Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Modified Pectins
2.2. Preparation of Modified Pectins Films
2.3. Preparation of Modified Pectin Nanoparticles
2.4. Characterization of Modified Pectin Films
2.5. Characterization of Modified Pectin Nanoparticles
2.6. In Vitro Cell Proliferation Effect
2.7. Statistical Analysis
3. Results
3.1. Characterization of Modified Pectin Films
3.1.1. Films Morphology
3.1.2. Viscoelastic Properties of Films
3.2. Characterization of Modified Pectin Nanoparticles
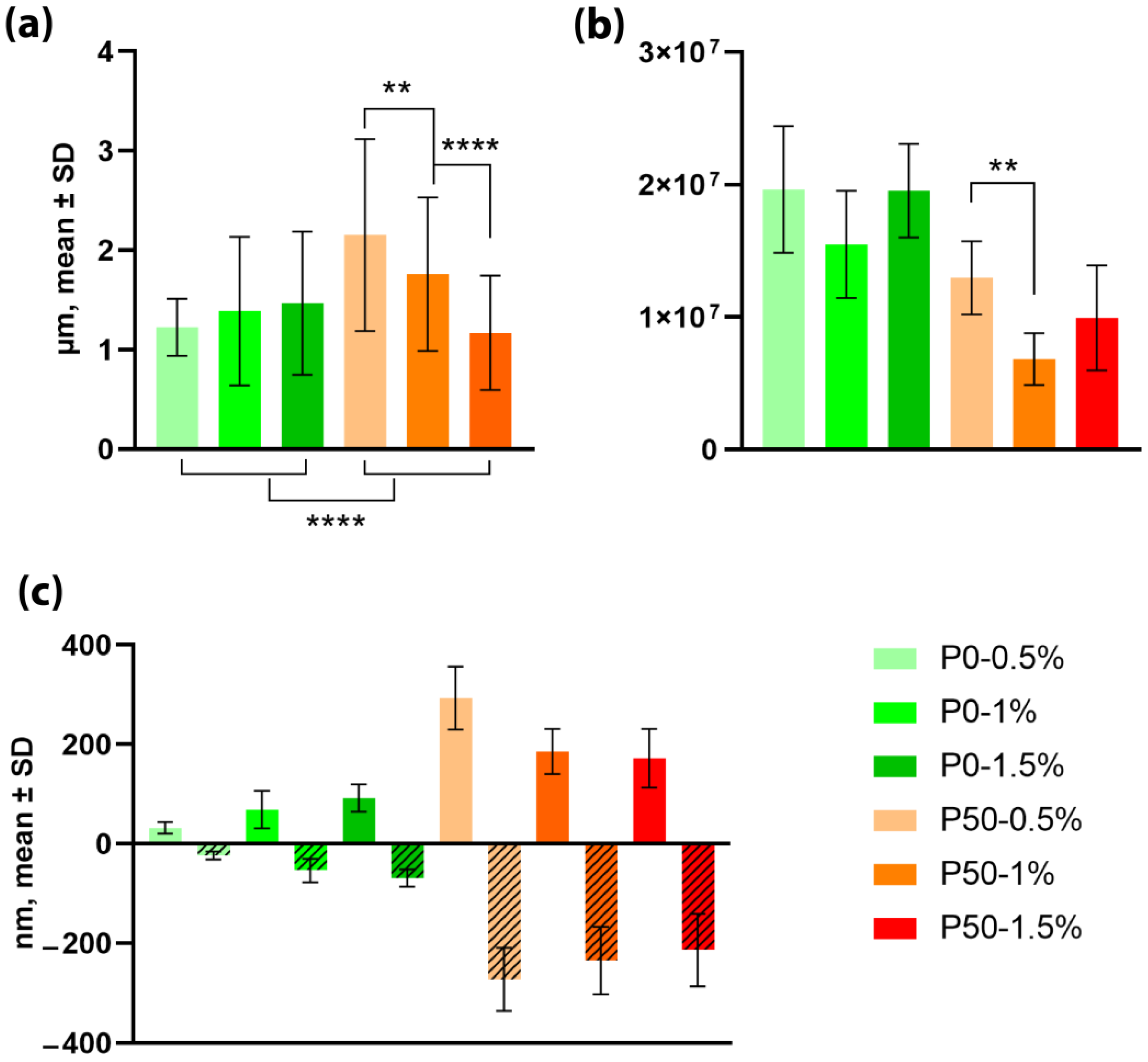
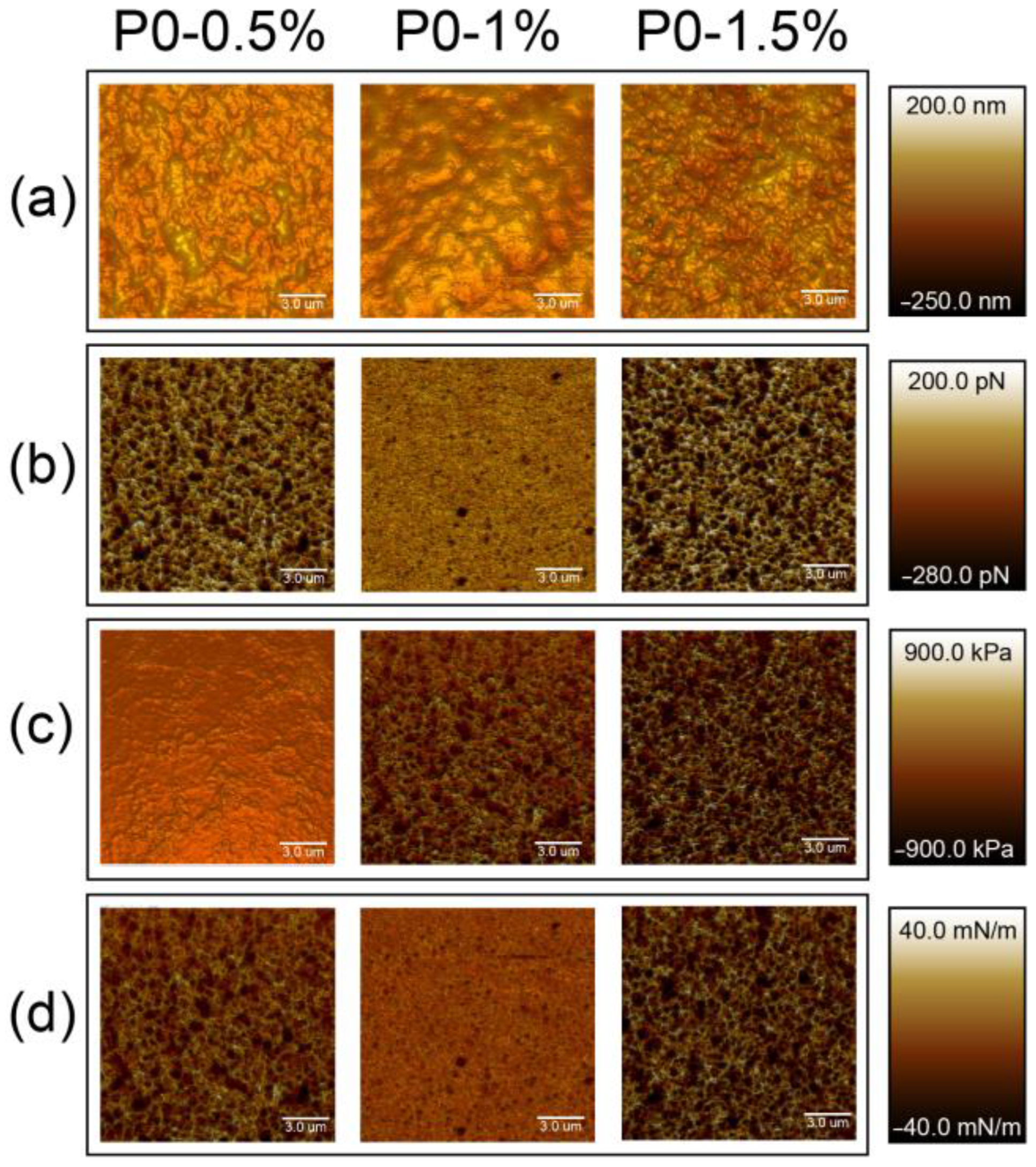
3.2.1. Nanoparticles’ Morphology
3.2.2. Mechanical Properties of Nanoparticles
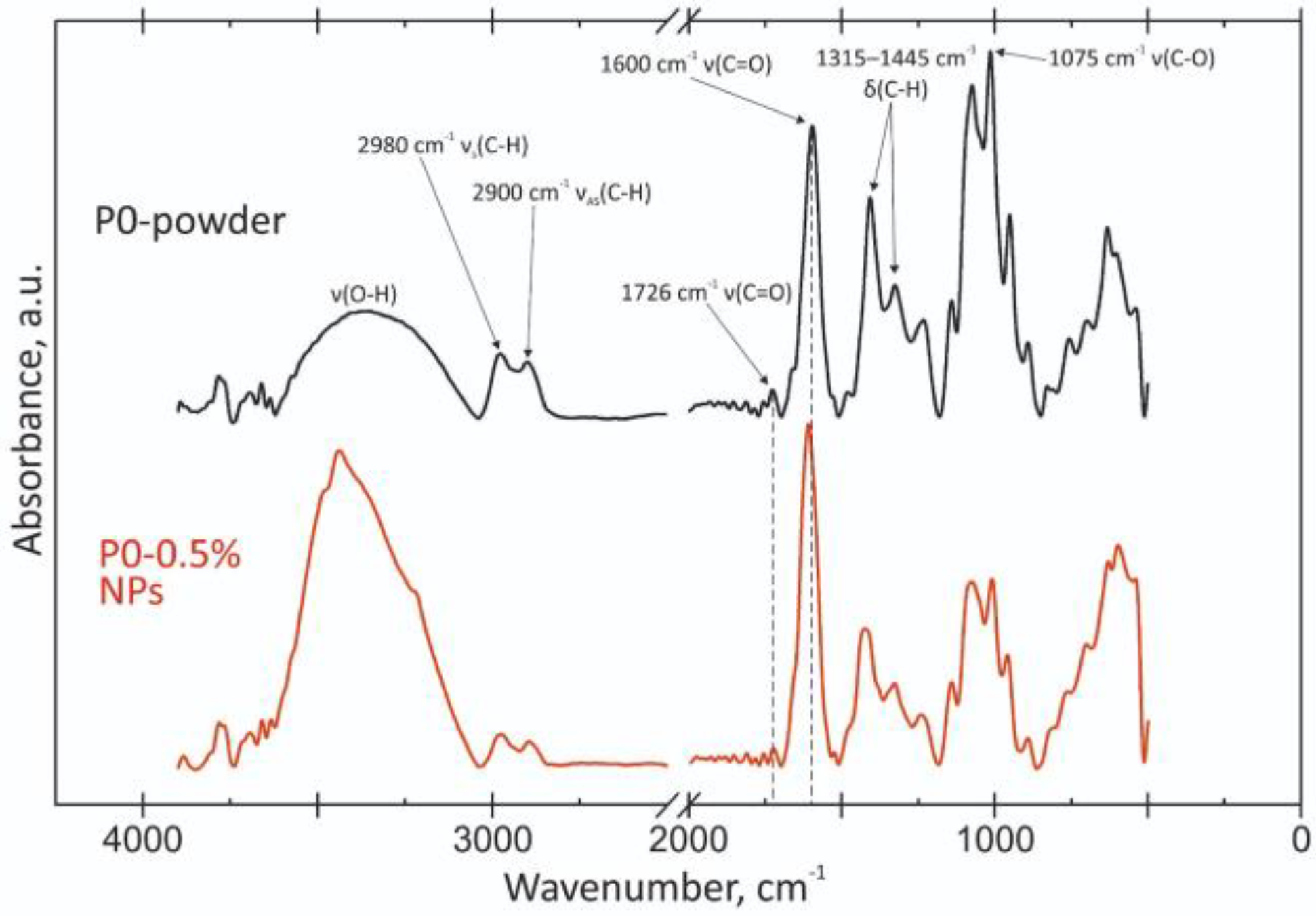
3.2.3. FTIR Spectroscopy of Nanoparticles
3.3. Biocompatibility of Modified Pectin Films and Nanoparticles
3.3.1. Effect on Metabolic Activity of Cells
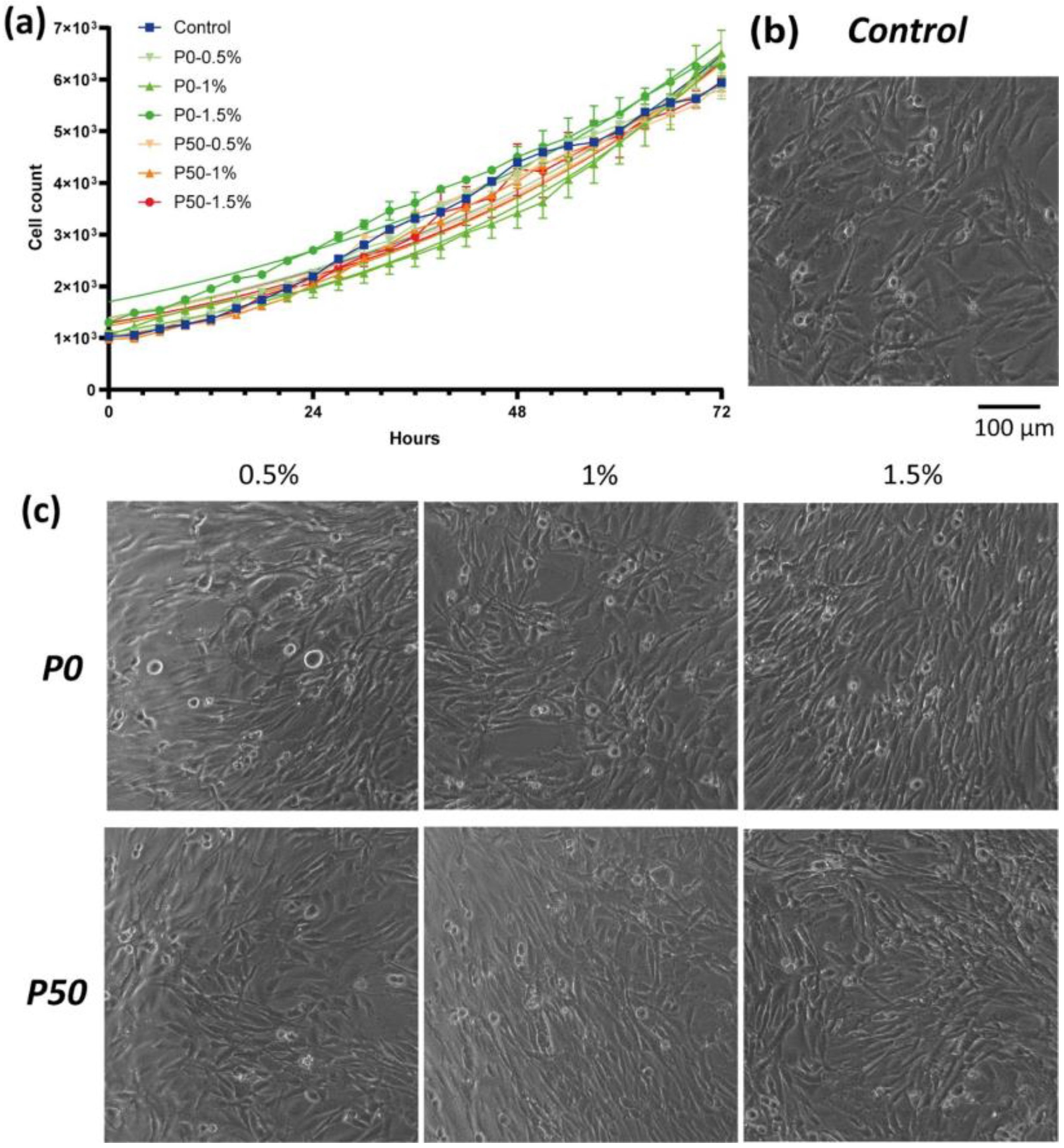
3.3.2. Effect on Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscope |
| BBB | blood–brain barrier |
| DE | esterification degree |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FTIR | Fourier-transform infrared spectroscopy |
| HA | hyaluronic acid |
| HEPES | N-(2-hydroxyethyl)-piperazine-N’-2-ethanesulfonic acid |
| NP | nanoparticle |
| P0 | pectins with an esterification degree of 0% |
| P50 | pectins with an esterification degree of 50% |
References
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int. J. Mol. Sci. 2021, 22, 11654. [Google Scholar] [CrossRef] [PubMed]
