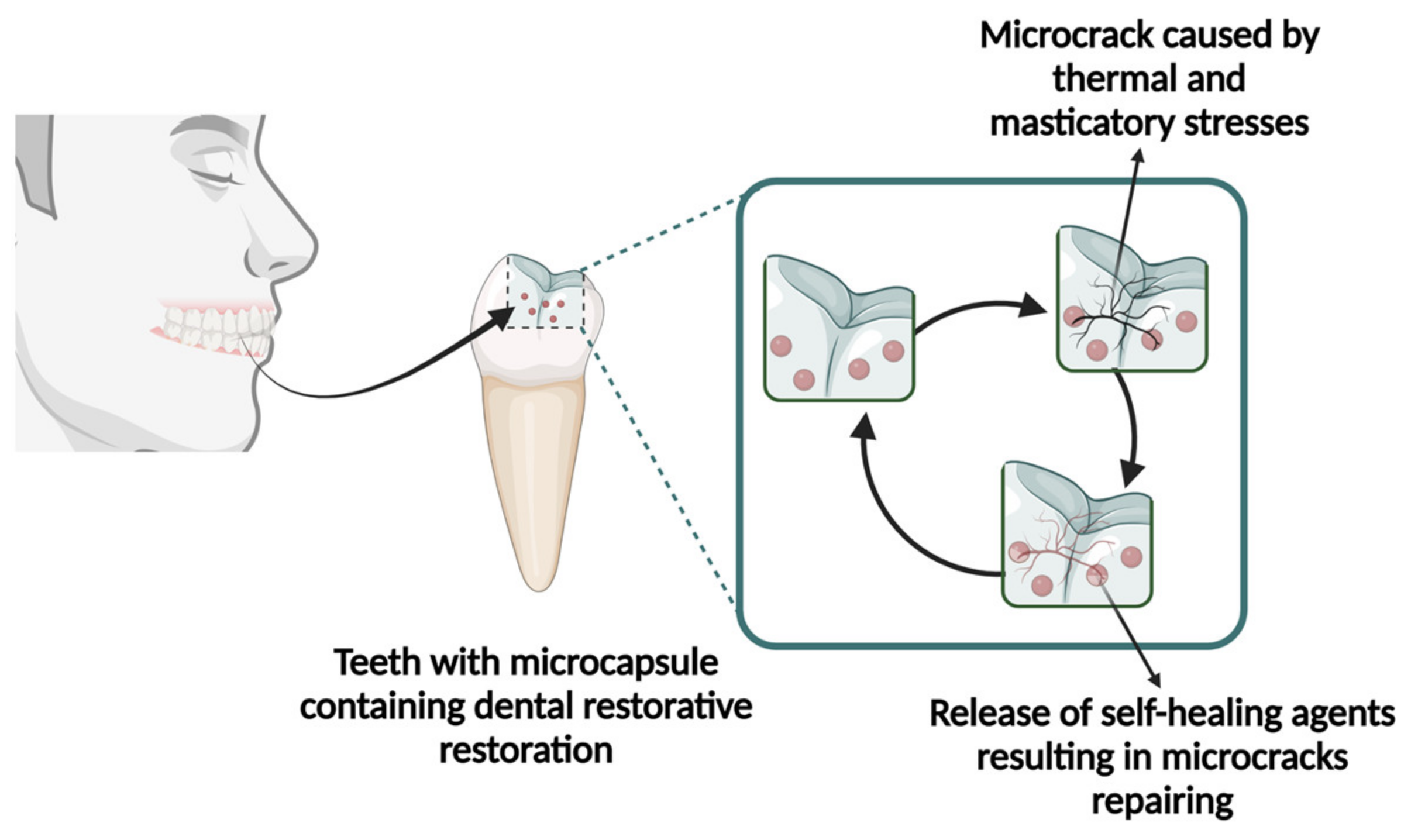
2.1.1. Mechanical
2.1.2. Temperature
2.1.3. Light
2.1.4. Magnetic/Electric Field
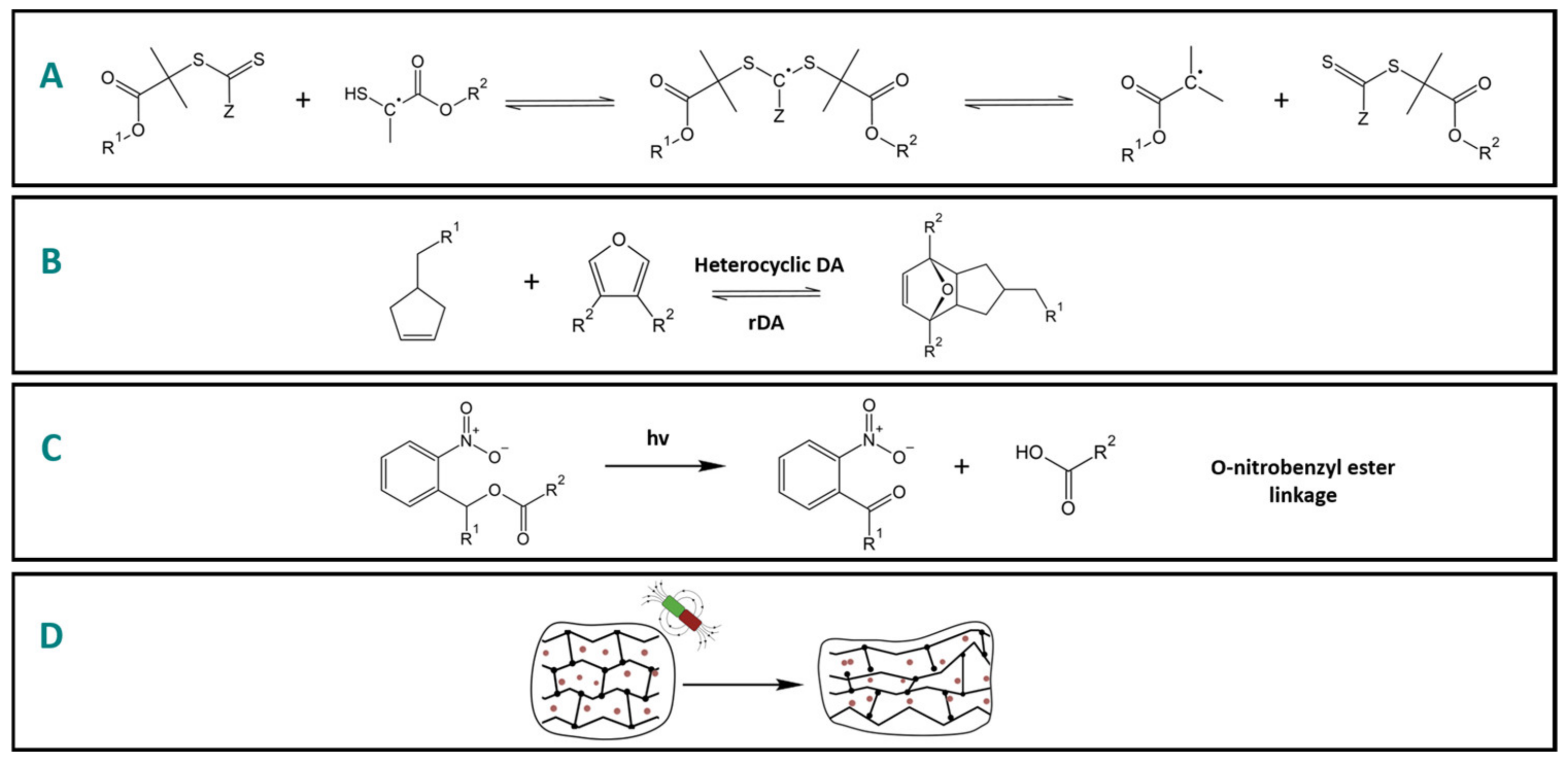
2.2. Chemical-Stimuli-Responsive Polymers
2.2.1. pH
2.2.2. Redox
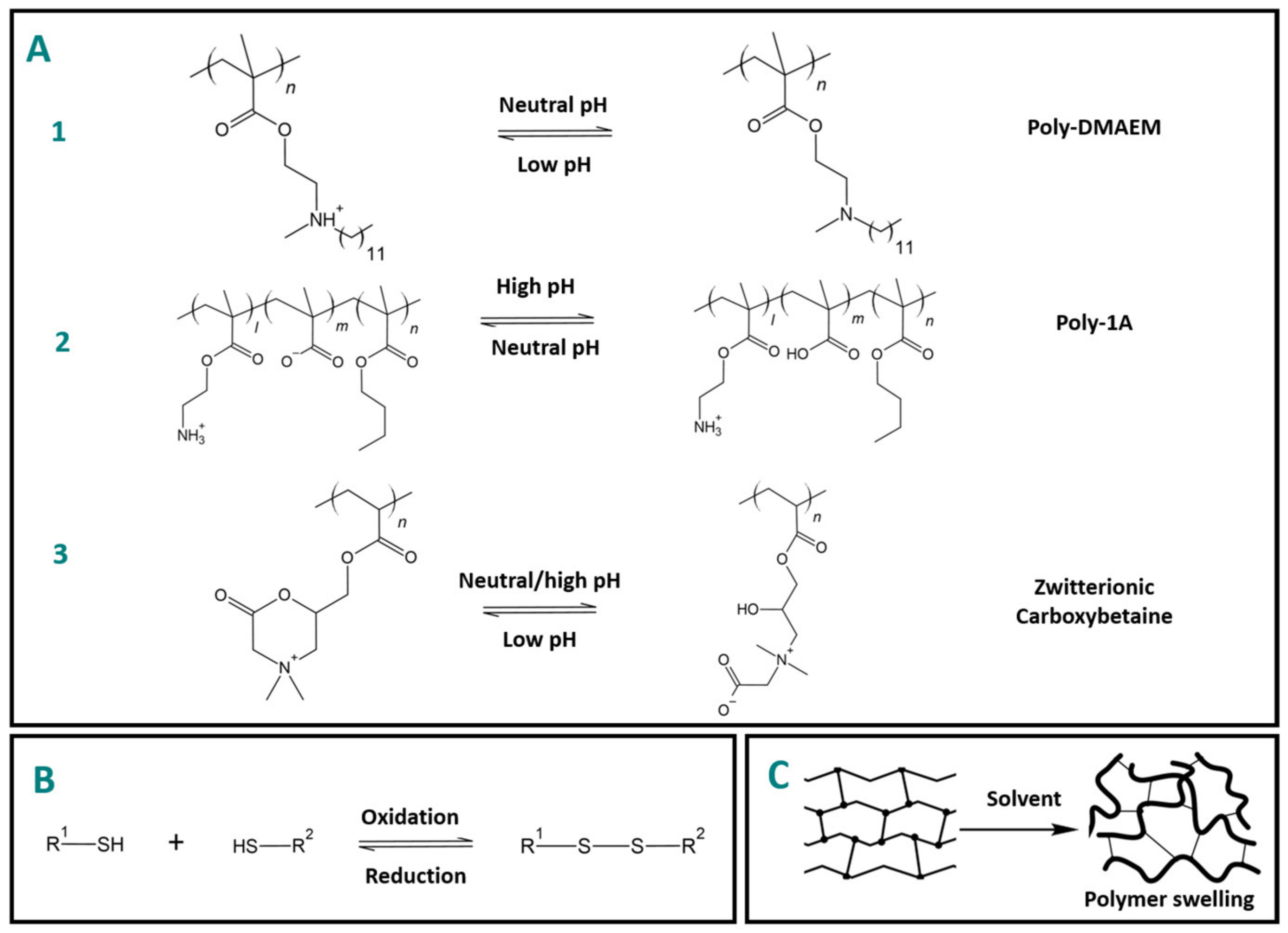
2.2.3. Solvent
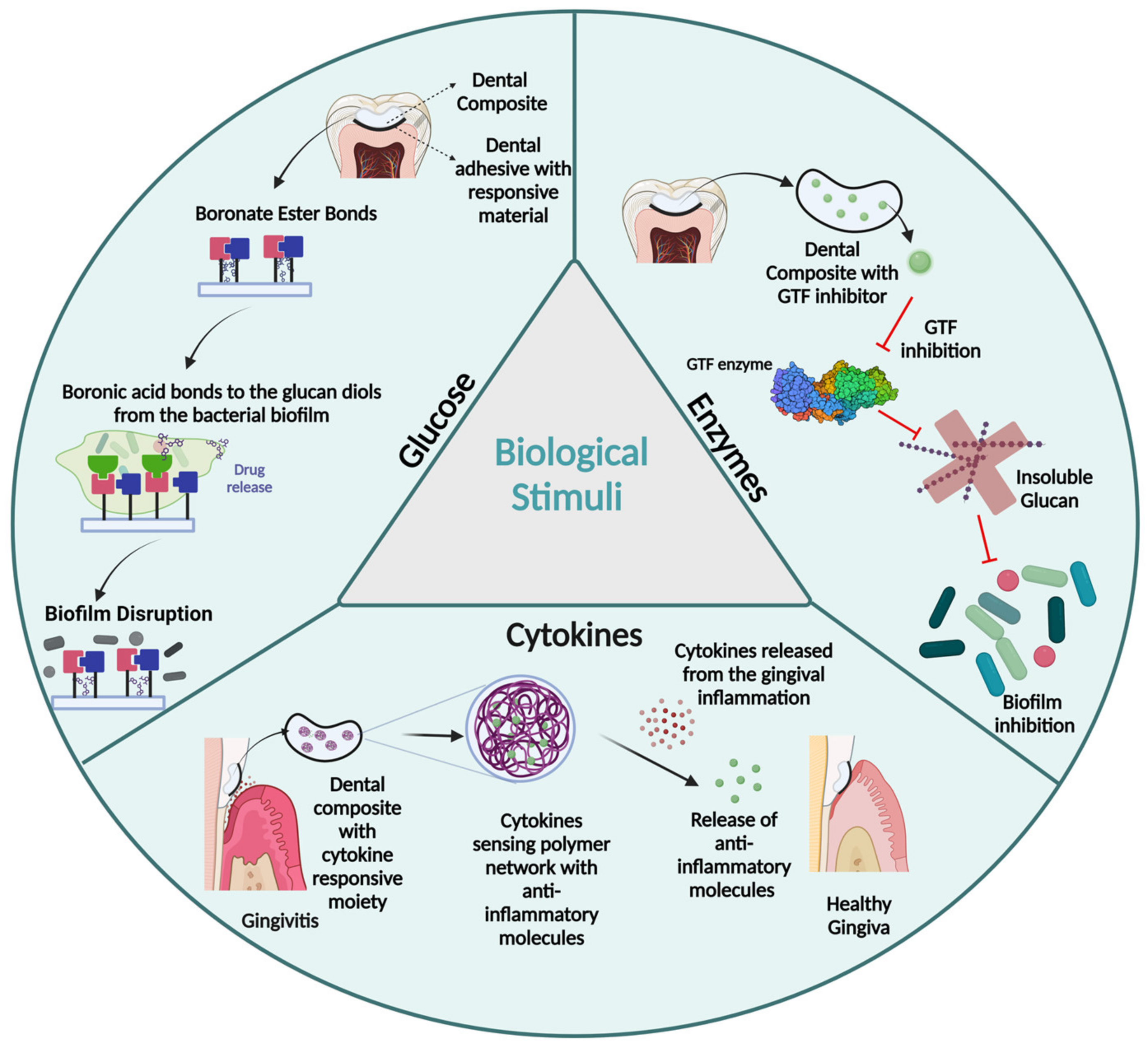
2.3. Biological-Stimuli-Responsive Polymers
2.3.1. Glucose
2.3.2. Enzymes
2.3.3. Cytokines
2.4. Multi-Stimuli Responsive Systems
3. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganesh, V.A.; Baji, A.; Ramakrishna, S. Smart functional polymers—A new route towards creating a sustainable environment. RSC Adv. 2014, 4, 53352–53364. [Google Scholar] [CrossRef]
- Urban, M.W. Stimuli-Responsive Materials: From Molecules to Nature Mimicking Materials Design; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Wiggins, K.M.; Brantley, J.N.; Bielawski, C.W. Methods for activating and characterizing mechanically responsive polymers. Chem. Soc. Rev. 2013, 42, 7130–7147. [Google Scholar] [CrossRef]
- Blaiszik, B.; Kramer, S.; Olugebefola, S.; Moore, J.; Sottos, N.; White, S. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Melo, M.A.S.; Strassler, H.E.; Xu, H.H. Effects of water-aging on self-healing dental composite containing microcapsules. J. Dent. 2016, 47, 86–93. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Ferracane, J.L.; Pfeifer, C.S. Fatigue-Crack Propagation Behavior in Microcapsule-Containing Self-Healing Polymeric Networks. Mater. Des. 2022, 223, 111142. