Smart Polymers for Soft Materials: From Solution Processing to Organic Solids
Abstract
:1. Overview
2. Co-Nonsolvency
2.1. Flory–Huggins Mean Field Description
2.2. Cooperativity Effect
2.3. Preferential Interactions
2.3.1. Solvation Thermodynamics: Kirkwood–Buff Theory of Solution
2.3.2. Competitive Displacement of Solvents by Cosolvents
3. Cosolvency
Flory–Huggins Mean Field Theory
4. Design of Complex Copolymers in Mixed Solvents
5. Heat Flow in Smart Polymers
5.1. Thermal Switching in Smart Responsive Polymers
5.2. Smart Polymers for Organic Solids
5.2.1. Tuning via Nonbonded Interactions
5.2.2. Tuning via Bonded Interactions
5.3. Classical Simulations and Comparing with the Experimental Data
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staudinger, H. Uber Polymerisation. Eur. J. Inorg. Chem. 1920, 53, 1073–1085. [Google Scholar]
- Köger, M. Simple models for complex nonequilibrium fluids. Phys. Rep. 2004, 390, 453–551. [Google Scholar] [CrossRef]
- Cohen-Stuart, M.A.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Gao, H.; Bettscheider, S.; Kraus, T.; Müser, M.H. Entropy Can Bundle Nanowires in Good Solvents. Nano Lett. 2019, 19, 6993–6999. [Google Scholar] [CrossRef]
- Müller, M. Process-directed self-assembly of copolymers: Results of and challenges for simulation studies. Prog. Polym. Sci. 2020, 101, 101198. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Smart responsive polymers: Fundamentals and design principles. Annu. Rev. Condens. Matter Phys. 2020, 11, 271–299. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ng, D.Y.W.; Weil, T. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. Prog. Polym. Sci. 2020, 105, 101241. [Google Scholar] [CrossRef]
- Forero-Martinez, N.C.; Lin, K.H.; Kremer, K.; Andrienko, D. Virtual Screening for Organic Solar Cells and Light Emitting Diodes. Adv. Sci. 2022, 9, 2200825. [Google Scholar] [CrossRef]
- Mendrek, B.; Oleszko-Torbus, N.; Teper, P.; Kowalczuk, A. Towards next generation polymer surfaces: Nano- and microlayers of star macromolecules and their design for applications in biology and medicine. Prog. Polym. Sci. 2023, 139, 101657. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford Science Publications: Oxford, UK, 1986. [Google Scholar]
- de Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Cloizeaux, J.D.; Jannink, G. Polymers in Solution: Their Modelling and Structure; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Meyer, D.E.; Chilkoti, A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Buurma, N.J.; Haq, I.; Turner, C.; Armes, S.P.; Castelletto, V.; Hamley, I.W.; Lewis, A.L. Synthesis and Characterization of Biocompatible, Thermoresponsive ABC and ABA Triblock Copolymer Gelators. Langmuir 2005, 21, 11026–11033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific Ion Effects on the Water Solubility of Macromolecules: PNIPAM and the Hofmeister Series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Cui, S.; Pang, X.; Zhang, S.; Yu, Y.; Ma, H.; Zhang, X. Unexpected Temperature-Dependent Single Chain Mechanics of Poly(N-isopropyl-acrylamide) in Water. Langmuir 2012, 28, 5151–5157. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen Bridges in Crystal Engineering: Interactions without Borders. Accounts Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Bogdanowicz, D.R.; Lu, H.H.; Koberstein, J.T. Polyacetals: Water-Soluble, pH-Degradable Polymers with Extraordinary Temperature Response. Macromolecules 2016, 49, 1858–1864. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of stereochemistry and copolymerization on the LCST of PNIPAm. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Zhang, W. A new thermoresponsive polymer of poly(N-acryloylsarcosine methyl ester) with a tunable LCST. Polym. Chem. 2017, 8, 3090–3101. [Google Scholar] [CrossRef]
- Kratz, K.; Hellweg, T.; Eimer, W. Structural changes in PNIPAM microgel particles as seen by SANS, DLS, and EM techniques. Polymer 2001, 42, 6631–6639. [Google Scholar] [CrossRef]