- Koziara, J.M.; Lockman, P.R.; Allen, D.D.; Mumper, R.J. Paclitaxel Nanoparticles for the Potential Treatment of Brain Tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ningaraj, N.S. Drug Delivery to Brain Tumours: Challenges and Progress. Expert Opin. Drug Deliv. 2006, 3, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Mooney, D.J. Biomaterials and Emerging Anticancer Therapeutics: Engineering the Microenvironment. Nat. Rev. Cancer 2016, 16, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Kwok, J.C.F.; Fawcett, J.W. Neural ECM in Regeneration and Rehabilitation. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 214, pp. 179–192. ISBN 978-0-444-63486-3. [Google Scholar]
- Ruoslahti, E. Brain Extracellular Matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Burnside, E.R.; Bradbury, E.J. Review: Manipulating the Extracellular Matrix and Its Role in Brain and Spinal Cord Plasticity and Repair: Manipulating the Matrix for CNS Repair. Neuropathol. Appl. Neurobiol. 2014, 40, 26–59. [Google Scholar] [CrossRef]
- Belousov, A.; Titov, S.; Shved, N.; Malykin, G.; Kovalev, V.; Suprunova, I.; Khotimchenko, Y.; Kumeiko, V. Hydrogels Based on Modified Pectins Capable of Modulating Neural Cell Behavior as Prospective Biomaterials in Glioblastoma Treatment. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 151, pp. 111–138. ISBN 978-0-12-821114-4. [Google Scholar]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate Nanocarriers in Biomedical Applications: Functionalization and Construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [Green Version]
- Belousov, A.; Titov, S.; Shved, N.; Garbuz, M.; Malykin, G.; Gulaia, V.; Kagansky, A.; Kumeiko, V. The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment. Front. Bioeng. Biotechnol. 