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Loomans, B.; Yeung, C.; Li, J.; Yang, F.; Leeuwenburgh, S. Influence of microcapsule parameters and initiator concentration on the self-healing capacity of resin-based dental composites. Dent. Mater. 2021, 37, 403–412. [Google Scholar] [CrossRef]
- Park, H.Y.; Kloxin, C.J.; Fordney, M.F.; Bowman, C.N. Stress relaxation of trithiocarbonate-dimethacrylate-based dental composites. Dent. Mater. 2012, 28, 888–893. [Google Scholar] [CrossRef][Green Version]
- Fugolin, A.P.P.; Costa, A.R.; Lewis, S.H.; Goulart, M.; Erhardt, M.C.; Pfeifer, C.S. Probing stress relaxation behavior in glassy methacrylate networks containing thio-carbamate additives. J. Mater. Chem. B 2021, 9, 3015–3024. [Google Scholar] [CrossRef]
- Schenzel, A.M.; Klein, C.; Rist, K.; Moszner, N.; Barner-Kowollik, C. Reversing Adhesion: A Triggered Release Self-Reporting Adhesive. Adv. Sci. 2016, 3, 1500361. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Nuruzzaman, M.; Mondal, M.I.H. 10—Photo-responsive hydrogel-treated fabrics for smart drug delivery systems. In Medical Textiles from Natural Resources; Mondal, M.I.H., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 315–338. [Google Scholar]
- Francis, R.; Gopalan, G.P.; Sivadas, A.; Joy, N. Properties of Stimuli-Responsive Polymers. In Biomedical Applications of Polymeric Materials and Composites; Wiley: Hoboken, NJ, USA, 2016; pp. 187–231. [Google Scholar]