- Scherzinger, C.; Holderer, O.; Richter, D.; Richtering, W. Polymer dynamics in responsive microgels: Influence of cononsolvency and microgel architecture. Phys. Chem. Chem. Phys. 2012, 14, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Backes, S.; Krause, P.; Tabaka, W.; Witt, M.U.; Mukherji, D.; Kremer, K.; von Klitzing, R. Poly(N-isopropylacrylamide) Microgels under Alcoholic Intoxication: When a LCST Polymer Shows Swelling with Increasing Temperature. ACS Macro Lett. 2017, 6, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Nakamura, T.; Miyamoto, K.; Tokita, M.; Komai, T. Multiple volume phase transition of nonionic thermosensitive gel. J. Chem. Phys. 1995, 103, 6241–6247. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Marques, C.M.; Netz, P.A. Molecular dynamics study of the LCST transition in aqueous poly(N-n-propylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 10100–10107. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics, 3rd ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Jeppesen, C.; Kremer, K. Single-chain collapse as a first-order transition: Model for PEO in water. Europhys. Lett. 1996, 34, 563. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yang, H.E.; Bae, Y.C. Molecular simulations and thermodynamic modeling for closed-loop phase miscibility of aqueous PEO solutions. Macromol. Res. 2013, 21, 921–930. [Google Scholar] [CrossRef]
- Hocine, S.; Li, M.H. Thermoresponsive self-assembled polymer colloids in water. Soft Matter 2013, 9, 5839–5861. [Google Scholar] [CrossRef]
- Badi, N. Non-linear PEG-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Gernandt, J.; Frenning, G.; Richtering, W.; Hansson, P. A model describing the internal structure of core/shell hydrogels. Soft Matter 2011, 7, 10327–10338. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, K.; Song, Q.; Liu, S. Thermo-Induced Formation of Unimolecular and Multimolecular Micelles from Novel Double Hydrophilic Multiblock Copolymers of N,N-Dimethylacrylamide and N-Isopropylacrylamide. Langmuir 2007, 23, 13076–13084. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.G.; Smart, T.P.; Jackson, A.J.; Sullivan, M.O.; Epps, T.H. Structural changes in block copolymer micelles induced by cosolvent mixtures. Soft Matter 2011, 7, 7094–7102. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch It Inside-Out: “Schizophrenic” Behavior of All Thermoresponsive UCST–LCST Diblock Copolymers. Langmuir 2019, 35, 9660–9676. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.; Chen, J.; Cheung, C.; Chilkoti, A.; Ding, Z.; Dong, L.; Fong, R.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar] [CrossRef]
- Shen, Z.; Terao, K.; Maki, Y.; Dobashi, T.; Ma, G.; Yamamoto, T. Synthesis and phase behavior of aqueous poly(N-isopropylacrylamide-co-acrylamide), poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) and poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate). Colloid Polym. Sci. 2006, 284, 1001. [Google Scholar] [CrossRef] [Green Version]
- De Silva, C.C.; Leophairatana, P.; Ohkuma, T.; Koberstein, J.T.; Kremer, K.; Mukherji, D. Sequence transferable coarse-grained model of amphiphilic copolymers. J. Chem. Phys. 2017, 147, 064904. [Google Scholar] [CrossRef]
- Tschöp, W.; Kremer, K.; Batoulis, J.; Bürger, T.; Hahn, O. Simulation of polymer melts. I. Coarse-graining procedure for polycarbonates. Acta Polym. 1998, 49, 61–74. [Google Scholar] [CrossRef]
- Tschöp, W.; Kremer, K.; Hahn, O.; Batoulis, J.; Bürger, T. Simulation of polymer melts. II. From coarse-grained models back to atomistic description. Acta Polym. 1998, 49, 75–79. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Netz, P.A.; Kremer, K.; Junghans, C.; Mukherji, D. C-IBI: Targeting cumulative coordination within an iterative protocol to derive coarse-grained models of (multi-component) complex fluids. J. Chem. Phys. 2016, 144, 174106. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Genetically Encoded Synthesis of Protein-Based Polymers with Precisely Specified Molecular Weight and Sequence by Recursive Directional Ligation: Examples from the Elastin-like Polypeptide System. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef]
- McPherson, D.T.; Xu, J.; Urry, D.W. Product Purification by Reversible Phase Transition FollowingEscherichia coliExpression of Genes Encoding up to 251 Repeats of the Elastomeric Pentapeptide GVGVP. Protein Expr. Purif. 1996, 7, 51–57. [Google Scholar] [CrossRef]