2019, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Curcio, M.; Cirillo, G.; Rouaen, J.R.C.; Saletta, F.; Nicoletta, F.P.; Vittorio, O.; Iemma, F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics 2020, 12, 1183. [Google Scholar] [CrossRef]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-Tumor Biomacromolecules —A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Cui, X.T. Carbohydrate Based Biomaterials for Neural Interface Applications. J. Mater. Chem. B 2022, 10, 4714–4740. [Google Scholar] [CrossRef] [PubMed]
- Koleva, P.M.; Keefer, J.H.; Ayala, A.M.; Lorenzo, I.; Han, C.E.; Pham, K.; Ralston, S.E.; Kim, K.D.; Lee, C.C. Hyper-Crosslinked Carbohydrate Polymer for Repair of Critical-Sized Bone Defects. BioRes. Open Access 2019, 8, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombino, S.; Curcio, F.; Poerio, T.; Pellegrino, M.; Russo, R.; Cassano, R. Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer. Molecules 2021, 26, 1889. [Google Scholar] [CrossRef]
- Stealey, S.; Guo, X.; Majewski, R.; Dyble, A.; Lehman, K.; Wedemeyer, M.; Steeber, D.A.; Kaltchev, M.G.; Chen, J.; Zhang, W. Calcium-Oligochitosan-Pectin Microcarrier for Colonic Drug Delivery. Pharm. Dev. Technol. 2020, 25, 260–265. [Google Scholar] [CrossRef]
- Potaś, J.; Wilczewska, A.Z.; Misiak, P.; Basa, A.; Winnicka, K. Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery. Int. J. Mol. Sci. 2022, 23, 8092. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and Biocompatibility Evaluation of Pectin and Chitosan Cryogels for Biomedical Application. J. Biomed. Mater. Res. 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Hiremath, L.; Vantagodi, S.; Hegde, S.S.; Anitha, G.S.; Keshamma, E. Development and Characterization of Pectin and Chitosan Based Biocomposite Material for Bio-Medical Application. J. Mater. Sci. Eng. 2021, 10, 9. [Google Scholar]
