Multicomponent Acrylic Formulation Design for Corrosion Casting with Controlled Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Coformulations as Case in Five Groups
2.3. Initial Testing Phase for Mechanical Performance
2.4. Pot Life Determination
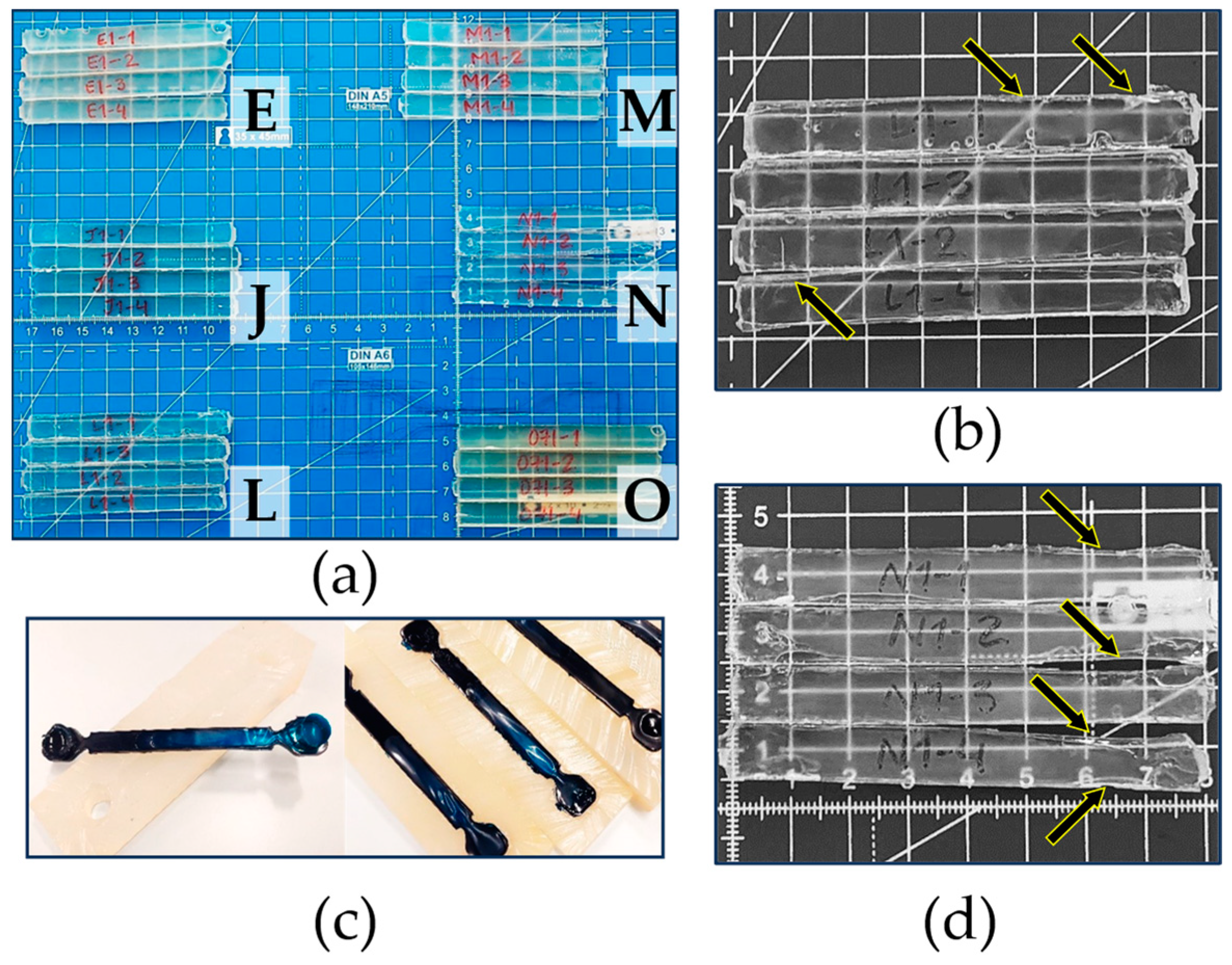
2.5. Rigorous Testing Phase for Mechanical Properties
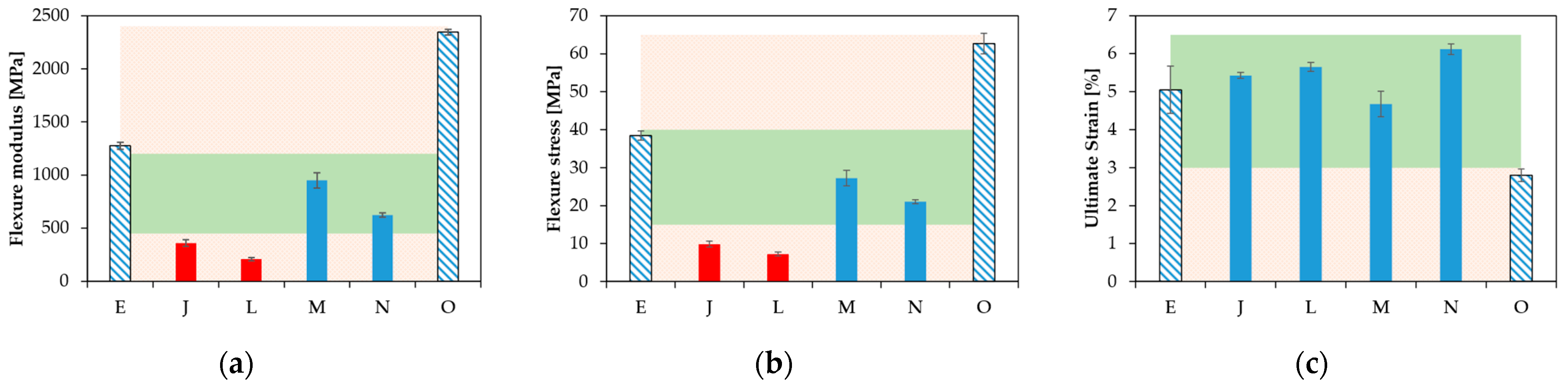
3. Results and Discussion
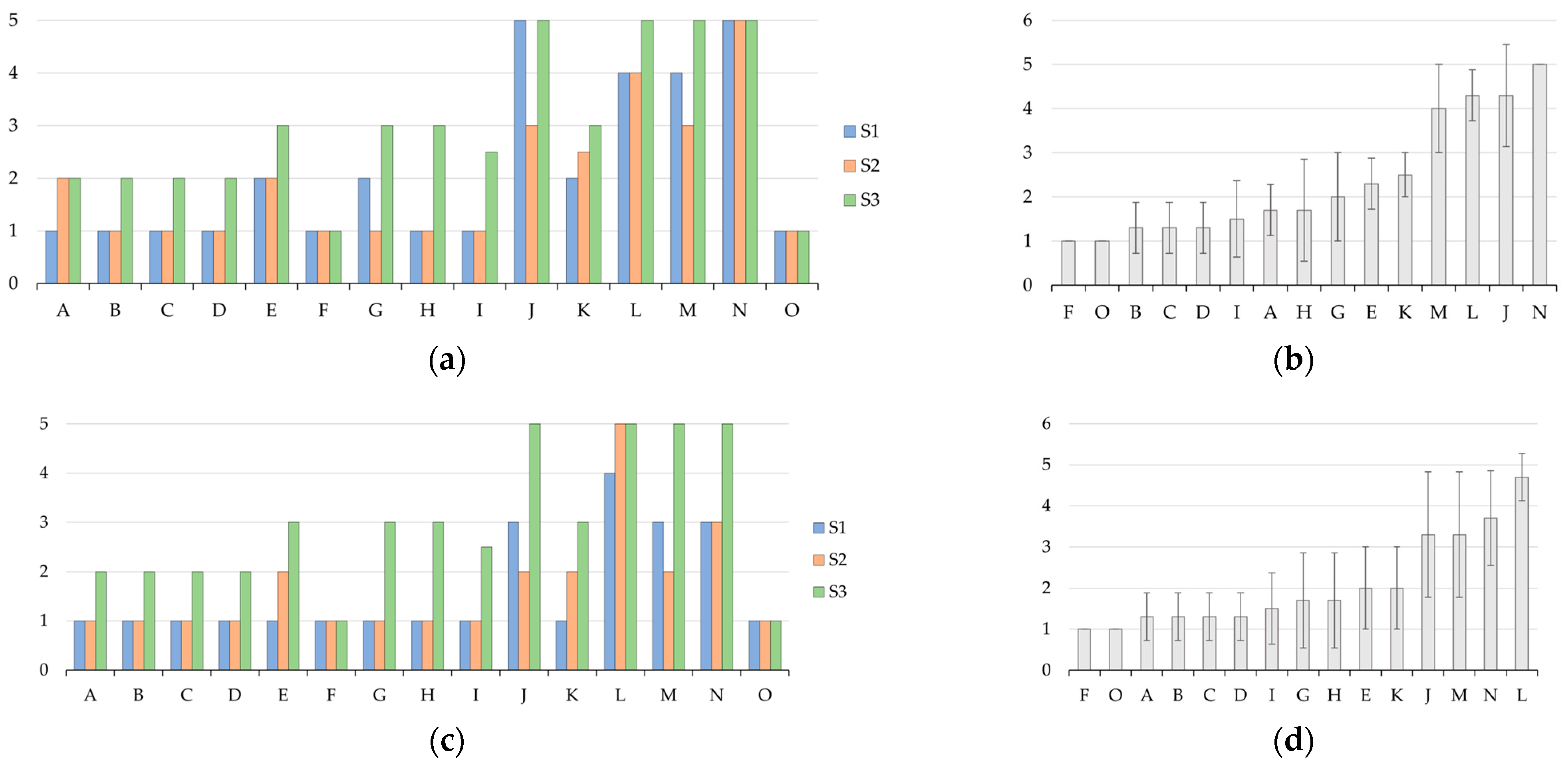
3.1. Preliminary Screening of Mechanical Performance
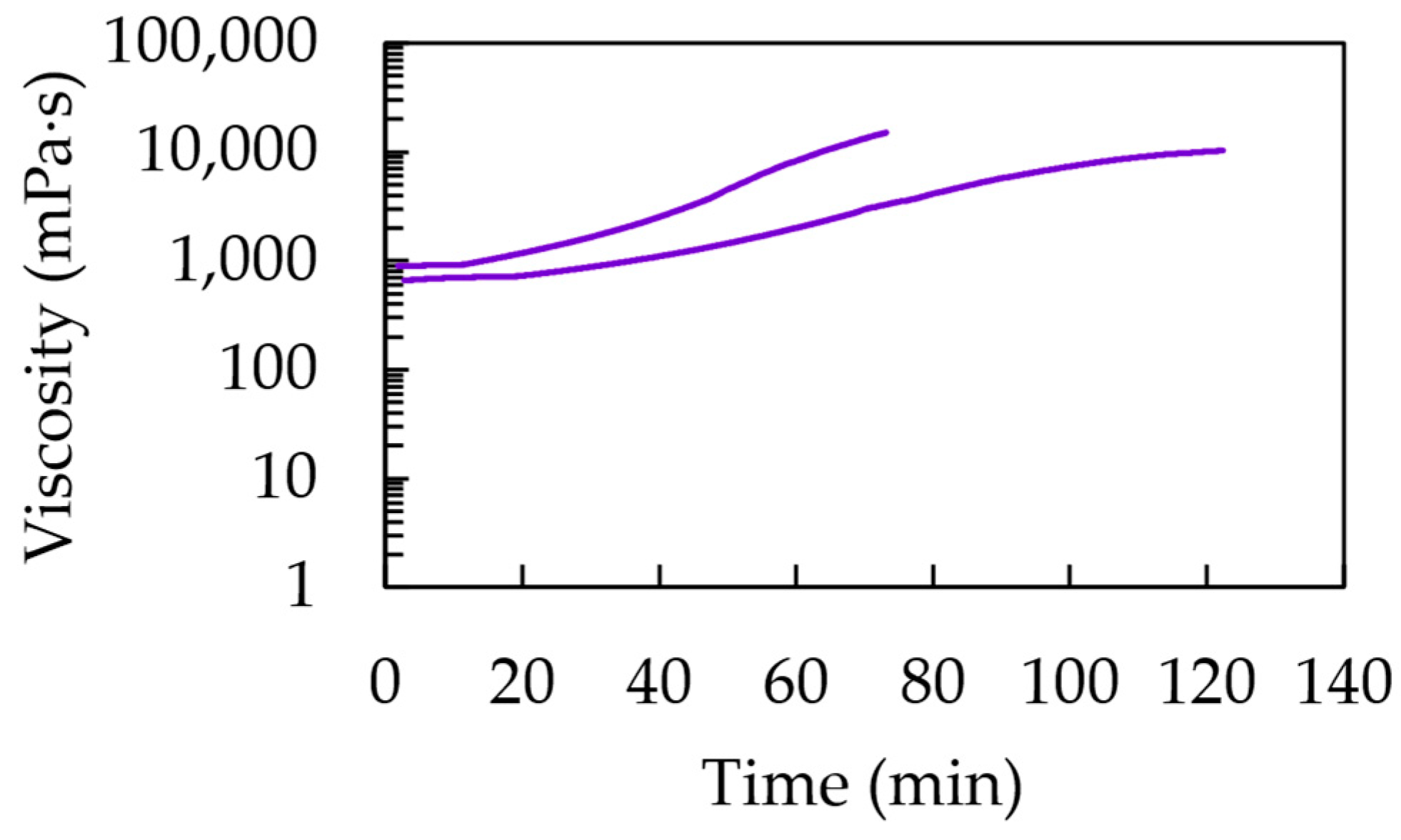
3.2. Pot Life Determination
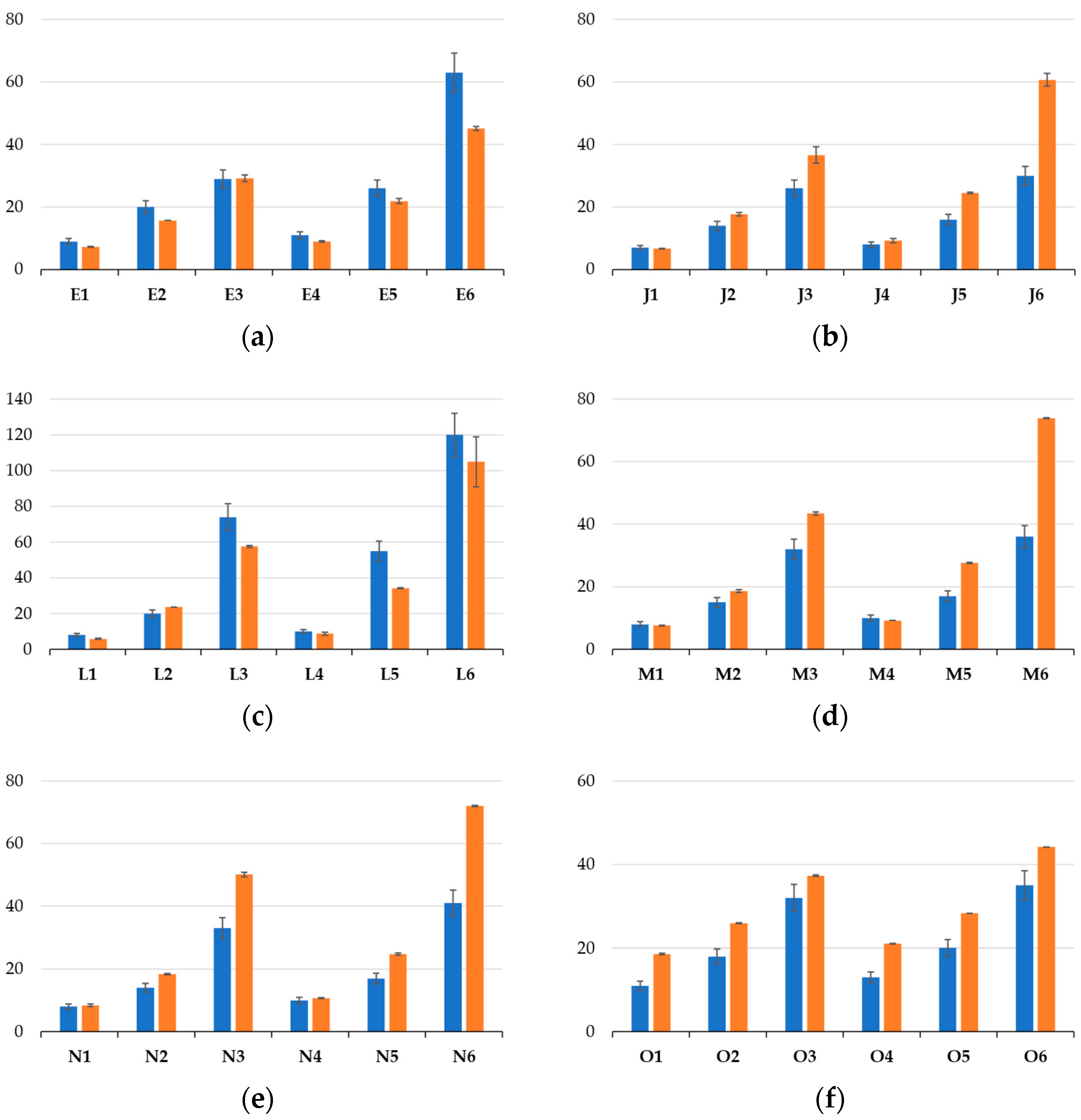
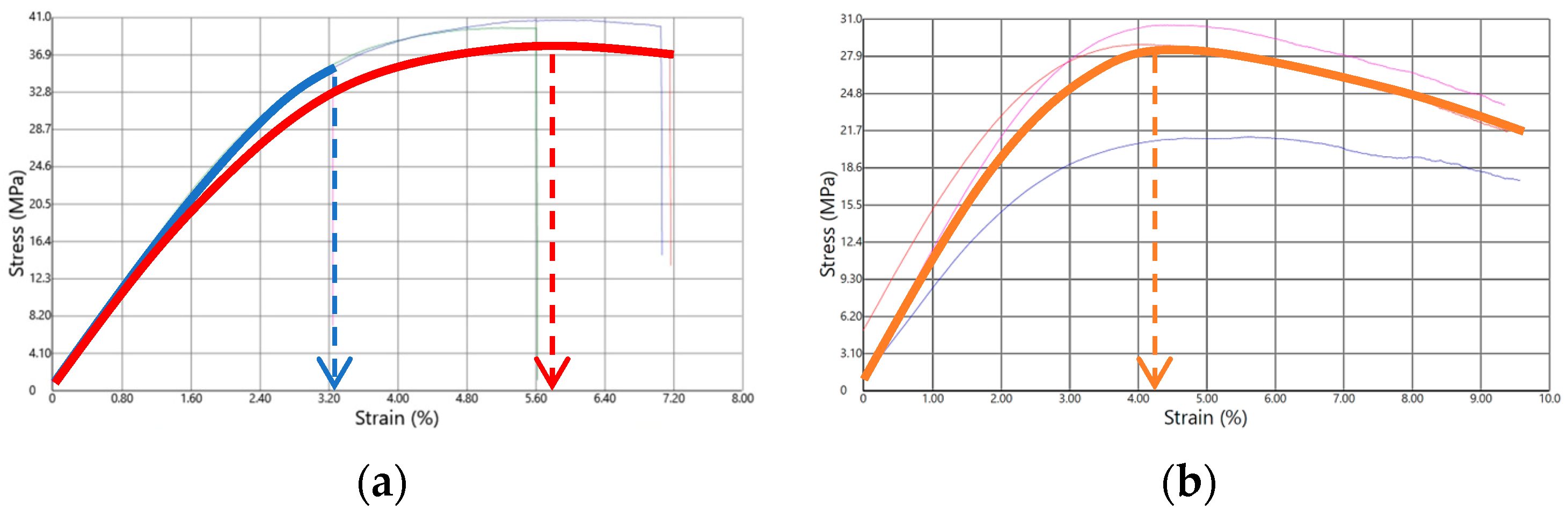
3.3. Rigorous Testing Phase for Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De Keer, L.; Kilic, K.I.; Van Steenberge, P.H.M.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational Prediction of the Molecular Configuration of Three-Dimensional Network Polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st Century: Synthetic Polymers in Drug Delivery Applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Efremova, O.A.; Tsygankova, A.R.; Brylev, K.A.; Edeleva, M.V.; Kurskaya, O.G.; Sutherland, A.J.; Shestopalov, A.M.; Mironov, Y.V.; Shestopalov, M.A. Characterization and Cytotoxicity Studies of Thiol-Modified Polystyrene Microbeads Doped with [{Mo6X8}(NO3)6]2 − (X = Cl, Br, I). Polym. Adv. Technol. 2016, 27, 922–928. [Google Scholar] [CrossRef]
- Hovorun, T.P.; Berladir, K.V.; Pererva, V.I.; Rudenko, S.G.; Martynov, A.I. Modern Materials for Automotive Industry. J. Eng. Sci. 2017, 4, f8–f18. [Google Scholar] [CrossRef]
- Alavi, S.; Thomas, S.; Sandeep, K.P.; Kalarikkal, N.; Varghese, J.; Yaragalla, S. (Eds.) Polymers for Packaging Applications; Apple Academic Press: Waretown, NJ, USA, 2014; ISBN 9781926895772. [Google Scholar]
- Moens, E.K.C.; de Smit, K.; Marien, Y.W.; Trigilio, A.D.; van Steenberge, P.H.M.; van Geem, K.M.; Dubois, J.-L.L.; D’Hooge, D.; D’Hooge, D.R. Progress in Reaction Mechanisms and Reactor Technologies for Thermochemical Recycling of Poly(Methyl Methacrylate). Polymers 2020, 12, 1667. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly(Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastic Materials, 7th ed.; Butterworth-Heinemann: Oxford, UK, 1999; ISBN 0750641320. [Google Scholar]