- Zhao, B.; Li, N.K.; Yingling, Y.G.; Hall, C.K. LCST Behavior is Manifested in a Single Molecule: Elastin-like polypeptide (VPGVG)n. Biomacromolecules 2016, 17, 111–118. [Google Scholar] [CrossRef]
- Prhashanna, A.; Taylor, P.A.; Qin, J.; Kiick, K.L.; Jayaraman, A. Effect of Peptide Sequence on the LCST-like Transition of Elastin-like Peptides and Elastin-like Peptide–Collagen-like Peptide Conjugates: Simulations and Experiments. Biomacromolecules 2019, 20, 1178–1189. [Google Scholar] [CrossRef]
- Dugave, C.; Demange, L. Cis-Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef]
- Zhao, Y.; Kremer, K. Proline Isomerization Regulates the Phase Behavior of Elastin-like Polypeptides in Water. J. Phys. Chem. B 2021, 125, 9751–9756. [Google Scholar] [CrossRef]
- Schild, H.G.; Muthukumar, M.; Tirrell, D.A. Cononsolvency in mixed aqueous solutions of poly(N-isopropylacrylamide). Macromolecules 1991, 24, 948–952. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ringsdorf, H.; Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 1990, 23, 2415–2416. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, C. Reentrant Coil-to-Globule-to-Coil Transition of a Single Linear Homopolymer Chain in a Water-Methanol Mixture. Phys. Rev. Lett. 2001, 86, 822–825. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, A.; Maekawa, Y.; Yoshida, M.; Kubota, K.; Katakai, R. Volume phase transitions of poly(acryloyl-l-proline methyl ester) gels in response to water–alcohol composition. Polymer 2001, 42, 1863–1867. [Google Scholar] [CrossRef]
- Kiritoshi, Y.; Ishihara, K. Preparation of cross-linked biocompatible poly(2-methacryloyloxyethyl phosphorylcholine) gel and its strange swelling behavior in water/ethanol mixture. J. Biomater. Sci. Polym. Ed. 2002, 13, 213–224. [Google Scholar] [CrossRef]
- Tanaka, F.; Koga, T.; Winnik, F.m.c.M. Temperature-Responsive Polymers in Mixed Solvents: Competitive Hydrogen Bonds Cause Cononsolvency. Phys. Rev. Lett. 2008, 101, 028302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagle, L.B.; Zhang, Y.; Litosh, V.A.; Chen, X.; Cho, Y.; Cremer, P.S. Investigating the Hydrogen-Bonding Model of Urea Denaturation. J. Am. Chem. Soc. 2009, 131, 9304–9310. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F.; Scherzinger, C.; Richtering, W. Temperature dependent phase behavior of PNIPAM microgels in mixed water/methanol solvents. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1100–1111. [Google Scholar] [CrossRef]
- Walter, J.; Sehrt, J.; Vrabec, J.; Hasse, H. Molecular Dynamics and Experimental Study of Conformation Change of Poly(N-isopropylacrylamide) Hydrogels in Mixtures of Water and Methanol. J. Phys. Chem. B 2012, 116, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Heyda, J.; Muzdalo, A.; Dzubiella, J. Rationalizing Polymer Swelling and Collapse under Attractive Cosolvent Conditions. Macromolecules 2013, 46, 1231–1238. [Google Scholar] [CrossRef]
- Mukherji, D.; Kremer, K. Coil–Globule–Coil Transition of PNIPAm in Aqueous Methanol: Coupling All-Atom Simulations to Semi-Grand Canonical Coarse-Grained Reservoir. Macromolecules 2013, 46, 9158–9163. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; Trappe, V. Co-nonsolvency of PNiPAM at the transition between solvation mechanisms. Soft Matter 2014, 10, 8288–8295. [Google Scholar] [CrossRef] [Green Version]
- Dudowicz, J.; Freed, K.F.; Douglas, J.F. Communication: Cosolvency and cononsolvency explained in terms of a Flory-Huggins type theory. J. Chem. Phys. 2015, 143, 131101. [Google Scholar] [CrossRef] [PubMed]
- Kyriakos, K.; Philipp, M.; Lin, C.H.; Dyakonova, M.; Vishnevetskaya, N.; Grillo, I.; Zaccone, A.; Miasnikova, A.; Laschewsky, A.; Müller-Buschbaum, P.; et al. Quantifying the Interactions in the Aggregation of Thermoresponsive Polymers: The Effect of Cononsolvency. Macromol. Rapid Commun. 2016, 37, 420–425. [Google Scholar] [CrossRef]