- Meneguin, A.B.; Ferreira Cury, B.S.; Dos Santos, A.M.; Franco, D.F.; Barud, H.S.; Da Silva Filho, E.C. Resistant Starch/Pectin Free-Standing Films Reinforced with Nanocellulose Intended for Colonic Methotrexate Release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, Y.; Li, J.; Liu, J.; Liu, Z.; Li, D. A Novel and Simple Oral Colon-Specific Drug Delivery System Based on the Pectin/Modified Nano-Carbon Sphere Nanocomposite Gel Films. Int. J. Biol. Macromol. 2020, 157, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Gyasi, M.E.; Owusu, F.W.A.; Entsie, P.; Agbenorhevi, J.K.; Banful, B.K.B.; Bayor, M.T. Pectin from Okra (Abelmoschus esculentus L.) Has Potential as a Drug Release Modifier in Matrix Tablets. Sci. World J. 2021, 2021, 6672277. [Google Scholar] [CrossRef] [PubMed]
- Riccio, B.V.F.; Silvestre, A.L.P.; Meneguin, A.B.; Ribeiro, T.D.C.; Klosowski, A.B.; Ferrari, P.C.; Chorilli, M. Exploiting Polymeric Films as a Multipurpose Drug Delivery System: A Review. AAPS PharmSciTech 2022, 23, 269. [Google Scholar] [CrossRef]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the Mechanobiology of Malignant Brain Tumors Using a Brain Matrix-Mimetic Hyaluronic Acid Hydrogel Platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef] [Green Version]
- Streitberger, K.-J.; Lilaj, L.; Schrank, F.; Braun, J.; Hoffmann, K.-T.; Reiss-Zimmermann, M.; Käs, J.A.; Sack, I. How Tissue Fluidity Influences Brain Tumor Progression. Proc. Natl. Acad. Sci. USA 2020, 117, 128–134. [Google Scholar] [CrossRef]
- Khotimchenko, Y.; Khozhaenko, E.; Kovalev, V.; Khotimchenko, M. Cerium Binding Activity of Pectins Isolated from the Seagrasses Zostera Marina and Phyllospadix Iwatensis. Mar. Drugs 2012, 10, 834–848. [Google Scholar] [CrossRef] [Green Version]