- Zhao, Y.-L.; Stoddart, J.F. Azobenzene-based light-responsive hydrogel system. Langmuir 2009, 25, 8442–8446. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Tamura, A.; Fushimi, M.; Santa, H.; Arisaka, Y.; Nikaido, T.; Tagami, J.; Yui, N. Light-embrittled dental resin cements containing photodegradable polyrotaxane cross-linkers for attenuating debonding strength. ACS Appl. Polym. Mater. 2020, 2, 5756–5766. [Google Scholar] [CrossRef]
- Filipcsei, G.; Csetneki, I.; Szilágyi, A.; Zrínyi, M. Magnetic field-responsive smart polymer composites. In Oligomers-Polymer Composites-Molecular Imprinting; Springer: Berlin/Heidelberg, Germany, 2007; pp. 137–189. [Google Scholar]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Nithin, K.S.; Sachhidananda, S.; Shilpa, K.N.; Sandeep, S.; Karthik, C.S.; Raj, B.M.J.; Siddaramaiah, H. Polymer-based smart composites and/or nanocomposites for optical, optoelectronic, and energy applications: A brief introduction. In Polymer-Based Advanced Functional Composites for Optoelectronic and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–29. [Google Scholar]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Zhou, X.; Zhu, C.; Han, Q.; Wang, H.; Xu, H.H.; Ren, B.; Cheng, L. Intelligent pH-responsive dental sealants to prevent long-term microleakage. Dent. Mater. 2021, 37, 1529–1541. [Google Scholar] [CrossRef]
- Bhat, R.; Godovikova, V.; Flannagan, S.E.; Li, Y.; Seseogullari-Dirihan, R.; González-Cabezas, C.; Kuroda, K. Targeting Cariogenic Streptococcus mutans in Oral Biofilms with Charge-Switching Smart Antimicrobial Polymers. ACS Biomater. Sci. Eng. 2023, 9, 318–328. [Google Scholar] [CrossRef]