- Micciulla, S.; Michalowsky, J.; Schroer, M.A.; Holm, C.; von Klitzing, R.; Smiatek, J. Concentration dependent effects of urea binding to poly(N-isopropylacrylamide) brushes: A combined experimental and numerical study. Phys. Chem. Chem. Phys. 2016, 18, 5324–5335. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.w.; Chen, L. Effects of cosolvent partitioning on conformational transitions and chain flexibility of thermoresponsive microgels. Phys. Rev. E 2019, 99, 022501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.W. Effects of cosolvent partitioning on conformational transitions and tethered chain flexibility in spherical polymer brushes. Soft Matter 2021, 17, 6817–6832. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, H.A.; Haro-Pérez, C.; Vázquez-Contreras, E.; Klapp, J.; Bautista-Carbajal, G.; Odriozola, G. P-NIPAM in water–acetone mixtures: Experiments and simulations. Phys. Chem. Chem. Phys. 2019, 21, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Sakota, K.; Tabata, D.; Sekiya, H. Macromolecular Crowding Modifies the Impact of Specific Hofmeister Ions on the Coil–Globule Transition of PNIPAM. J. Phys. Chem. B 2015, 119, 10334–10340. [Google Scholar] [CrossRef] [PubMed]
- Okur, H.I.; Hladílková, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef]
- Shultz, A.R.; Flory, P.J. Polymer chain dimensions in mixed-solvent media. J. Polym. Sci. 1955, 15, 231–242. [Google Scholar] [CrossRef]
- Read, B.E. A light-scattering study of preferential adsorption in the system benzene + cyclohexane + polystyrene. Trans. Faraday Soc. 1960, 56, 382–390. [Google Scholar] [CrossRef]
- Wolf, B.A.; Willms, M.M. Measured and calculated solubility of polymers in mixed solvents: Co-nonsolvency. Die Makromol. Chem. 1978, 179, 2265–2277. [Google Scholar] [CrossRef]
- Mukherji, D.; Wagner, M.; Watson, M.D.; Winzen, S.; de Oliveira, T.E.; Marques, C.M.; Kremer, K. Relating side chain organization of PNIPAm with its conformation in aqueous methanol. Soft Matter 2016, 12, 7995–8003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.E.; Ding, E.; Olsen, B.D. Cononsolvency of Elastin-like Polypeptides in Water/Alcohol Solutions. Biomacromolecules 2019, 20, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
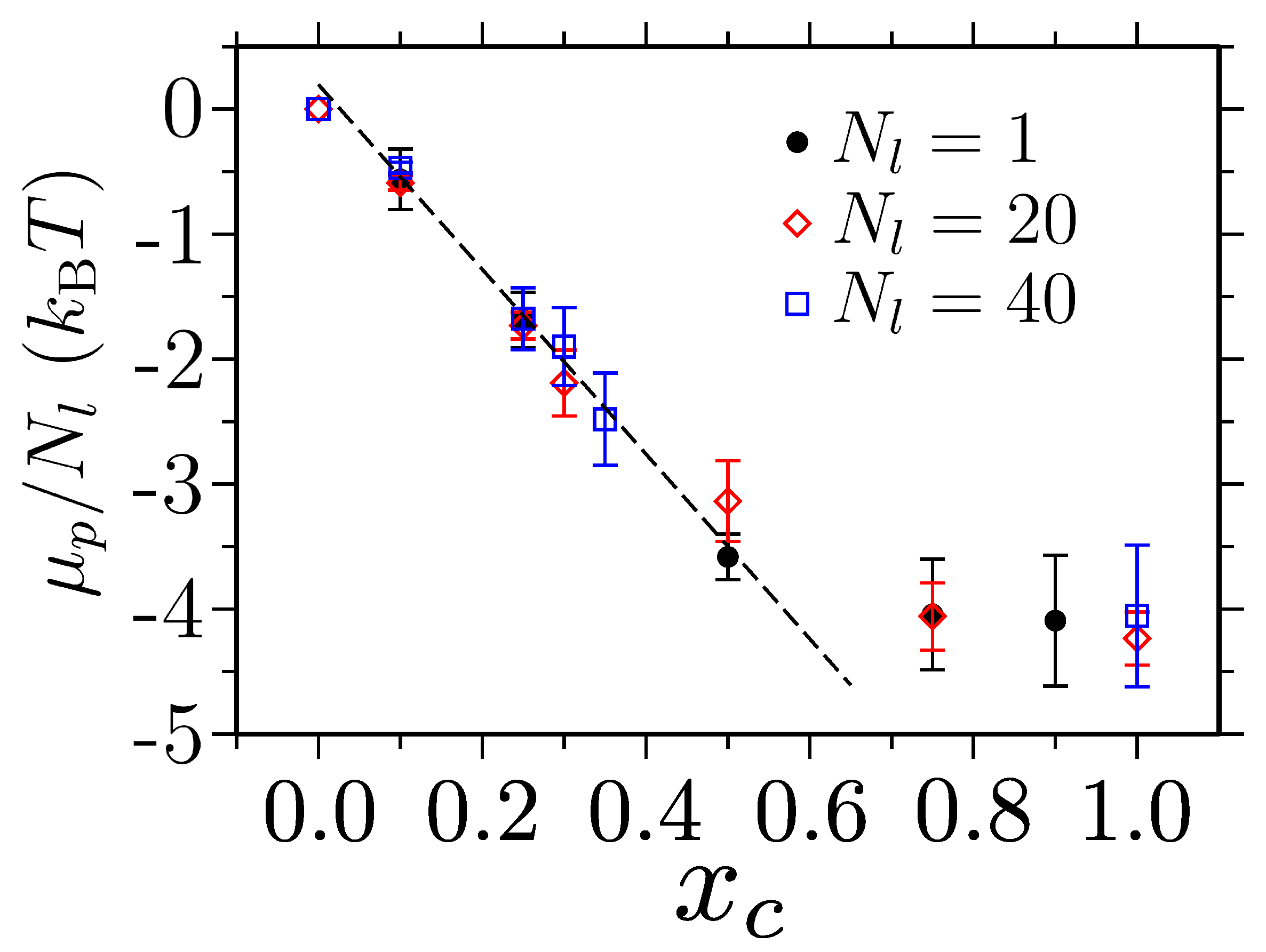
- Zhao, Y.; Singh, M.K.; Kremer, K.; Cortes-Huerto, R.; Mukherji, D. Why Do Elastin-like Polypeptides Possibly Have Different Solvation Behaviors in Water-Ethanol and Water-Urea Mixtures? Macromolecules 2020, 53, 2101–2110. [Google Scholar] [PubMed]
- Fernandez-Pierola, I.; Horta, A. Co–nonsolvency of PMMA. Polym. Bull. 1980, 3, 273–278. [Google Scholar] [CrossRef]
- Lund, R.; Willner, L.; Stellbrink, J.; Radulescu, A.; Richter, D. Role of Interfacial Tension for the Structure of PEP-PEO Polymeric Micelles. A Combined SANS and Pendant Drop Tensiometry Investigation. Macromolecules 2004, 37, 9984–9993. [Google Scholar] [CrossRef] [Green Version]
- Ohkura, M.; Kanaya, T.; Keisuke, K. Gels of poly(vinyl alcohol) from dimethyl sulphoxide/water solutions. Polymer 1992, 33, 3686–3690. [Google Scholar] [CrossRef]
- Jia, D.; Zuo, T.; Rogers, S.; Cheng, H.; Hammouda, B.; Han, C.C. Re-entrance of Poly(N,N-diethylacrylamide) in D2O/d-Ethanol Mixture at 27 °C. Macromolecules 2016, 49, 5152–5159. [Google Scholar] [CrossRef]
- Higaki, Y.; Kuraoka, N.; Masuda, T.; Nakamura, M.; Hifumi, E. Cononsolvency of poly(carboxybetaine methacrylate) in water–ethanol mixed solvents. Polym. J. 2023, 55, 869–876. [Google Scholar] [CrossRef]
- Scherzinger, C.; Lindner, P.; Keerl, M.; Richtering, W. Cononsolvency of Poly(N,N-diethylacrylamide) (PDEAAM) and Poly(N-isopropylacrylamide) (PNIPAM) Based Microgels in Water/Methanol Mixtures: Copolymer vs Core-Shell Microgel. Macromolecules 2010, 43, 6829–6833. [Google Scholar] [CrossRef]
- Tanaka, F.; Koga, T.; Kojima, H.; Xue, N.; Winnik, F.M. Preferential Adsorption and Co-nonsolvency of Thermoresponsive Polymers in Mixed Solvents of Water/Methanol. Macromolecules 2011, 44, 2978–2989. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Collapse in two good solvents, swelling in two poor solvents: Defying the laws of polymer solubility? J. Phys. Condens. Matter 2017, 30, 024002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, H.; Merlitz, H.; Fery, A.; Sommer, J.U. Polymer Brushes and Gels in Competing Solvents: The Role of Different Interactions and Quantitative Predictions for Poly(N-isopropylacrylamide) in Alcohol–Water Mixtures. Macromolecules 2020, 53, 2323–2335. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Polymer collapse in miscible good solvents is a generic phenomenon driven by preferential adsorption. Nat. Commun. 2014, 5, 4882. [Google Scholar]