- Khotimchenko, M.Y.; Kolenchenko, E.A.; Khotimchenko, Y.S.; Khozhaenko, E.V.; Kovalev, V.V. Cerium Binding Activity of Different Pectin Compounds in Aqueous Solutions. Colloids Surf. B Biointerfaces 2010, 77, 104–110. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Pappas, C.S.; Malovikova, A.; Hromadkova, Z.; Tarantilis, P.A.; Ebringerova, A.; Polissiou, M.G. Determination of the Degree of Esterification of Pectinates with Decyl and Benzyl Ester Groups by Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) and Curve-Fitting Deconvolution Method. Carbohydr. Polym. 2004, 56, 465–469. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of Pectin-Sodium Alginate Based Films for Potential Healthcare Application: Study of Chemico-Physical Interactions between the Components of Films and Assessment of Their Antimicrobial Activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.I.; Ng, S.-F. Optimization, Characterization, and in Vitro Assessment of Alginate-Pectin Ionic Cross-Linked Hydrogel Film for Wound Dressing Applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and Characterization of Low Methoxyl Pectin/Gelatin/Carboxymethyl Cellulose Absorbent Hydrogel Film for Wound Dressing Applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocaaga, B.; Kurkcuoglu, O.; Tatlier, M.; Batirel, S.; Guner, F.S. Low-methoxyl Pectin–Zeolite Hydrogels Controlling Drug Release Promote in Vitro Wound Healing. J. Appl. Polym. Sci. 2019, 136, 47640. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Kim, H.; Yoo, J.-W. Development of Clindamycin-Loaded Alginate/Pectin/Hyaluronic Acid Composite Hydrogel Film for the Treatment of MRSA-Infected Wounds. J. Pharm. Investig. 2021, 51, 597–610. [Google Scholar] [CrossRef]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential Use of Propolis-Loaded Quaternized Chitosan/Pectin Hydrogel Films as Wound Dressings: Preparation, Characterization, Antibacterial Evaluation, and in Vitro Healing Assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef]