- Cheng, G.; Li, G.; Xue, H.; Chen, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef]
- Jin, J.; Kim, J.-Y.; Choi, W.; Lee, M.-J.; Seo, J.-Y.; Yu, J.; Kwon, J.-S.; Hong, J.; Choi, S.-H. Incorporation of carboxybetaine methacrylate into poly (methyl methacrylate) to prevent multi-species biofilm formation. J. Ind. Eng. Chem. 2020, 86, 194–204. [Google Scholar] [CrossRef]
- Kim, D.; Lee, M.J.; Kim, J.Y.; Lee, D.; Kwon, J.S.; Choi, S.H. Incorporation of zwitterionic materials into light-curable fluoride varnish for biofilm inhibition and caries prevention. Sci. Rep. 2019, 9, 19550. [Google Scholar] [CrossRef]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent progress and advances in stimuli-responsive polymers for cancer therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Tirelli, N. Oxidation-responsive polymers: Which groups to use, how to make them, what to expect from them (biomedical applications). Macromol. Chem. Phys. 2013, 214, 143–158. [Google Scholar]
- Liu, J.; Jia, B.; Li, Z.; Li, W. Reactive oxygen species-responsive polymer drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1115603. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.-W.; Zhang, W.-J.; Hong, C.-Y.; Pan, C.-Y. pH-and reductant-responsive polymeric vesicles with robust membrane-cross-linked structures: In situ cross-linking in polymerization-induced self-assembly. Macromolecules 2019, 52, 1140–1149. [Google Scholar]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Hollmann, B.; Perkins, M.; Chauhan, V.M.; Aylott, J.W.; Hardie, K.R. Fluorescent nanosensors reveal dynamic pH gradients during biofilm formation. NPJ Biofilms Microbiomes 2021, 7, 50. [Google Scholar] [CrossRef]