- Mukherji, D.; Marques, C.M.; Stuehn, T.; Kremer, K. Co-non-solvency: Mean-field polymer theory does not describe polymer collapse transition in a mixture of two competing good solvents. J. Chem. Phys. 2015, 142, 114903. [Google Scholar] [CrossRef]
- Jia, D.; Muthukumar, M.; Cheng, H.; Han, C.C.; Hammouda, B. Concentration Fluctuations near Lower Critical Solution Temperature in Ternary Aqueous Solutions. Macromolecules 2017, 50, 7291–7298. [Google Scholar] [CrossRef]
- Naim, A.B. Molecular Theory of Solutions; Oxford University Press: London, UK, 2006. [Google Scholar]
- Marcus, Y. Preferential solvation in mixed solvents Part 8. Aqueous methanol from sub-ambient to elevated temperatures. Phys. Chem. Chem. Phys. 1999, 1, 2975–2983. [Google Scholar] [CrossRef]
- Mukherji, D.; van der Vegt, N.F.A.; Kremer, K.; Delle Site, L. Kirkwood–Buff Analysis of Liquid Mixtures in an Open Boundary Simulation. J. Chem. Theory Comput. 2012, 8, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.U. Adsorption–Attraction Model for Co-Nonsolvency in Polymer Brushes. Macromolecules 2017, 50, 2219–2228. [Google Scholar] [CrossRef]
- Sommer, J.U. Gluonic and Regulatory Solvents: A Paradigm for Tunable Phase Segregation in Polymers. Macromolecules 2018, 51, 3066–3074. [Google Scholar] [CrossRef]
- Okada, Y.; Tanaka, F. Cooperative Hydration, Chain Collapse, and Flat LCST Behavior in Aqueous Poly(N-isopropylacrylamide) Solutions. Macromolecules 2005, 38, 4465–4471. [Google Scholar] [CrossRef]
- Tanaka, F. Thermoreversible Gelation of Associating Polymers in Hydrogen-Bonding Mixed Solvents. Langmuir 2022, 38, 5098–5110. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Wagner, M.; Watson, M.D.; Winzen, S.; de Oliveira, T.E.; Marques, C.M.; Kremer, K. Reply to the ‘Comment on “Relating side chain organization of PNIPAm with its conformation in aqueous methanol”’ by A Pica and G. Graziano. Soft Matter 2017, 13, 7701–7703. [Google Scholar] [CrossRef]
- Magda, J.J.; Fredrickson, G.H.; Larson, R.G.; Helfand, E. Dimensions of a polymer chain in a mixed solvent. Macromolecules 1988, 21, 726–732. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ottaviani, M.F.; Bossmann, S.H.; Garcia-Garibay, M.; Turro, N.J. Consolvency of poly(N-isopropylacrylamide) in mixed water-methanol solutions: A look at spin-labeled polymers. Macromolecules 1992, 25, 6007–6017. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Liu, B.; Bai, J.; Gong, P.; Ru, G.; Feng, J. Preferential adsorption of the additive is not a prerequisite for cononsolvency in water-rich mixtures. Phys. Chem. Chem. Phys. 2017, 19, 30097–30106. [Google Scholar] [CrossRef]
- Scholtz, J.; Barrick, D.; York, E.; Stewart, J.; Baldwin, R. Urea unfolding of peptide helices as a model for interpreting protein unfolding. Proc. Natl. Acad. Sci. USA 1995, 92, 185–189. [Google Scholar] [CrossRef]
- Stumpe, M.C.; Grubmüller, H. Interaction of urea with amino acids: Implications for urea-induced protein denaturation. J. Am. Chem. Soc. 2007, 129, 16126–16131. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.; Zhou, R.; Thirumalai, D.; Berne, B.J. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc. Natl. Acad. Sci. USA 2008, 105, 16928–16933. [Google Scholar] [CrossRef]
- Zangi, R.; Zhou, R.; Berne, B.J. Urea’s Action on Hydrophobic Interactions. J. Am. Chem. Soc. 2009, 131, 1535–1541. [Google Scholar] [CrossRef]
- England, J.L.; Haran, G. Role of Solvation Effects in Protein Denaturation: From Thermodynamics to Single Molecules and Back. Annu. Rev. Phys. Chem. 2011, 62, 257–277. [Google Scholar] [CrossRef] [Green Version]
- Elam, W.A.; Schrank, T.P.; Campagnolo, A.J.; Hilser, V.J. Temperature and urea have opposing impacts on polyproline II conformational bias. Biochemistry 2013, 52, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, S.; Jaganade, T.; Priyakumar, U.D. Urea-aromatic interactions in biology. Biophys. Rev. 2020, 12, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Udgaonkar, J.B. Heterogeneity in Protein Folding and Unfolding Reactions. Chem. Rev. 2022, 122, 8911–8935. [Google Scholar] [CrossRef] [PubMed]
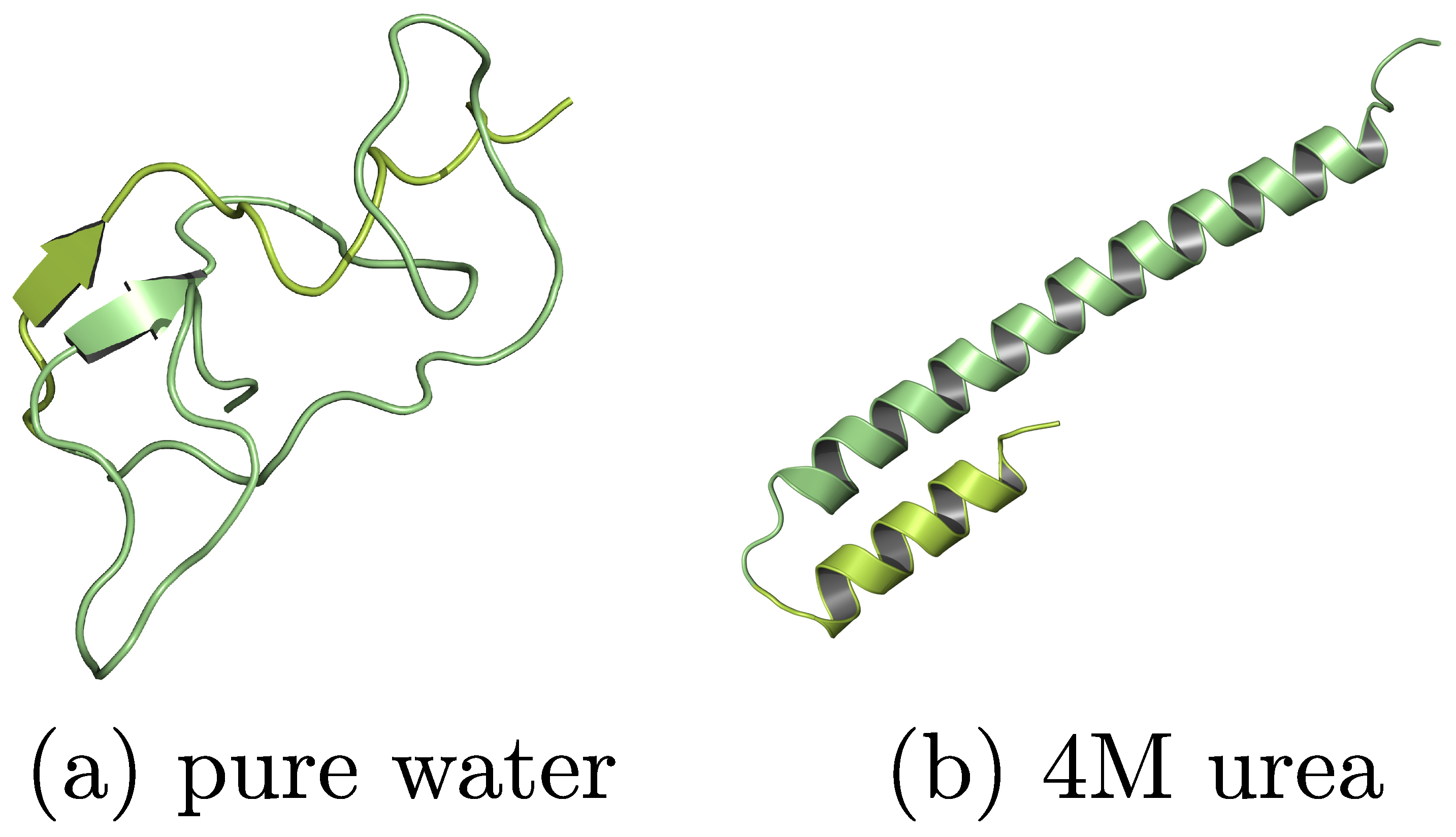