- Clifford, A.; D’Elia, A.; Deering, J.; Lee, B.E.J.; Grandfield, K.; Zhitomirsky, I. Electrochemical Fabrication and Characterization of Pectin Hydrogel Composite Materials for Bone Tissue Repair. ACS Appl. Polym. Mater. 2020, 2, 3390–3396. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of Pectin in Oral Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Moideen, M.M.J.; Karuppaiyan, K.; Kandhasamy, R.; Seetharaman, S. Skimmed Milk Powder and Pectin Decorated Solid Lipid Nanoparticle Containing Soluble Curcumin Used for the Treatment of Colorectal Cancer. J. Food Process Eng. 2020, 43. [Google Scholar] [CrossRef]
- Katas, H.; Mohd Amin, M.C.I.; Moideen, N.; Ng, L.Y.; Megat Baharudin, P.A.A. Cell Growth Inhibition Effect of DsiRNA Vectorised by Pectin-Coated Chitosan-Graphene Oxide Nanocomposites as Potential Therapy for Colon Cancer. J. Nanomater. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-P.; Jiang, M.-C.; Chen, B.; Gao, P.; Yang, S.; Liu, Y.-F.; Ye, P.-J.; He, D.-X.; Huang, H.-L.; Yu, C.-Y. Fabrication and Characterization of a Novel Self-Assembling Micelle Based on Chitosan Cross-Linked Pectin–Doxorubicin Conjugates Macromolecular pro-Drug for Targeted Cancer Therapy. RSC Adv. 2018, 8, 12004–12016. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.-J.; Huang, C.; Yang, S.; Gao, P.; Li, Z.-P.; Tang, S.-Y.; Xiang, Y.; Liu, Y.-F.; Chen, Y.-P.; He, D.