- Harper, C.A. Handbook of Plastics, Elastomers, and Composites, 4th ed.; Harper, C.A., Ed.; McGRAW-HILL: New York, NY, USA, 2002; ISBN 9780071384766. [Google Scholar]
- Ram, A. Fundamentals of Polymer Engineering; Springer US: New York, NY, USA, 1997; ISBN 978-1-4899-1824-6. [Google Scholar]
- Zhang, K.; Ma, C.; Zheng, Y.; Zhou, F.; Xiao, Y.; Xing, W.; Hu, Y. A Novel Coating of Hyperbranched Poly(Urethane-Phosphine Oxide) for Poly(Methyl Methacrylate) with High Fire Safety, Excellent Adhesion and Transparency. Prog. Org. Coat. 2021, 161, 106481. [Google Scholar] [CrossRef]
- Siwal, S.S.; Chaudhary, G.; Saini, A.K.; Kaur, H.; Saini, V.; Mokhta, S.K.; Chand, R.; Chandel, U.K.; Christie, G.; Thakur, V.K. Key Ingredients and Recycling Strategy of Personal Protective Equipment (PPE): Towards Sustainable Solution for the COVID-19 like Pandemics. J. Environ. Chem. Eng. 2021, 9, 106284. [Google Scholar] [CrossRef] [PubMed]
- Rendeki, S.; Nagy, B.; Bene, M.; Pentek, A.; Toth, L.; Szanto, Z.; Told, R.; Maroti, P. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers 2020, 12, 2703. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. Nanotechnology-Based Self-Sterilizing Surfaces and Their Potential in Combating COVID-19. Nanomedicine 2021, 16, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- De Smit, K.; Marien, Y.W.; van Geem, K.M.; van Steenberge, P.H.M.M.; D’hooge, D.R. Connecting Polymer Synthesis and Chemical Recycling on a Chain-by-Chain Basis: A Unified Matrix-Based Kinetic Monte Carlo Strategy. React. Chem. Eng. 2020, 5, 1909–1928. [Google Scholar] [CrossRef]
- Scheirs, J.; Kaminsky, W. (Eds.) Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780470021545. [Google Scholar]
- Kikuchi, Y.; Hirao, M.; Ookubo, T.; Sasaki, A. Design of Recycling System for Poly(Methyl Methacrylate) (PMMA). Part 1: Recycling Scenario Analysis. Int. J. Life Cycle Assess. 2014, 19, 120–129. [Google Scholar] [CrossRef]