- Baptista, L.A.; Zhao, Y.; Kremer, K.; Mukherji, D.; Cortes-Huerto, R. Stabilizing α-Helicity of a Polypeptide in Aqueous Urea: Dipole Orientation or Hydrogen Bonding? ACS Macro Lett. 2023, 12, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Konstantinovsky, D.; Perets, E.A.; Santiago, T.; Velarde, L.; Hammes-Schiffer, S.; Yan, E.C.Y. Detecting the First Hydration Shell Structure around Biomolecules at Interfaces. ACS Cent. Sci. 2022, 8, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.G.; Buff, F.P. The Statistical Mechanical Theory of Solutions. I. J. Chem. Phys. 1951, 19, 774–777. [Google Scholar] [CrossRef]
- Galata, A.A.; Anogiannakis, S.D.; Theodorou, D.N. Thermodynamic analysis of Lennard-Jones binary mixtures using Kirkwood-Buff theory. Fluid Phase Equilibria 2018, 470, 25–37. [Google Scholar] [CrossRef]
- Sevilla, M.; Cortes-Huerto, R. Connecting density fluctuations and Kirkwood–Buff integrals for finite-size systems. J. Chem. Phys. 2022, 156, 044502. [Google Scholar] [CrossRef]
- Kang, M.; Smith, P.E. Preferential interaction parameters in biological systems by Kirkwood–Buff theory and computer simulation. Fluid Phase Equilibria 2007, 256, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Pillai, V.V.S.; Gobbo, D.; Ballone, P.; Benedetto, A. The transition from salt-in-water to water-in-salt nanostructures in water solutions of organic ionic liquids relevant for biological applications. Phys. Chem. Chem. Phys. 2021, 23, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Forero-Martinez, N.C.; Cortes-Huerto, R.; Benedetto, A.; Ballone, P. Thermoresponsive Ionic Liquid/Water Mixtures: From Nanostructuring to Phase Separation. Molecules 2022, 27, 1647. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; de Oliveira, T.E.; Ruscher, C.; Rottler, J. Thermodynamics, morphology, mechanics, and thermal transport of PMMA-PLA blends. Phys. Rev. Mater. 2022, 6, 025606. [Google Scholar] [CrossRef]
- Venetsanos, F.; Anogiannakis, S.D.; Theodorou, D.N. Mixing Thermodynamics and Flory–Huggins Interaction Parameter of Polyethylene Oxide/Polyethylene Oligomeric Blends from Kirkwood–Buff Theory and Molecular Simulations. Macromolecules 2022, 55, 4852–4862. [Google Scholar] [CrossRef]
- Müller, M.; Binder, K. An algorithm for the semi-grand-canonical simulation of asymmetric polymer mixtures. Comput. Phys. Commun. 1994, 84, 173–185. [Google Scholar] [CrossRef]
- Krüger, P.; Schnell, S.K.; Bedeaux, D.; Kjelstrup, S.; Vlugt, T.J.H.; Simon, J.M. Kirkwood–Buff Integrals for Finite Volumes. J. Phys. Chem. Lett. 2013, 4, 235–238. [Google Scholar] [CrossRef]
- Cortes-Huerto, R.; Kremer, K.; Potestio, R. Communication: Kirkwood-Buff integrals in the thermodynamic limit from small-sized molecular dynamics simulations. J. Chem. Phys. 2016, 145, 141103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rösgen, J.; Pettitt, B.M.; Bolen, D.W. Protein Folding, Stability, and Solvation Structure in Osmolyte Solutions. Biophys. J. 2005, 89, 2988–2997. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.L. An Introduction to Statistical Thermodynamics; Courier Dover Publications: Mineola, NY, USA, 1960. [Google Scholar]
- Wolf, B.A.; Blaum, G. Measured and calculated solubility of polymers in mixed solvents: Monotony and cosolvency. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1115–1132. [Google Scholar] [CrossRef]
- Yu, Y.; Kieviet, B.D.; Kutnyanszky, E.; Vancso, G.J.; de Beer, S. Cosolvency-Induced Switching of the Adhesion between Poly(methyl methacrylate) Brushes. ACS Macro Lett. 2015, 4, 75–79. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Becer, C.R.; Guerrero-Sanchez, C.; Hoeppener, S.; Schubert, U.S. Solubility and Thermoresponsiveness of PMMA in Alcohol-Water Solvent Mixtures. Aust. J. Chem. 2010, 63, 1173–1178. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, Y.C. Enhanced solvation effect of re-collapsing behavior for cross-linked PMMA particle gel in aqueous alcohol solutions. Polymer 2014, 55, 4684–4692. [Google Scholar] [CrossRef]
- Asadujjaman, A.; Ahmadi, V.; Yalcin, M.; ten Brummelhuis, N.; Bertin, A. Thermoresponsive functional polymers based on 2,6-diaminopyridine motif with tunable UCST behaviour in water/alcohol mixtures. Polym. Chem. 2017, 8, 3140–3153. [Google Scholar] [CrossRef]