-X.; et al. Facile Fabrication of a Novel Hybrid Nanoparticles by Self-Assembling Based on Pectin-Doxorubicin Conjugates for Hepatocellular Carcinoma Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Xu, W.; Liang, H.; He, L.; Liu, S.; Li, Y.; Li, B.; Chen, Y. Construction of PH-Sensitive Lysozyme/Pectin Nanogel for Tumor Methotrexate Delivery. Colloids Surf. B Biointerfaces 2015, 126, 459–466. [Google Scholar] [CrossRef]
- Amaral, S.D.C.; Barbieri, S.F.; Ruthes, A.C.; Bark, J.M.; Brochado Winnischofer, S.M.; Silveira, J.L.M. Cytotoxic Effect of Crude and Purified Pectins from Campomanesia Xanthocarpa Berg on Human Glioblastoma Cells. Carbohydr. Polym. 2019, 224, 115140. [Google Scholar] [CrossRef]
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M.; et al. Etoposide and Olaparib Polymer-Coated Nanoparticles within a Bioadhesive Sprayable Hydrogel for Post-Surgical Localised Delivery to Brain Tumours. Eur. J. Pharm. Biopharm. 2020, 157, 108–120. [Google Scholar] [CrossRef]
- Ouyang, J.; Yang, M.; Gong, T.; Ou, J.; Tan, Y.; Zhang, Z.; Li, S. Doxorubicin-Loading Core-Shell Pectin Nanocell: A Novel Nanovehicle for Anticancer Agent Delivery with Multidrug Resistance Reversal. PLoS ONE 2020, 15, e0235090. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Qin, Z.; Yang, B.; Zhang, E.; Dong, D.; Wang, J.; Wen, Y.; Tian, L.; Yao, F. Engineering Pectin-Based Hollow Nanocapsules for Delivery of Anticancer Drug. Carbohydr. Polym. 2017, 177, 86–96. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle Crossing of Blood–Brain Barrier: A Road to New Therapeutic Approaches to Central Nervous System Diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Tamilselvi, S.; Kavitha, R.; Usharani, M.; Mumjitha, M.; Mohanapriya, S.; MohanaPriya, S. Mechanical Characterization of Bio Composite Films as a Novel Drug Carrier Platform for Sustained Release of 5-Fluorouracil for Colon Cancer: Methodological Investigation. J. Mech. Behav. Biomed. Mater. 