- Kaminsky, W.; Predel, M.; Sadiki, A. Feedstock Recycling of Polymers by Pyrolysis in a Fluidised Bed. Polym. Degrad. Stab. 2004, 85, 1045–1050. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Dewil, R. Recycling and Recovery of Post-Cet Alonsumer Plastic Solid Waste in a European Context. Therm. Sci. 2012, 16, 669–685. [Google Scholar] [CrossRef]
- Smolders, K.; Baeyens, J. Thermal Degradation of PMMA in Fluidised Beds. Waste Manag. 2004, 24, 849–857. [Google Scholar] [CrossRef] [PubMed]
- de Tommaso, J.; Dubois, J.-L. Risk Analysis on PMMA Recycling Economics. Polymers 2021, 13, 2724. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; Matyjaszewski, K., Davis, T.P., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 978-0-471-39274-3. [Google Scholar]
- Achilias, D.S.; Sideridou, I. Study of the Effect of Two BPO/Amine Initiation Systems on the Free-Radical Polymerization of MMA Used in Dental Resins and Bone Cements. J. Macromol. Sci. Part A 2002, 39, 1435–1450. [Google Scholar] [CrossRef]
- Alhareb, A.O.; Akil, H.M.; Ahmad, Z.A. Impact Strength, Fracture Toughness and Hardness Improvement of PMMA Denture Base through Addition of Nitrile Rubber/Ceramic Fillers. Saudi J. Dent. Res. 2017, 8, 26–34. [Google Scholar] [CrossRef]
- Chen, T.; Kusy, R.P. Effect of Methacrylic Acid:Methyl Methacrylate Monomer Ratios on Polymerization Rates and Properties of Polymethyl Methacrylates. J. Biomed. Mater. Res. 1997, 36, 190–199. [Google Scholar] [CrossRef]
- Okuyama, Y.; Shiraishi, T.; Yoshida, K.; Kurogi, T.; Watanabe, I.; Murata, H. Influence of Composition and Powder/Liquid Ratio on Setting Characteristics and Mechanical Properties of Autopolymerized Hard Direct Denture Reline Resins Based on Methyl Methacrylate and Ethylene Glycol Dimethacrylate. Dent. Mater. J. 2014, 33, 522–529. [Google Scholar] [CrossRef]
- Johnson, J.A.; Jones, D.W. The Mechanical Properties of PMMA and Its Copolymers with Ethyl Methacrylate and Butyl Methacrylate. J. Mater. Sci. 1994, 29, 870–876. [Google Scholar] [CrossRef]
