Valorization of Glucose-Derived Humin as a Low-Cost, Green, Reusable Adsorbent for Dye Removal, and Modeling the Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Collection of GDH Byproduct
2.3. Characterization of GDH
2.4. Sample Reactor for Dye Adsorption and Analysis
2.5. Use GDH as a Dye Adsorbent
2.6. Effect of Parameters on Dye Adsorption
2.7. Adsorption Kinetics and Thermodynamics
2.8. Adsorption Isotherm Models
2.9. Response Surface Methodology
2.10. Regeneration and Reuse
3. Results and Discussion
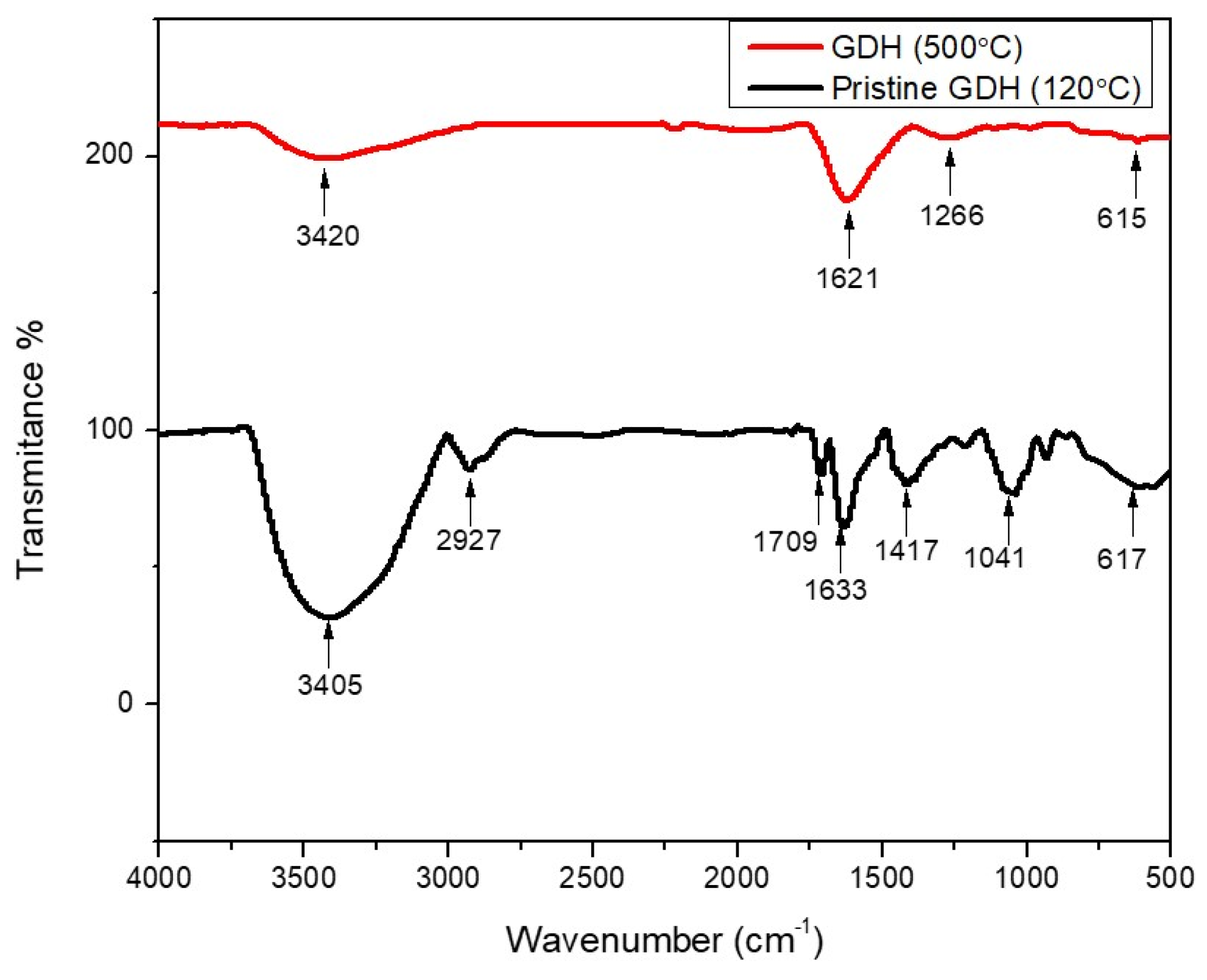
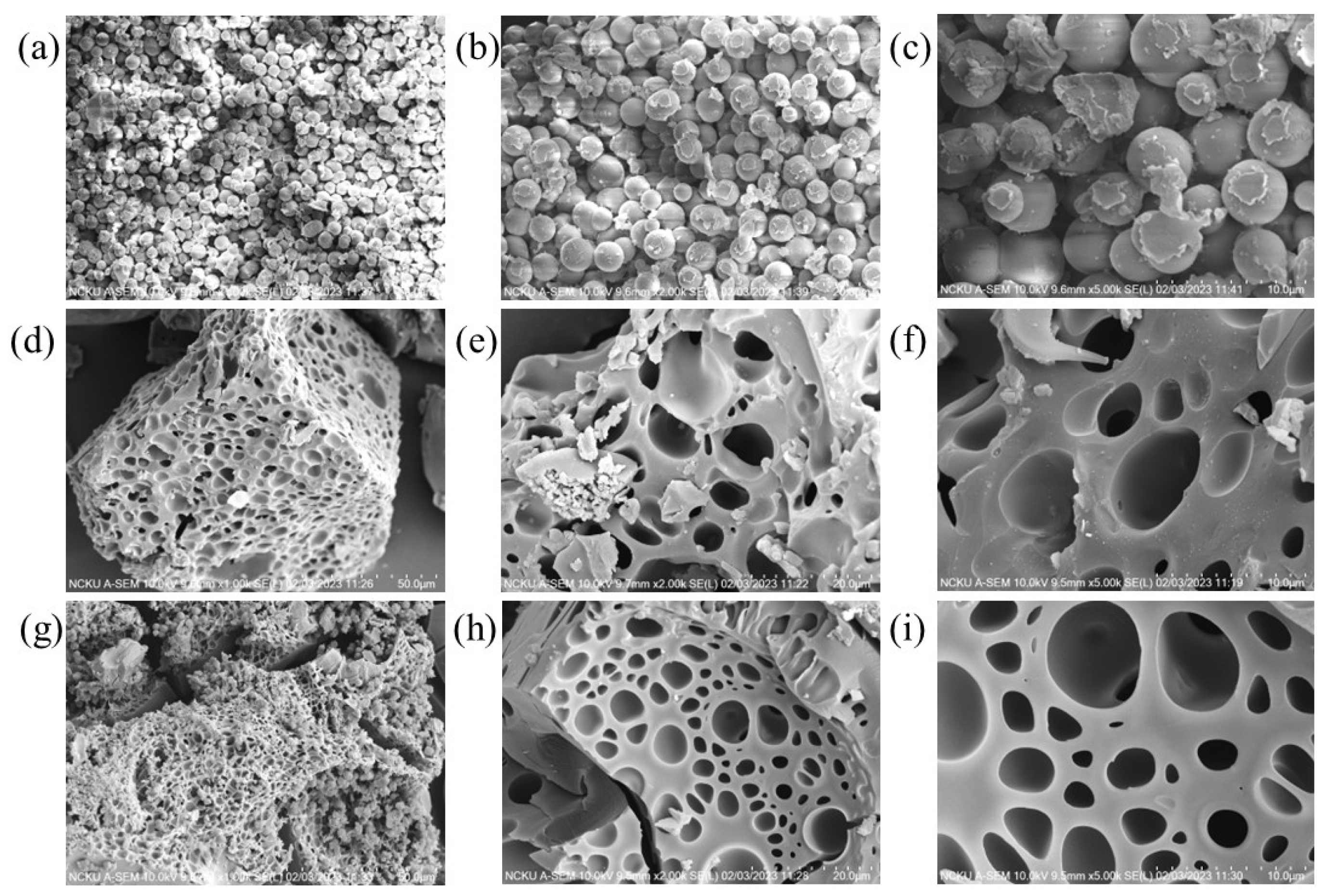
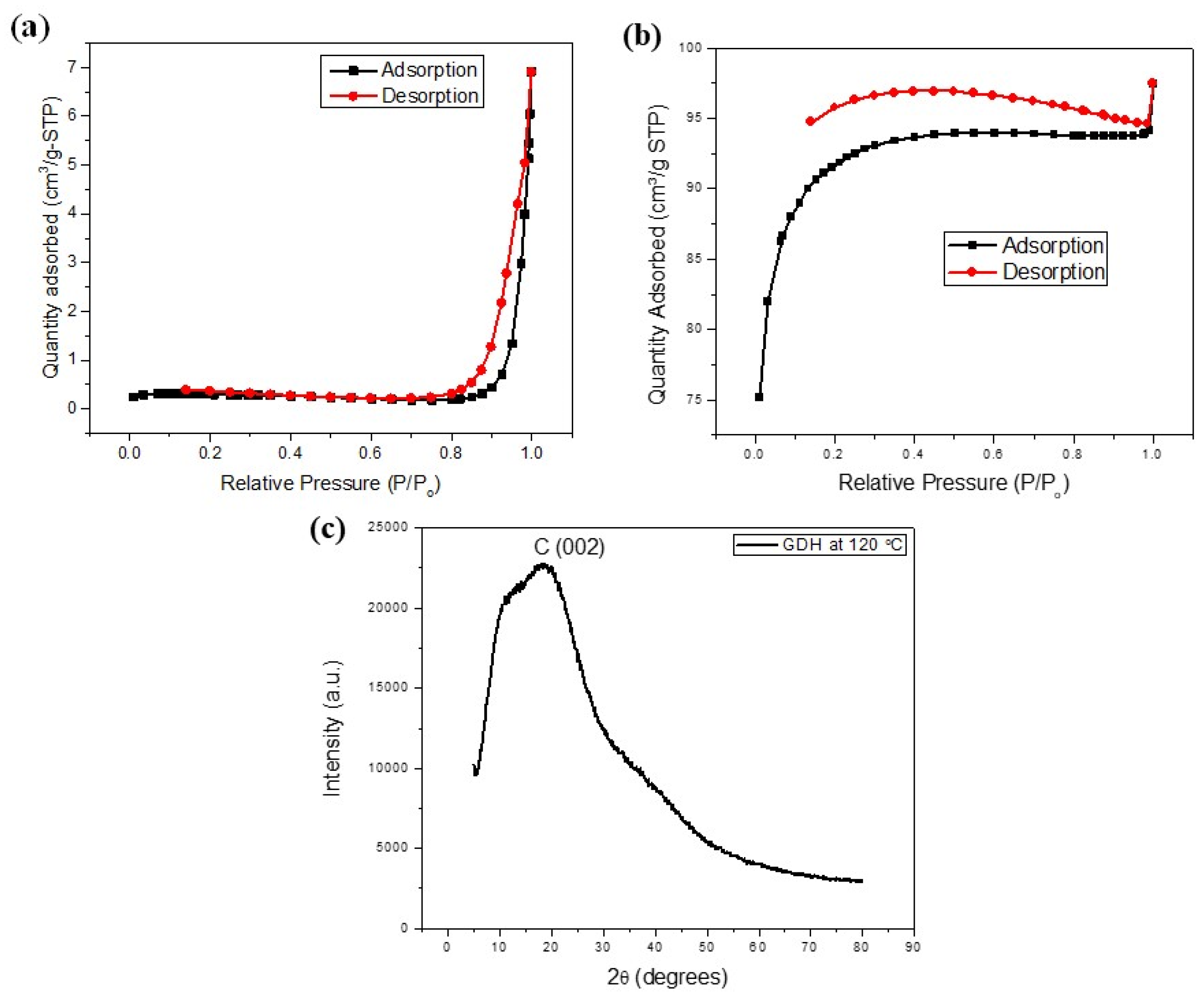
3.1. Characterization of GDH
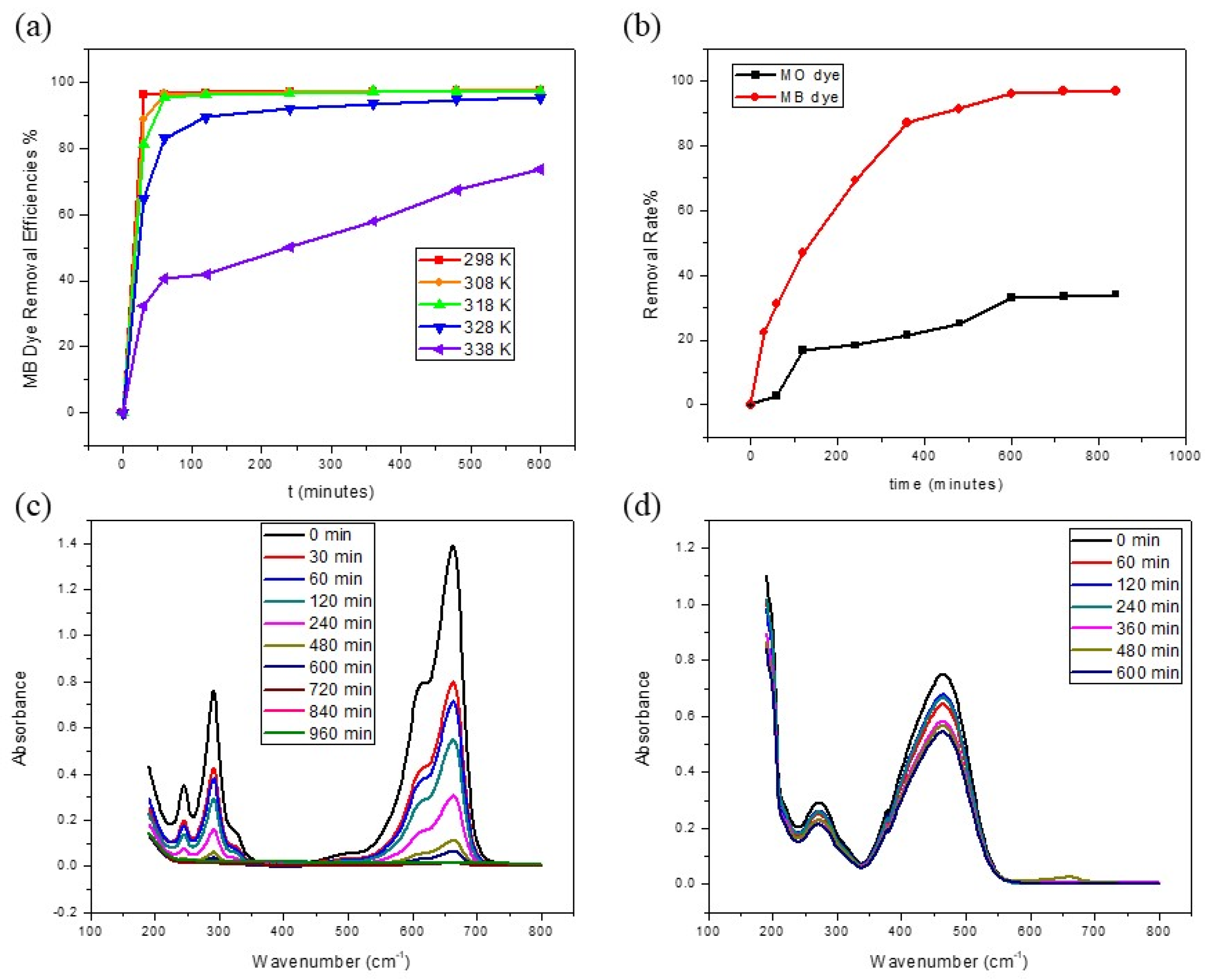
3.2. Effects of Different Parameters on MB Dye Adsorption
3.2.1. Effect of Adsorbent Amount
3.2.2. Effect of PH
3.2.3. Effect of Temperature
3.2.4. Comparison of Adsorption of MB and MO Dyes
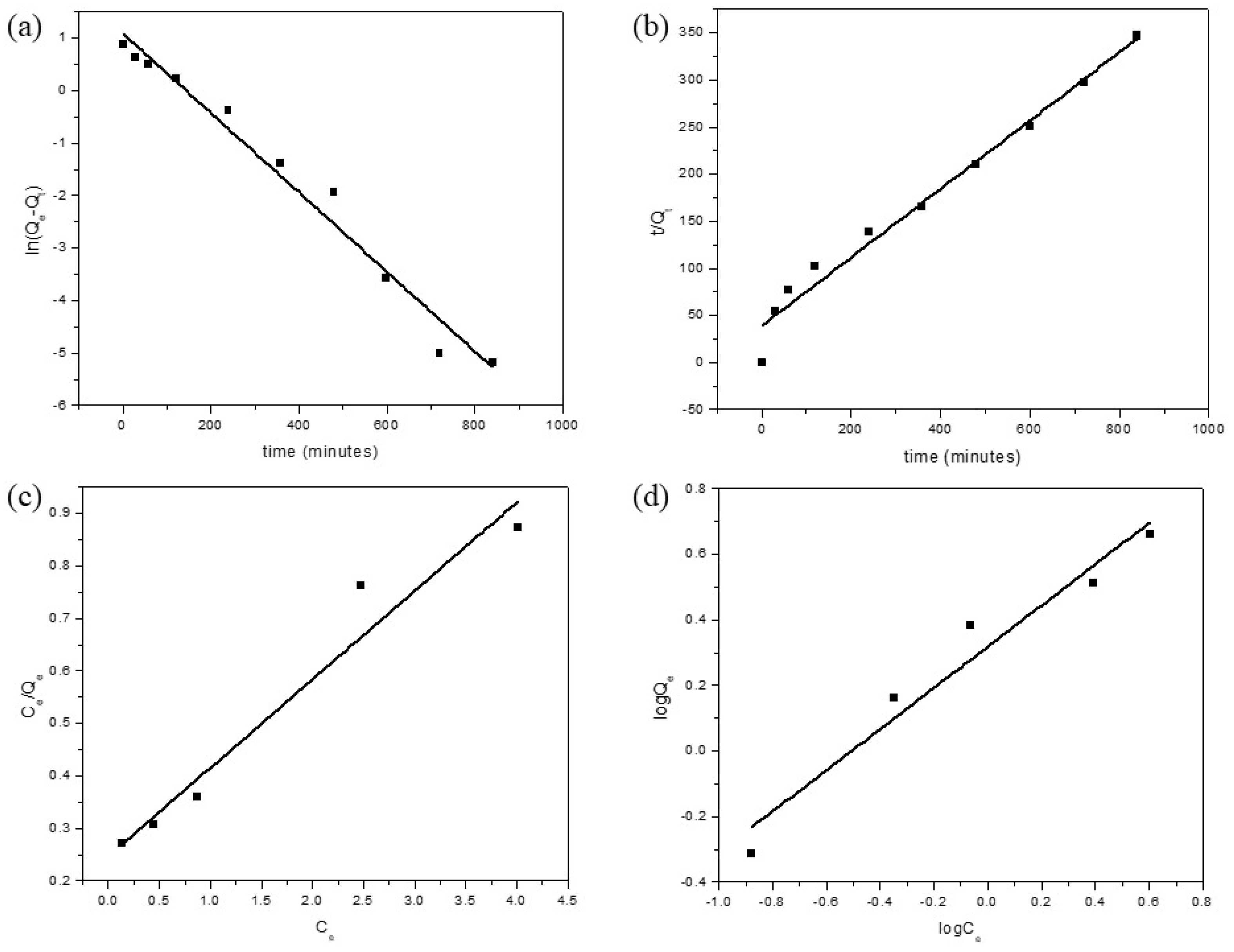
3.3. Adsorption Kinetics and Thermodynamics
3.4. Adsorption Isotherm Models
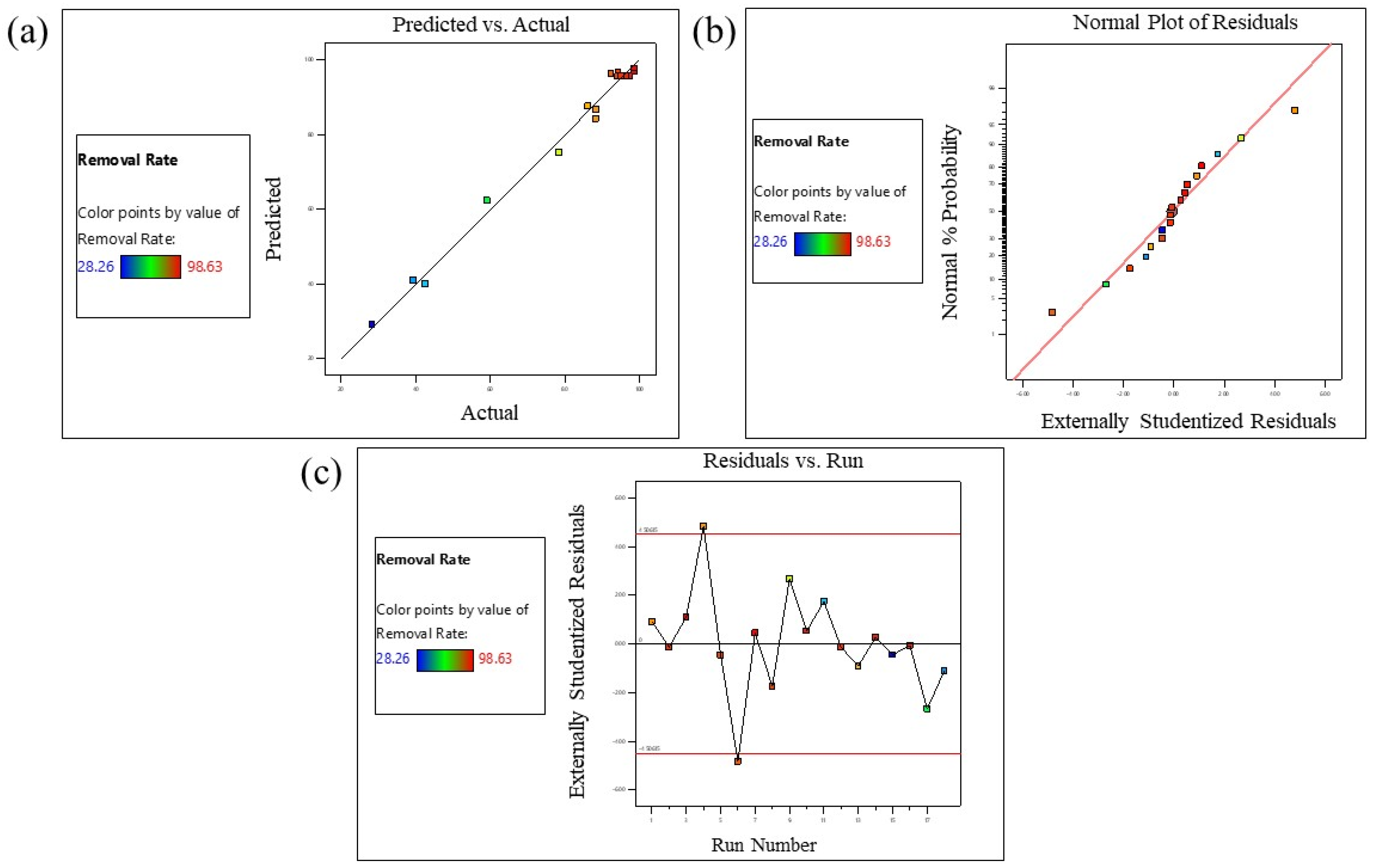
3.5. Response Surface Methodology
3.6. Regeneration and Reuse
3.7. Literature Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Niu, X.; Wu, H.; Zhang, H.; Ao, Z.; Zhang, S. Valorization of humin as a glucose derivative to fabricate a porous carbon catalyst for esterification and hydroxyalkylation/alkylation. Waste Manag. 2020, 103, 407–415. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Wu, S.-Y.; Chang, K.-L.; Huang, P.-J. Hydrogel-based heterogeneous-acid-catalysts for converting carbohydrates into the platform chemical: 5-hydroxymethylfurfural. J. Taiwan Inst. Chem. Eng. 2023, 149, 104997. [Google Scholar] [CrossRef]
- van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.; Bruijnincx, P.C.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Yan, P.; Xia, Z.; Liu, X.; Zhang, Z.C. Mechanistic understanding of humin formation in the conversion of glucose and fructose to 5-hydroxymethylfurfural in [BMIM] Cl ionic liquid. RSC Adv. 2020, 10, 34732–34737. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- Zuo, M.; Wang, X.; Wang, Q.; Zeng, X.; Lin, L. Aqueous-Natural Deep Eutectic Solvent-Enhanced 5-Hydroxymethylfurfural Production from Glucose, Starch, and Food Wastes. ChemSusChem 2022, 15, e202101889. [Google Scholar] [CrossRef]