2021, 115, 104266. [Google Scholar] [CrossRef]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of Polymer Micelles Affects Interactions with Tumor Cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable Rigidity of (Polymeric Core)-(Lipid Shell) Nanoparticles for Regulated Cellular Uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]








| Sample | Ca2+ (mM) |
|---|---|
| P0-0.5% | 1.33 |
| P0-1% | 2.66 |
| P0-1.5% | 4 |
| P50-0.5% | 4 |
| P50-1% | 8 |
| P50-1.5% | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patlay, A.A.; Belousov, A.S.; Silant’ev, V.E.; Shatilov, R.A.; Shmelev, M.E.; Kovalev, V.V.; Perminova, I.V.; Baklanov, I.N.; Kumeiko, V.V. Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications. Polymers 2023, 15, 3280. https://doi.org/10.3390/polym15153280
Patlay AA, Belousov AS, Silant’ev VE, Shatilov RA, Shmelev ME, Kovalev VV, Perminova IV, Baklanov IN, Kumeiko VV. Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications. Polymers. 2023; 15(15):3280. https://doi.org/10.3390/polym15153280
Chicago/Turabian StylePatlay, Aleksandra A., Andrei S. Belousov, Vladimir E. Silant’ev, Roman A. Shatilov, Mikhail E. Shmelev, Valeri V. Kovalev, Irina V. Perminova, Ivan N. Baklanov, and Vadim V. Kumeiko. 2023. "Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications" Polymers 15, no. 15: 3280. https://doi.org/10.3390/polym15153280
APA StylePatlay, A. A., Belousov, A. S., Silant’ev, V. E., Shatilov, R. A., Shmelev, M. E., Kovalev, V. V., Perminova, I. V., Baklanov, I. N., & Kumeiko, V. V. (2023). Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications. Polymers, 15(15), 3280. https://doi.org/10.3390/polym15153280