- Kema Ajekwene, K. Properties and Applications of Acrylates. In Acrylate Polymers for Advanced Applications; Serrano-Aroca, Á., Deb, S., Eds.; IntechOpen: London, UK, 2020; p. 13. [Google Scholar]
- Silikas, N.; Al-Kheraif, A.; Watts, D.C. Influence of P/L Ratio and Peroxide/Amine Concentrations on Shrinkage-Strain Kinetics during Setting of PMMA/MMA Biomaterial Formulations. Biomaterials 2005, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vallo, C.I. Flexural Strength Distribution of a PMMA-Based Bone Cement. J. Biomed. Mater. Res. 2002, 63, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Vazquez, B.; Bonfield, W. Effect of Crosslinking Agents on Acrylic Bone Cements Based on Poly(Methylmethacrylate). J. Biomed. Mater. Res. 1997, 37, 465–473. [Google Scholar] [CrossRef]
- Doomernik, D.E.; Kruse, R.R.; Reijnen, M.M.P.J.; Kozicz, T.L.; Kooloos, J.G.M. A Comparative Study of Vascular Injection Fluids in Fresh-Frozen and Embalmed Human Cadaver Forearms. J. Anat. 2016, 229, 582–590. [Google Scholar] [CrossRef]
- Christoffersonm, R.H.; Nilsson, B.O. Microvascular Corrosion Casting with Analysis in the Scanning Electron Microscope. Scanning 1988, 10, 43–63. [Google Scholar] [CrossRef]
- Weiger, T.; Lametschwandtner, A.; Adam, H. Methylmethacrylate and Mercox CL in the Scanning Electron Microscopy of Corrosion Casts. Mikroskopie 1982, 39, 187–197. [Google Scholar] [PubMed]
- Cornillie, P.; Casteleyn, C.; von Horst, C.; Henry, R. Corrosion Casting in Anatomy: Visualizing the Architecture of Hollow Structures and Surface Details. Anat. Histol. Embryol. 2019, 48, 591–604. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Ohta, Y.; Tajiri, S. New Improved Method for Injection of Acrylic Resin. Okajimas Folia Anat. Jpn. 1952, 24, 259–267. [Google Scholar] [CrossRef]
- Murakami, T. Application of the Scanning Electron Microscope to the Study of the Fine Distribution of the Blood Vessels. Arch. Histol. Jpn. 1971, 32, 445–454. [Google Scholar] [CrossRef]