- Zuo, M.; Jia, W.; Feng, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew. Energy 2021, 164, 23–33. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Zhao, Y.; Chen, J.; Zhou, J. Structural characterization and pyrolysis behavior of humin by-products from the acid-catalyzed conversion of C6 and C5 carbohydrates. J. Anal. Appl. Pyrolysis 2016, 118, 259–266. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Lange, H.; Rova, U.; Christakopoulos, P.; Matsakas, L. Covalently bound humin-lignin hybrids as important novel substructures in organosolv spruce lignins. Int. J. Biol. Macromol. 2023, 233, 123471. [Google Scholar] [CrossRef]
- Hu, X.; Lievens, C.; Larcher, A.; Li, C.-Z. Reaction pathways of glucose during esterification: Effects of reaction parameters on the formation of humin type polymers. Bioresour. Technol. 2011, 102, 10104–10113. [Google Scholar] [CrossRef]
- Kalusulingam, R.; Gajula, S.; Koilraj, P.; Shanthana Lakshmi, D.; Tayade, R.J.; Srinivasan, K. Biomass-derived humin-like furanic polymers as an effective UV-shielding agent for optically transparent thin-film composites. ACS Appl. Polym. Mater. 2021, 3, 1932–1942. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; van Eck, E.R.H.; Bula, W.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Wang, Y.; Agarwal, S.; Kloekhorst, A.; Heeres, H.J. Catalytic hydrotreatment of humins in mixtures of formic acid/2-propanol with supported ruthenium catalysts. ChemSusChem 2016, 9, 951–961. [Google Scholar] [CrossRef]
- Pin, J.-M.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; van der Waal, J.C.; de Jong, E. Valorization of biorefinery side-stream products: Combination of humins with polyfurfuryl alcohol for composite elaboration. ACS Sustain. Chem. Eng. 2014, 2, 2182–2190. [Google Scholar] [CrossRef]
- Mija, A.; van der Waal, J.C.; Pin, J.-M.; Guigo, N.; de Jong, E. Humins as promising material for producing sustainable carbohydrate-derived building materials. Constr. Build. Mater. 2017, 139, 594–601. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodríguez-Castellón, E.; van der Waal, J.C.; Luque, R. Benign-by-design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef] [Green Version]
- Andreas, R.; Zhang, J. Characteristics of adsorption interactions of cadmium (II) onto humin from peat soil in freshwater and seawater media. Bull. Environ. Contam. Toxicol. 2014, 92, 352–357. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zou, X.; Shu, R.; Ding, L.; Yao, K.; Lv, W.; Liu, G. Impact of humin on soil adsorption and remediation of Cd (II), Pb (II), and Cu (II). Soil Sediment Contam. Int. J. 2016, 25, 700–715. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, Q.; Wang, N.; Li, C.; Wang, L. First determination of Cu adsorption on soil humin. Environ. Chem. Lett. 2013, 11, 41–46. [Google Scholar] [CrossRef]