- Nagy, P. Kinetics and mechanisms of thiol–disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Grocke, G.L.; Zhang, H.; Kopfinger, S.S.; Patel, S.N.; Rowan, S.J. Synthesis and Characterization of Redox-Responsive Disulfide Cross-Linked Polymer Particles for Energy Storage Applications. ACS Macro Lett. 2021, 10, 1637–1642. [Google Scholar] [CrossRef]
- Cimatu, K.L.; Ambagaspitiya, T.D.; Premadasa, U.I.; Adhikari, N.M.; Kruse, A.; Robertson, E.; Guan, S.; Rong, L.; Advincula, R.; Bythell, B.J. Polymer-solvent interaction and conformational changes at a molecular level: Implication to solvent-assisted deformation and aggregation at the polymer surface. J. Colloid Interface Sci. 2022, 616, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Bandyopadhyay, A. Solvent Responsive Shape Memory Polymers-Evolution, Current Status, and Future Outlook. Macromol. Chem. Phys. 2021, 222, 2100195. [Google Scholar] [CrossRef]
- Asha, A.B.; Srinivas, S.; Hao, X.; Narain, R. Chapter 5—Enzyme-Responsive Polymers: Classifications, Properties, Synthesis Strategies, and Applications. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 155–189. [Google Scholar]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhang, Y.; Zhang, X.; Kahkoska, A.R.; Chen, G.; Wang, Z.; Sun, W.; Cai, L.; Chen, Z.; et al. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 2019, 5, eaaw4357. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.; Sauer, U. Systems biology of microbial metabolism. Curr. Opin. Microbiol. 2010, 13, 337–343. [Google Scholar] [CrossRef]