- Kratky, R.G.; Roach, M.R. Shrinkage of Batson’s and Its Relevance to Vascular Casting. Atherosclerosis 1984, 51, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Hossler, F.E. Vascular Corrosion Casting Can Provide Quantitative as Well as Morphological Information on the Microvasculature of Organs and Tissues. Micros. Today 1998, 6, 14–15. [Google Scholar] [CrossRef]
- Hodde, K.C.; Steeber, D.A.; Albrecht, R.M. Advances in Corrosion Casting Methods. Scanning Microsc. 1990, 4, 693–704. [Google Scholar] [PubMed]
- Sugiyama, K.; Gu, Z.-B.; Kawase, C.; Yamamoto, T.; Kitazawa, Y. Optic Nerve and Peripapillary Choroidal Microvasculature of the Rat Eye. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3084–3090. [Google Scholar]
- Reyes, P.; Edeleva, M.; D’hooge, D.R.; Cardon, L.; Cornillie, P. Combining Chromatographic, Rheological, and Mechanical Analysis to Study the Manufacturing Potential of Acrylic Blends into Polyacrylic Casts. Materials 2021, 14, 6939. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, M.; Marien, Y.W.; van Steenberge, P.H.M.M.; D’hooge, D.R. Impact of Side Reactions on Molar Mass Distribution, Unsaturation Level and Branching Density in Solution Free Radical Polymerization of n -Butyl Acrylate under Well-Defined Lab-Scale Reactor Conditions. Polym. Chem. 2021, 12, 2095–2114. [Google Scholar] [CrossRef]
- Edeleva, M.; Marien, Y.W.; van Steenberge, P.H.M.; D’hooge, D.R. Jacket Temperature Regulation Allowing Well-Defined Non-Adiabatic Lab-Scale Solution Free Radical Polymerization of Acrylates. React. Chem. Eng. 2021, 6, 1053–1069. [Google Scholar] [CrossRef]
- van Steenberge, P.H.M.M.; D’Hooge, D.R.; Wang, Y.; Zhong, M.; Reyniers, M.-F.F.; Konkolewicz, D.; Matyjaszewski, K.; Marin, G.B.; D’hooge, D.R.; Wang, Y.; et al. Linear Gradient Quality of ATRP Copolymers. Macromolecules 2012, 45, 8519–8531. [Google Scholar] [CrossRef]
- Shipp, D.A.; Wang, J.-L.; Matyjaszewski, K. Synthesis of Acrylate and Methacrylate Block Copolymers Using Atom Transfer Radical Polymerization. Macromolecules 1998, 31, 8005–8008. [Google Scholar] [CrossRef]