- Rosa, L.M.T.; Botero, W.G.; Santos, J.C.C.; Cacuro, T.A.; Waldman, W.R.; do Carmo, J.B.; de Oliveira, L.C. Natural organic matter residue as a low cost adsorbent for aluminum. J. Environ. Manag. 2018, 215, 91–99. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Q.; Fu, D.; Chen, P.; Li, R.; Liu, H.; Wang, Y.; Liu, Y.; Lv, W. Effect of tartaric acid on the adsorption of Pb (Ⅱ) via humin: Kinetics and mechanism. J. Taiwan Inst. Chem. Eng. 2020, 107, 79–88. [Google Scholar] [CrossRef]
- Chen, B.; Peng, Z.; Li, C.; Feng, Y.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Catalytic conversion of biomass to furanic derivatives with deep eutectic solvents. ChemSusChem 2021, 14, 1496–1506. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Glucose to fructose isomerization in aqueous media over homogeneous and heterogeneous catalysts. ChemCatChem 2016, 8, 1100–1110. [Google Scholar] [CrossRef]
- Marullo, S.; Rizzo, C.; D’Anna, F. Activity of a heterogeneous catalyst in deep eutectic solvents: The case of carbohydrate conversion into 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 13359–13368. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Chatel, G.; Jérôme, F. Contribution of deep eutectic solvents for biomass processing: Opportunities, challenges, and limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–108. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, P.-J. Sono-assisted rapid dye removal by chromium-based metal organic frameworks derived from waste PET bottles: Characterization, kinetics and adsorption isotherms. J. Environ. Chem. Eng. 2021, 9, 106766. [Google Scholar]
- Sayjumpa, J.; Jomhataikool, B.; Faungnawakij, K.; Kuboon, S.; Kraithong, W.; Fuji, M.; Eiad-ua, A. Porous carbon adsorbent from humin derived from thai leonardite for methylene blue dye adsorption. Curr. Appl. Sci. Technol. 2019, 19, 1–8. [Google Scholar]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Ajith, M.; Aswathi, M.; Priyadarshini, E.; Rajamani, P. Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresour. Technol. 2021, 342, 126000. [Google Scholar] [CrossRef]
- Amode, J.O.; Santos, J.H.; Md. Alam, Z.; Mirza, A.H.; Mei, C.C. Adsorption of methylene blue from aqueous solution using untreated and treated (Metroxylon spp.) waste adsorbent: Equilibrium and kinetics studies. Int. J. Ind. Chem. 2016, 7, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly efficient removal of methylene blue dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [Green Version]
- Albayati, T.M.; Sabri, A.A.; Alazawi, R.A. Separation of methylene blue as pollutant of water by SBA-15 in a fixed-bed column. Arab. J. Sci. Eng. 2016, 41, 2409–2415. [Google Scholar] [CrossRef]