- Oyarte Gálvez, L.; de Beer, S.; van der Meer, D.; Pons, A. Dramatic effect of fluid chemistry on cornstarch suspensions: Linking particle interactions to macroscopic rheology. Phys. Rev. E 2017, 95, 030602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
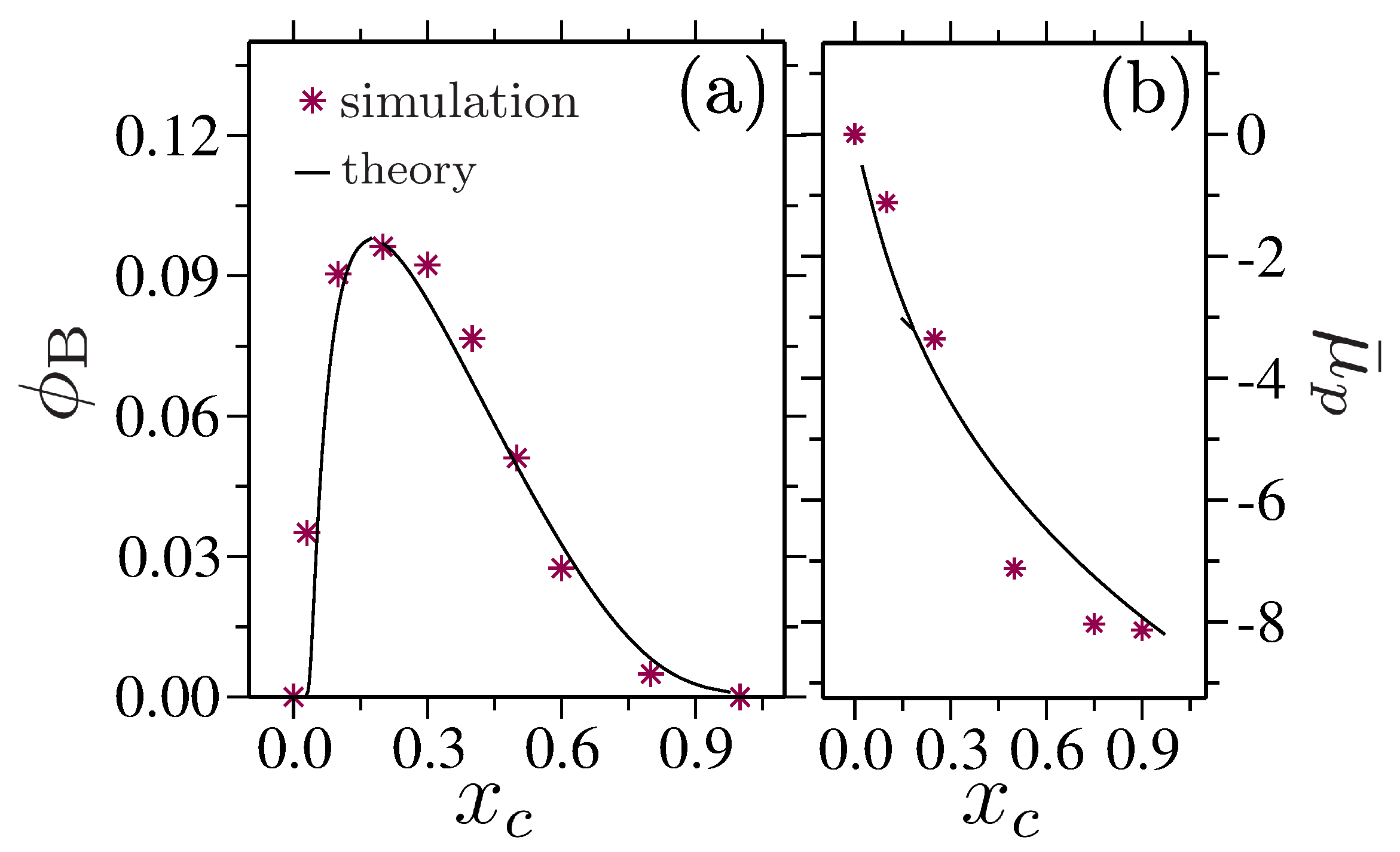
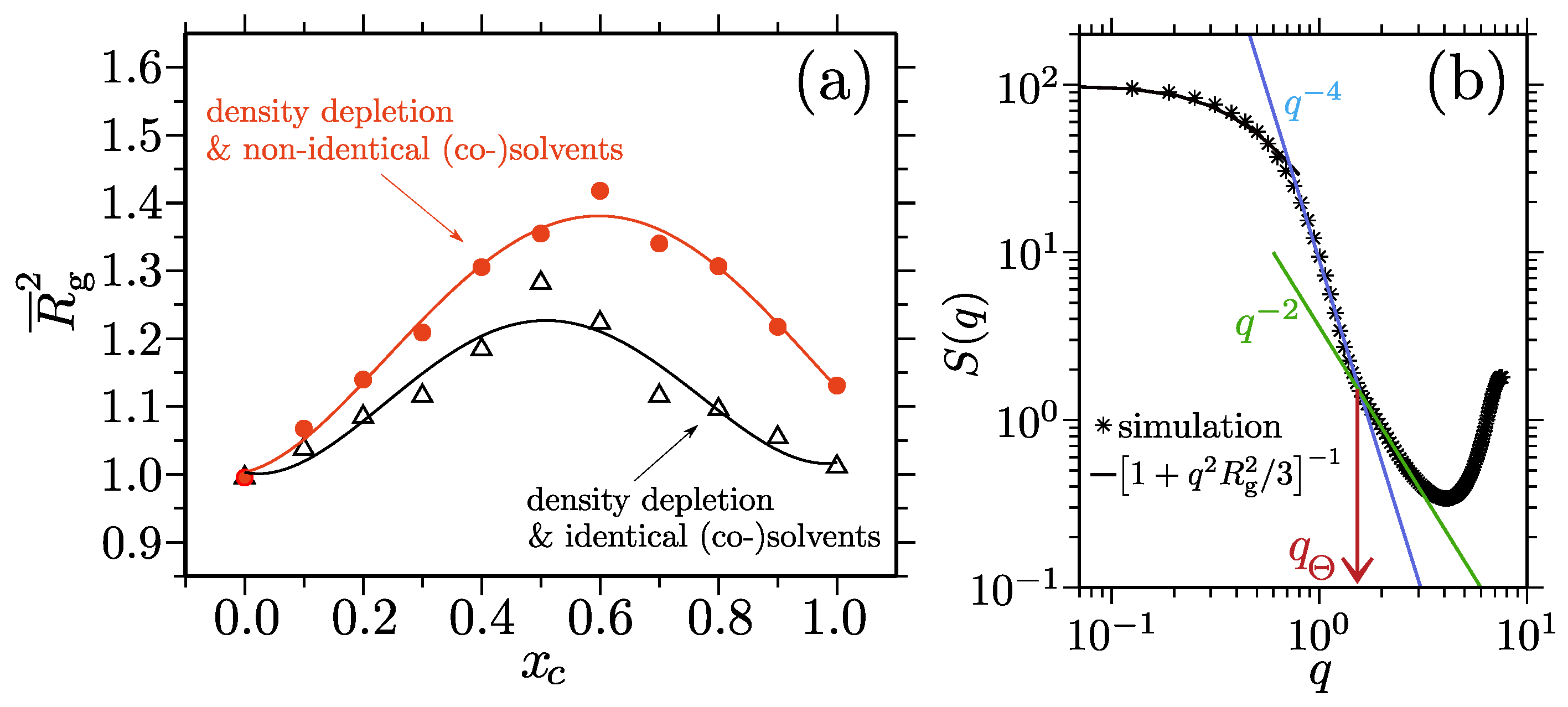
- Mukherji, D.; Marques, C.M.; Stühn, T.; Kremer, K. Depleted depletion drives polymer swelling in poor solvent mixtures. Nat. Commun. 2017, 8, 1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekkerkerker, H.N.W.; Tuinier, R. Colloids and the Depletion Interaction; Clarendon: Oxford, UK, 1990. [Google Scholar]
- Mao, Y.; Cates, M.E.; Lekkerkerker, H.N.W. Depletion Stabilization by Semidilute Rods. Phys. Rev. Lett. 1995, 75, 4548–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Cates, M.; Lekkerkerker, H. Depletion force in colloidal systems. Phys. A Stat. Mech. Its Appl. 1995, 222, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Crocker, J.C.; Matteo, J.A.; Dinsmore, A.D.; Yodh, A.G. Entropic Attraction and Repulsion in Binary Colloids Probed with a Line Optical Tweezer. Phys. Rev. Lett. 1999, 82, 4352–4355. [Google Scholar] [CrossRef]
- Phillips, R.; Kondev, J.; Theriot, J.; Garcia, H. Physical Biology of the Cell, 2nd ed.; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Perera, A.; Sokolić, F.; Almásy, L.; Koga, Y. Kirkwood-Buff integrals of aqueous alcohol binary mixtures. J. Chem. Phys. 2006, 124, 124515. [Google Scholar] [CrossRef]
- Chang, D.P.; Dolbow, J.E.; Zauscher, S. Switchable Friction of Stimulus-Responsive Hydrogels. Langmuir 2007, 23, 250–257. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Moehwald, H. Adhesion and Mechanical Properties of PNIPAM Microgel Films and Their Potential Use as Switchable Cell Culture Substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Beer, S.; Kutnyanszky, E.; Schön, P.M.; Vancso, G.J.; Müser, M.H. Solvent-induced immiscibility of polymer brushes eliminates dissipation channels. Nat. Commun. 2014, 5, 3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, M.J.; Steen, P.H. Capillarity-based switchable adhesion. Proc. Natl. Acad. Sci. USA 2010, 107, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Watson, M.D.; Morsbach, S.; Schmutz, M.; Wagner, M.; Marques, C.M.; Kremer, K. Soft and Smart: Co-nonsolvency-Based Design of Multiresponsive Copolymers. Macromolecules 2019, 52, 3471–3478. [Google Scholar] [CrossRef]
- Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the Inside and the Outside of Aggregates of Water-Soluble Block Copolymers with Double Thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Hietala, S.; Nuopponen, M.; Kalliomäki, K.; Tenhu, H. Thermoassociating Poly(N-isopropylacrylamide) A-B-A Stereoblock Copolymers. Macromolecules 2008, 41, 2627–2631. [Google Scholar] [CrossRef]
- Plamper, F.A.; Steinschulte, A.A.; Hofmann, C.H.; Drude, N.; Mergel, O.; Herbert, C.; Erberich, M.; Schulte, B.; Winter, R.; Richtering, W. Toward Copolymers with Ideal Thermosensitivity: Solution Properties of Linear, Well-Defined Polymers of N-Isopropyl Acrylamide and N,N-Diethyl Acrylamide. Macromolecules 2012, 45, 8021–8026. [Google Scholar] [CrossRef]
- Yin, F.; Laborie, P.; Lonetti, B.; Gineste, S.; Coppel, Y.; Lauth-de Viguerie, N.; Marty, J.D. Dual Thermo- and pH-Responsive Block Copolymer of Poly(N-isopropylacrylamide)-block-Poly(N,N-diethylamino Ethyl Acrylamide): Synthesis, Characterization, Phase Transition, and Self-Assembly Behavior in Aqueous Solution. Macromolecules 2023, 56, 3703–3720. [Google Scholar] [CrossRef]
- Ko, C.H.; Henschel, C.; Meledam, G.P.; Schroer, M.A.; Guo, R.; Gaetani, L.; Müller-Buschbaum, P.; Laschewsky, A.; Papadakis, C.M. Co-Nonsolvency Effect in Solutions of Poly(methyl methacrylate)-b-poly(N-isopropylacrylamide) Diblock Copolymers in Water/Methanol Mixtures. Macromolecules 2021, 54, 5825–5837. [Google Scholar] [CrossRef]
- Henry, A. Thermal transport in polymers. Annu. Rev. Heat Transf. 2014, 17, 485–520. [Google Scholar] [CrossRef]