- Nese, A.; Mosnáček, J.; Juhari, A.; Yoon, J.A.; Koynov, K.; Kowalewski, T.; Matyjaszewski, K. Synthesis, Characterization, and Properties of Starlike Poly( n -Butyl Acrylate)- b -Poly(Methyl Methacrylate) Block Copolymers. Macromolecules 2010, 43, 1227–1235. [Google Scholar] [CrossRef]
- Bhabhe, M.D.; Galvankar, P.S.; Desai, V.M.; Athawale, V.D. Copolymers of Methyl Methacrylate and Acrylate Comonomers: Synthesis and Characterization. J. Appl. Polym. Sci. 1995, 56, 485–494. [Google Scholar] [CrossRef]
- González, I.; Asua, J.M.; Leiza, J.R. The Role of Methyl Methacrylate on Branching and Gel Formation in the Emulsion Copolymerization of BA/MMA. Polymer 2007, 48, 2542–2547. [Google Scholar] [CrossRef]
- Fang, H.; Jiang, F.; Wu, Q.; Ding, Y.; Wang, Z. Supertough Polylactide Materials Prepared through in Situ Reactive Blending with PEG-Based Diacrylate Monomer. ACS Appl. Mater. Interfaces 2014, 6, 13552–13563. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Rai, J.S.P. Synthesis and Kinetic Study of Diacrylate and Dimethacrylate. Int. J. Plast. Technol. 2013, 17, 182–193. [Google Scholar] [CrossRef]
- Asua, J.M. Polymer Reaction Engineering, 1st ed.; Asua, J.M., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2007; ISBN 9780470692134. [Google Scholar]
- De Keer, L.; Van Steenberge, P.H.M.; Reyniers, M.-F.; D’hooge, D.R. Going Beyond the Carothers, Flory and Stockmayer Equation by Including Cyclization Reactions and Mobility Constraints. Polymers 2021, 13, 2410. [Google Scholar] [CrossRef] [PubMed]
- Sangwai, J.S.; Bhat, S.A.; Gupta, S.; Saraf, D.N.; Gupta, S.K. Bulk Free Radical Polymerizations of Methyl Methacrylateunder Non-Isothermal Conditions and with Intermediate Additionof Initiator: Experiments and Modeling. Polymer 2005, 46, 11451–11462. [Google Scholar] [CrossRef]
- Balke, S.T.; Hamielec, A.E. Bulk Polymerization of Methyl Methacrylate; Wiley: Hoboken, NJ, USA, 1973; Volume 17. [Google Scholar]
- Zhan, P.; Chen, J.; Zheng, A.; Shi, H.; Chen, F.; Wei, D.; Xu, X.; Guan, Y. The Trommsdorff Effect under Shear and Bulk Polymerization of Methyl Methacrylate via Reactive Extrusion. Eur. Polym. J. 2020, 122, 109272. [Google Scholar] [CrossRef]