- Saeed, M.; Jamal, M.A.; Haq, A.-U.; Ilyas, M.; Younas, M.; Shahzad, M.A. Oxidative degradation of methylene blue in aqueous medium catalyzed by lab prepared nickel hydroxide. Int. J. Chem. React. Eng. 2016, 14, 45–51. [Google Scholar] [CrossRef]
- Kazemi, F.; Mohamadnia, Z.; Kaboudin, B.; Karimi, Z. Photodegradation of methylene blue with a titanium dioxide/polyacrylamide photocatalyst under sunlight. J. Appl. Polym. Sci. 2016, 133, 43386. [Google Scholar] [CrossRef]
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative degradation of methylene blue via PDS-based advanced oxidation process using natural pyrite. Int. J. Environ. Res. Public Health 2019, 16, 4773. [Google Scholar] [CrossRef] [Green Version]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-removal of methylene blue from aqueous solution by Galactomyces geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Eljiedi, A.A.A.; Kamari, A. Removal of methyl orange and methylene blue dyes from aqueous solution using lala clam (Orbicularia orbiculata) shell. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Porri, A.; Baroncelli, R.; Guglielminetti, L.; Sarrocco, S.; Guazzelli, L.; Forti, M.; Catelani, G.; Valentini, G.; Bazzichi, A.; Franceschi, M. Fusarium oxysporum degradation and detoxification of a new textile-glycoconjugate azo dye (GAD). Fungal Biol. 2011, 115, 30–37. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Liang, Y.; Pervez, M.N.; Ye, X.; Dong, X.; Hassan, M.M.; Cai, Y. Effluent-free deep dyeing of cotton fabric with cacao husk extracts using the Taguchi optimization method. Cellulose 2021, 28, 517–532. [Google Scholar] [CrossRef]
- Xu, X.; Gong, J.; Li, Z.; Li, Q.; Zhang, J.; Wang, L.; Huang, J. Mordant free dyeing and functionalization of wool fabrics with biocolorants derived from Apocynum venetum L. Bast. ACS Sustain. Chem. Eng. 2020, 8, 12686–12695. [Google Scholar] [CrossRef]
- Shafiq, F.; Siddique, A.; Pervez, M.N.; Hassan, M.M.; Naddeo, V.; Cai, Y.; Hou, A.; Xie, K.; Khan, M.Q.; Kim, I.-S. Extraction of natural dye from aerial parts of argy wormwood based on optimized taguchi approach and functional finishing of cotton fabric. Materials 2021, 14, 5850. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, T.; Xiao, L.; Pervez, M.N.; Cai, X.; Naddeo, V.; Cai, Y. Sustainable fashion: Eco-friendly dyeing of wool fiber with novel mixtures of biodegradable natural dyes. Sci. Rep. 2022, 12, 21040. [Google Scholar] [CrossRef]
- Santosa, S.J.; Kunarti, E.S.; Aprilita, N.H.; Wulandari, B.; Bawani, D.N. Sorption mechanism and performance of peat soil humin for Methylene blue and p-Nitrophenol. Indones. J. Chem. 2019, 19, 198–210. [Google Scholar] [CrossRef]