- Scaffa, P.M.C.; Kendall, A.; Icimoto, M.Y.; Fugolin, A.P.P.; Logan, M.G.; DeVito-Moraes, A.G.; Lewis, S.H.; Zhang, H.; Wu, H.; Pfeifer, C.S. The potential use of glycosyl-transferase inhibitors for targeted reduction of S. mutans biofilms in dental materials. Sci. Rep. 2023, 13, 11889. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018, 76, 283–294. [Google Scholar] [CrossRef]
- Schattling, P.; Jochum, D.F.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Schönfeld, D.; Koss, S.; Vohl, N.; Friess, F.; Drescher, D.; Pretsch, T. Dual Stimuli-Responsive Orthodontic Aligners: An in Vitro Study. Materials 2023, 16, 3094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fugolin, A.P.P.; Huynh, B.; Rajasekaran, S.P. Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers. Polymers 2023, 15, 3346. https://doi.org/10.3390/polym15163346
Fugolin APP, Huynh B, Rajasekaran SP. Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers. Polymers. 2023; 15(16):3346. https://doi.org/10.3390/polym15163346
Chicago/Turabian StyleFugolin, Ana Paula P., Bao Huynh, and Sivashankari P. Rajasekaran. 2023. "Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers" Polymers 15, no. 16: 3346. https://doi.org/10.3390/polym15163346
APA StyleFugolin, A. P. P., Huynh, B., & Rajasekaran, S. P. (2023). Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers. Polymers, 15(16), 3346. https://doi.org/10.3390/polym15163346