- Ren, S.; Hinojosa-Castellanos, L.; Zhang, L.; Dubé, M.A. Bulk Free-Radical Copolymerization of n-Butyl Acrylate and n-Butyl Methacrylate: Reactivity Ratio Estimation. Macromol. React. Eng. 2017, 11, 1600050. [Google Scholar] [CrossRef]
- Bujak, P.; Henzel, N.; Matlengiewicz, M. Microstructure Study of Methyl Methacrylate/n -Butyl Acrylate Copolymer by 13 C NMR Spectroscopy. Int. J. Polym. Anal. Charact. 2008, 13, 149–162. [Google Scholar] [CrossRef]
- Grady, M.C.; Simonsick, W.J.; Hutchinson, R.A. Studies of Higher Temperature Polymerization of N-Butyl Methacrylate and n-Butyl Acrylate. Macromol. Symp. 2002, 182, 149–168. [Google Scholar] [CrossRef]
- Bowman, C.N.; Peppas, N.A. Polymers for Information Storage Systems. II. Polymerization Kinetics for Preparation of Highly Crosslinked Polydimethacrylates. J. Appl. Polym. Sci. 1991, 42, 2013–2018. [Google Scholar] [CrossRef]
- Gallow, K.C.; Jhon, Y.K.; Tang, W.; Genzer, J.; Loo, Y.-L.L. Cloud Point Suppression in Dilute Solutions of Model Gradient Copolymers with Prespecified Composition Profiles. J. Polym. Sci. B Polym. Phys. 2011, 49, 629–637. [Google Scholar] [CrossRef]
- Yu, J.; He, J. Crystallization Kinetics of Maleic Anhydride Grafted Polypropylene Ionomers. Polymer 2000, 41, 891–898. [Google Scholar] [CrossRef]
- Brauer, G.M.; Petrianyk, N.; Termini, D.J. Storage Stability of Dental Composites. J. Dent. Res. 1979, 58, 1791–1800. [Google Scholar] [CrossRef]
- De Keer, L.; Cavalli, F.; Estupiñán, D.; Krüger, A.J.D.; Rocha, S.; Van Steenberge, P.H.M.; Reyniers, M.F.; De Laporte, L.; Hofkens, J.; Barner, L.; et al. Synergy of Advanced Experimental and Modeling Tools to Underpin the Synthesis of Static Step-Growth-Based Networks Involving Polymeric Precursor Building Blocks. Macromolecules 2021, 54, 9280–9298. [Google Scholar] [CrossRef]
- International Organization for Standardization. Plastics—Determination of Charpy impact properties—Part 1: Non-instrumented impact test (ISO Standard N° ISO 179-1). 2023. Available online: https://www.iso.org/standard/84393.html (accessed on 5 July 2023).