- Jesus, A.M.D.; Romão, L.P.C.; Araújo, B.R.; Costa, A.S.; Marques, J.J. Use of humin as an alternative material for adsorption/desorption of reactive dyes. Desalination 2011, 274, 13–21. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Li, D.; Chung, Y.-C.; Huang, P.-J. Green synthesis of reusable adsorbents for the removal of heavy metal ions. ACS Omega 2021, 6, 30478–30487. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Shih, H.-Y.; Huang, P.-J. Facile synthesis of hydrogel-based ion-exchange resins for nitrite/nitrate removal and studies of adsorption behavior. Polymers 2022, 14, 1442. [Google Scholar] [CrossRef]
- Ajith, M.; Priyadarshini, E.; Rajamani, P. Effective and selective removal of heavy metals from industrial effluents using sustainable Si–CD conjugate based column chromatography. Bioresour. Technol. 2020, 314, 123786. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Lee, D.-Y.; Huang, P.-J. Novel reusable hydrogel adsorbents for precious metal recycle. Sci. Rep. 2021, 11, 19577. [Google Scholar] [CrossRef]
- Wu, Z.; Joo, H.; Lee, K. Kinetics and thermodynamics of the organic dye adsorption on the mesoporous hybrid xerogel. Chem. Eng. J. 2005, 112, 227–236. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.-L.; Zhou, P. Preparation of porous nano-calcium titanate microspheres and its adsorption behavior for heavy metal ion in water. J. Hazard. Mater. 2011, 186, 971–977. [Google Scholar] [CrossRef]
- Rajeswari, A.; Amalraj, A.; Pius, A. Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J. Water Process Eng. 2016, 9, 123–134. [Google Scholar] [CrossRef]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M. Adsorptive removal of anionic dye from aqueous solutions by Algerian kaolin: Characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci 2013, 4, 482–491. [Google Scholar]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef]
- Ali, R.; Mahmood, T.; Naeem, A.; Ullah, A.; Aslam, M.; Khan, S. Process optimization of Auramine O adsorption by surfactant-modified activated carbon using Box–Behnken design of response surface methodology. Desalin. Water Treat. 2021, 217, 367–390. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box–Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Rosly, N.Z.; Abdullah, A.H.; Ahmad Kamarudin, M.; Ashari, S.E.; Alang Ahmad, S.A. Adsorption of methylene blue dye by calix [6] arene-modified lead sulphide (Pbs): Optimisation using response surface methodology. Int. J. Environ. Res. Public Health 2021, 18, 397. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.-M.; Antonietti, M. Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13C NMR investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.M.C.; Lefferts, L.; Seshan, K. Valorization of humin-based byproducts from biomass processing—A route to sustainable hydrogen. ChemSusChem 2013, 6, 1651–1658. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.; Heeres, H. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Ghani, N.I.A.; Yusuf, N.Y.M.; Isahak, W.N.R.W.; Masdar, M.S. Modification of Activated Carbon from Biomass Nypa and Amine Functional Groups as Carbon Dioxide Adsorbent. J. Phys. Sci. 2017, 28, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, P.; Anitha, A. Refuse derived energy-tea derived boric acid activated carbon as an electrode material for electrochemical capacitors. Port. Electrochim. Acta 2013, 31, 165–174. [Google Scholar] [CrossRef]
- Santos, A.M.; Bertoli, A.C.; Borges, A.C.C.; Gomes, R.A.; Garcia, J.S.; Trevisan, M.G. New organomineral complex from humic substances extracted from poultry wastes: Synthesis, characterization and controlled release study. J. Braz. Chem. Soc. 2018, 29, 140–150. [Google Scholar]
- Xie, Z.; Guan, W.; Ji, F.; Song, Z.; Zhao, Y. Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology. J. Chem. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Santosa, S.J.; Sudiono, S.; Sujandi, S. Peat Soil Humic Acid Immobilization on Silica Gel and Its Application as an Adsorbent for the Selective Adsorption of Copper. E-J. Surf. Sci. Nanotechnol. 2006, 4, 602–608. [Google Scholar] [CrossRef]
- Santosa, S.J.; Siswanta, D.; Sudiono, S.; Utarianingrum, R. Chitin–humic acid hybrid as adsorbent for Cr (III) in effluent of tannery wastewater treatment. Appl. Surf. Sci. 2008, 254, 7846–7850. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Fernando, P.S.A.; Ahmed, R.T.; Srinath, R.; Priyadharshini, M.; Vignesh, A.; Thanjiappan, A. Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid–treated orange peel. Chem. Eng. Commun. 2014, 201, 1526–1547. [Google Scholar] [CrossRef]
- Abbas, M. Removal of brilliant green (BG) by activated carbon derived from medlar nucleus (ACMN)–Kinetic, isotherms and thermodynamic aspects of adsorption. Adsorpt. Sci. Technol. 2020, 38, 464–482. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.; Ahmad, M.; Allen, S. Thermodynamic behaviour and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: A kinetic study. J. Colloid Interface Sci. 2005, 287, 6–13. [Google Scholar] [CrossRef]
- Potgieter, J.; Pardesi, C.; Pearson, S. A kinetic and thermodynamic investigation into the removal of methyl orange from wastewater utilizing fly ash in different process configurations. Environ. Geochem. Health 2021, 43, 2539–2550. [Google Scholar] [CrossRef]