- International Organization for Standardization. Plastics—Determination of flexural properties (ISO Standard N° ISO 178). 2023. Available online: https://www.iso.org/standard/70513.html (accessed on 5 July 2023).
- Reyes, P.; D’hooge, D.R.; Cardon, L.; Cornillie, P. From Identifying Polymeric Resins to Corrosion Casting Applications. J. Appl. Polym. Sci. 2022, 139, 52284. [Google Scholar] [CrossRef]


| Group | Case | Mon_a | Mon_b | Mon_c | Mon_d | Mon_e | Mon_f | cM0/cI0 | wA0/wI0 |
|---|---|---|---|---|---|---|---|---|---|
| I | A | 98.0 | --- | 1.0 | 1.0 | --- | --- | 100 | 1.0 |
| B | 90.0 | 8.0 | 1.0 | 1.0 | --- | --- | 80 | 0.5 | |
| C | 81.0 | 15.0 | 2.0 | 2.0 | --- | --- | 100 | 1.0 | |
| D | 82.0 | 8.0 | 5.0 | 5.0 | --- | --- | 100 | 1.0 | |
| II | E | 75.0 | 15.0 | 5.0 | 5.0 | --- | --- | 200 | 1.0 |
| F | 81.0 | 15.0 | 3.0 | 1.0 | --- | --- | 200 | 1.0 | |
| G | 81.0 | 15.0 | 1.0 | 3.0 | --- | --- | 200 | 1.0 | |
| III | H | 87.0 | 10.0 | 1.5 | 1.5 | --- | --- | 200 | 1.0 |
| I | 76.0 | 20.0 | 2.0 | 2.0 | --- | --- | 200 | 1.0 | |
| IV | J | 75.0 | 15.0 | --- | --- | 5.0 | 5.0 | 200 | 1.0 |
| K | 81.0 | 15.0 | --- | --- | 2.0 | 2.0 | 200 | 1.0 | |
| L | 65.0 | 15.0 | --- | --- | 10.0 | 10.0 | 250 | 1.0 | |
| V | M | 75.0 | 15.0 | 5.0 | --- | --- | 5.0 | 250 | 1.0 |
| N | 60.0 | 15.0 | 12.5 | --- | --- | 12.5 | 350 | 0.9 |
| Case | Subcase | cM0/cI0 | wA0/wI0 | Case | Subcase | cM0/cI0 | wA0/wI0 |
|---|---|---|---|---|---|---|---|
| E | E1 | 200 | 1 | M | M1 | 250 | 1 |
| E2 | 400 | 1 | M2 | 500 | 1 | ||
| E3 | 600 | 1 | M3 | 750 | 1 | ||
| E4 | 200 | 0.7 | M4 | 250 | 0.7 | ||
| E5 | 400 | 0.7 | M5 | 500 | 0.7 | ||
| E6 | 600 | 0.7 | M6 | 750 | 0.7 | ||
| J | J1 | 200 | 1 | N | N1 | 350 | 0.9 |
| J2 | 400 | 1 | N2 | 525 | 0.9 | ||
| J3 | 600 | 1 | N3 | 800 | 0.9 | ||
| J4 | 200 | 0.7 | N4 | 350 | 0.7 | ||
| J5 | 400 | 0.7 | N5 | 525 | 0.7 | ||
| J6 | 600 | 0.7 | N6 | 800 | 0.7 | ||
| L | L1 | 250 | 1 | O | O1 | 100 | 1 |
| L2 | 500 | 1 | O2 | 200 | 1 | ||
| L3 | 750 | 1 | O3 | 400 | 1 | ||
| L4 | 250 | 0.7 | O4 | 100 | 0.7 | ||
| L5 | 500 | 0.7 | O5 | 200 | 0.7 | ||
| L6 | 750 | 0.7 | O6 | 400 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, P.; Edeleva, M.; D’hooge, D.R.; Cardon, L.; Cornillie, P. Multicomponent Acrylic Formulation Design for Corrosion Casting with Controlled Mechanical Properties. Polymers 2023, 15, 3236. https://doi.org/10.3390/polym15153236
Reyes P, Edeleva M, D’hooge DR, Cardon L, Cornillie P. Multicomponent Acrylic Formulation Design for Corrosion Casting with Controlled Mechanical Properties. Polymers. 2023; 15(15):3236. https://doi.org/10.3390/polym15153236
Chicago/Turabian StyleReyes, Pablo, Mariya Edeleva, Dagmar R. D’hooge, Ludwig Cardon, and Pieter Cornillie. 2023. "Multicomponent Acrylic Formulation Design for Corrosion Casting with Controlled Mechanical Properties" Polymers 15, no. 15: 3236. https://doi.org/10.3390/polym15153236
APA StyleReyes, P., Edeleva, M., D’hooge, D. R., Cardon, L., & Cornillie, P. (2023). Multicomponent Acrylic Formulation Design for Corrosion Casting with Controlled Mechanical Properties. Polymers, 15(15), 3236. https://doi.org/10.3390/polym15153236