- Ebelegi, A.N.; Ayawei, N.; Wankasi, D. Interpretation of adsorption thermodynamics and kinetics. Open J. Phys. Chem. 2020, 10, 166–182. [Google Scholar] [CrossRef]





| Variables | Code | Units | Coded Variables Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Time | A | minutes | 30 | 315 | 600 |
| Concentration | B | ppm | 5 | 27.5 | 50 |
| Weight | C | g | 0.05 | 0.1 | 0.15 |
| Wave Number/cm−1 | Infrared Adsorption | Functional Groups and Structures |
|---|---|---|
| 3405, 3420 | O-H stretching | Hydroxyl group |
| 2927 | C-H stretching | Methyl and methylene structures |
| 2216, 1943 | O-H stretching | Phenolic group |
| 1709 | C=O stretching | Carbonyl group |
| 1621, 1633 | C=C stretching | Furan ring |
| 1417 | C-H bending | Methyl group |
| 1266, 1213, 1041 | C-O stretching | Aromatic ethers and aliphatic alcohols |
| 800–500 range | C-H bending | Furan ring |
| Temperature/°C | BET Surface Area/(m2/g) | Pore Volume/(cm3/g) | Pore Size/(nm) |
|---|---|---|---|
| 80 | 2.7672 | 0.0177 | 64.11 |
| 100 | 0.1513 | 0.0012 | 69.13 |
| 120 | 0.8065 | 0.0093 | 56.63 |
| 140 | 0.5126 | 0.0136 | 71.58 |
| 160 | 0.6953 | 0.0148 | 75.84 |
| After Carbonization | 331.9938 | 0.0949 | 36.33 |
| Pseudo-First Order Model | ||
| k1 (min−1) | Qe (mg/g) | R2 |
| 0.00757 | 2.42245 | 0.97413 |
| Pseudo-Second Order Model | ||
| k2 × 10−4 (g/mg min) | Qe (mg/g) | R2 |
| 418.145 | 0.02568 | 0.97543 |
| Temperature/K | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol K) |
|---|---|---|---|
| 298 | −94.3118 | −1670.28 | −5.23782 |
| 318 | −37.8638 | ||
| 338 | 105.5307 |
| Langmuir Isotherm Model | ||
| Qmax | KL | R2 |
| 5.93275 | 0.68219 | 0.94306 |
| Freundlich Isotherm Model | ||
| n | KF | R2 |
| 1.555932 | 2.0804 | 0.9416 |
| VARIABLES | RESPONSE | ||||
|---|---|---|---|---|---|
| Run | Time (A) | Concentration (B) | Weight (C) | MB Removal Rate% | |
| Actual Value | Predicted Value | ||||
| 1 | 600 | 27.5 | 0.05 | 88.26 | 86.78 |
| 2 | 315 | 27.5 | 0.1 | 95.23 | 95.63 |
| 3 | 600 | 5 | 0.1 | 98.63 | 96.89 |
| 4 | 30 | 5 | 0.1 | 88.24 | 84.27 |
| 5 | 315 | 27.5 | 0.1 | 94.21 | 95.63 |
| 6 | 600 | 50 | 0.1 | 92.36 | 96.33 |
| 7 | 600 | 27.5 | 0.15 | 98.56 | 97.81 |
| 8 | 315 | 5 | 0.15 | 94.28 | 96.77 |
| 9 | 315 | 50 | 0.15 | 78.46 | 75.24 |
| 10 | 315 | 27.5 | 0.1 | 97.25 | 95.63 |
| 11 | 315 | 50 | 0.05 | 42.53 | 40.04 |
| 12 | 315 | 27.5 | 0.1 | 95.19 | 95.63 |
| 13 | 30 | 27.5 | 0.15 | 86.15 | 87.63 |
| 14 | 315 | 27.5 | 0.1 | 96.48 | 95.63 |
| 15 | 30 | 27.5 | 0.05 | 28.26 | 29.01 |
| 16 | 315 | 27.5 | 0.1 | 95.42 | 95.63 |
| 17 | 315 | 5 | 0.05 | 59.12 | 62.34 |
| 18 | 30 | 50 | 0.1 | 39.25 | 40.99 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Comment |
|---|---|---|---|---|---|---|
| Model | 8748.48 | 9 | 972.05 | 94.83 | <0.0001 | significant |
| A-time | 2308.94 | 1 | 2308.94 | 225.26 | <0.0001 | |
| B-Concentration | 960.75 | 1 | 960.75 | 93.73 | <0.0001 | |
| C-Weight | 2424.86 | 1 | 2424.86 | 236.57 | <0.0001 | |
| AB | 456.25 | 1 | 456.25 | 44.51 | 0.0002 | |
| AC | 566.20 | 1 | 566.20 | 55.24 | <0.0001 | |
| BC | 0.1482 | 1 | 0.1482 | 0.0145 | 0.9072 | |
| A2 | 94.35 | 1 | 94.35 | 9.21 | 0.0162 | |
| B2 | 563.13 | 1 | 563.13 | 54.94 | <0.0001 | |
| C2 | 1071.83 | 1 | 1071.83 | 104.57 | <0.0001 | |
| Residual | 82.00 | 8 | 10.25 | |||
| Lack of Fit | 76.24 | 3 | 25.41 | 22.06 | 0.0026 | significant |
| Pure Error | 5.76 | 5 | 1.15 | |||
| Cor Total | 8830.48 | 17 | ||||
| Response (Y) | SD | CV | R2 | Adj. R2 | Pred. R2 | AP |
| MB Removal rate% | 3.20 | 3.93 | 0.9907 | 0.9803 | 0.8609 | 28.8300 |
| Sample Name | BET Surface Area/(m2/g) | Pore Volume/(cm3/g) | Pore Size/(nm) |
|---|---|---|---|
| Regenerated carbonized GDH | 113.01 | 0.05 | 4.80 |
| No. | Adsorbent | Name of the Dye and Concentration | Adsorbent Weight/(g) | Time/(min) | Removal Efficiency | Reference |
|---|---|---|---|---|---|---|
| 1 | Leonardite -humin | MB–25 ppm | 1 | 5 | 90% | [29] |
| 2 | Peat soil -humin | MB–10 ppm p-NP–10 ppm | 0.05 | 120 | N/A | [47] |
| 3 | Peat soil -humin | RO 16–50 ppm RR 120–50 ppm | 0.2 | 90 | RO 16–81.4% RR 120–66.8% | [48] |
| 4 | Glucose -derived humin (GDH) | MB dye–25 ppm | 0.1 | 600 | 97% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmapriya, T.N.; Chang, K.-L.; Huang, P.-J. Valorization of Glucose-Derived Humin as a Low-Cost, Green, Reusable Adsorbent for Dye Removal, and Modeling the Process. Polymers 2023, 15, 3268. https://doi.org/10.3390/polym15153268
Dharmapriya TN, Chang K-L, Huang P-J. Valorization of Glucose-Derived Humin as a Low-Cost, Green, Reusable Adsorbent for Dye Removal, and Modeling the Process. Polymers. 2023; 15(15):3268. https://doi.org/10.3390/polym15153268
Chicago/Turabian StyleDharmapriya, Thakshila Nadeeshani, Ken-Lin Chang, and Po-Jung Huang. 2023. "Valorization of Glucose-Derived Humin as a Low-Cost, Green, Reusable Adsorbent for Dye Removal, and Modeling the Process" Polymers 15, no. 15: 3268. https://doi.org/10.3390/polym15153268
APA StyleDharmapriya, T. N., Chang, K.-L., & Huang, P.-J. (2023). Valorization of Glucose-Derived Humin as a Low-Cost, Green, Reusable Adsorbent for Dye Removal, and Modeling the Process. Polymers, 15(15), 3268. https://doi.org/10.3390/polym15153268