- Kim, G.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.S.; Gidley, D.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Tsai, T.; Liu, J.; Braun, P.V.; Cahill, D.G. Thermal Conductivity, Heat Capacity, and Elastic Constants of Water-Soluble Polymers and Polymer Blends. Macromolecules 2016, 49, 972–978. [Google Scholar] [CrossRef]
- Bruns, D.; de Oliveira, T.E.; Rottler, J.; Mukherji, D. Tuning Morphology and Thermal Transport of Asymmetric Smart Polymer Blends by Macromolecular Engineering. Macromolecules 2019, 52, 5510–5517. [Google Scholar] [CrossRef] [Green Version]
- Keblinski, P. Modeling of Heat Transport in Polymers and Their Nanocomposites. In Handbook of Materials Modeling; Springer International Publishing: Cham, Switzerland, 2020; pp. 975–997. [Google Scholar]
- Smith, M.K.; Singh, V.; Kalaitzidou, K.; Cola, B.A. High Thermal and Electrical Conductivity of Template Fabricated P3HT/MWCNT Composite Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 14788–14794. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Shuai, Z.; Wang, D. Tuning Thermal Transport in Chain-Oriented Conducting Polymers for Enhanced Thermoelectric Efficiency: A Computational Study. Adv. Funct. Mater. 2017, 27, 1702847. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower Limit to the Thermal Conductivity of Disordered Crystals. Phys. Rev. B 1990, 46, 6131–6140. [Google Scholar] [CrossRef]
- Cahill, D.G.; Ford, W.K.; Goodson, K.E.; Mahan, G.D.; Majumdar, A.; Maris, H.J.; Merlin, R.; Phillpot, S.R. Nanoscale Thermal Transport. J. Appl. Phys. 2003, 93, 793–818. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.R.; Topp, K.A.; Pohl, R.O. Specific Heat and Thermal Conductivity of Solid Fullerenes. Science 1993, 259, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bernardi, M.; Lunt, R.R.; Bulovic, V.; Grossman, J.C.; Gradecak, S. Toward Efficient Carbon Nanotube/P3HT Solar Cells: Active Layer Morphology, Electrical, and Optical Properties. Nano Lett. 2011, 11, 5316–5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fugallo, G.; Colombo, L. Calculating Lattice Thermal Conductivity: A Synopsis. Phys. Scr. 2018, 93, 043002. [Google Scholar] [CrossRef]
- Braun, J.L.; Rost, C.M.; Lim, M.; Giri, A.; Olson, D.H.; Kotsonis, G.N.; Stan, G.; Brenner, D.W.; Maria, J.P.; Hopkins, P.E. Charge-Induced Disorder Controls the Thermal Conductivity of Entropy-Stabilized Oxides. Adv. Mater. 2018, 30, 1805004. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef]
- Tomko, J.A.; Pena-Francesch, A.; Jung, H.; Tyagi, M.; Allen, B.D.; Demirel, M.C.; Hopkins, P.E. Tunable thermal transport and reversible thermal conductivity switching in topologically networked bio-inspired materials. Nat. Nanotechnol. 2018, 13, 959–964. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Tian, Z. Thermal Switching of Thermoresponsive Polymer Aqueous Solutions. ACS Macro Lett. 2018, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Tang, N.; An, M.; Guo, R.; Ma, D.; Yu, X.; Zang, J.; Yang, N. Thermally-Responsive Hydrogels Poly(N-Isopropylacrylamide) as the Thermal Switch. J. Phys. Chem. C 2019, 123, 31003–31010. [Google Scholar] [CrossRef]
- Mukherji, D.; Singh, M.K. Tuning thermal transport in highly cross-linked polymers by bond-induced void engineering. Phys. Rev. Mater. 2021, 5, 025602. [Google Scholar] [CrossRef]
- Lv, G.; Jensen, E.; Evans, C.M.; Cahill, D.G. High Thermal Conductivity Semicrystalline Epoxy Resins with Anthraquinone-Based Hardeners. ACS Appl. Polym. Mater. 2021, 3, 4430–4435. [Google Scholar] [CrossRef]
- Maurya, M.K.; Wu, J.; Singh, M.K.; Mukherji, D. Thermal Conductivity of Semicrystalline Polymer Networks: Crystallinity or Cross-Linking? ACS Macro Lett. 2022, 11, 925–929. [Google Scholar] [CrossRef]
- Zheng, R.; Gao, J.; Wang, J.; Chen, G. Reversible temperature regulation of electrical and thermal conductivity using liquid–solid phase transitions. Nat. Commun. 2011, 2, 289. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Peng, Z.; Guo, R.; An, M.; Chen, X.; Li, X.; Yang, N.; Zang, J. Thermal Transport in Soft PAAm Hydrogels. Polymers 2017, 9, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallecchi, E.; Chen, Z.; Fernandes, G.E.; Wan, Y.; Kim, J.H.; Xu, J. A thermal diode and novel implementation in a phase-change material. Mater. Horiz. 2015, 2, 125–129. [Google Scholar] [CrossRef]
- Wu, J.; Mukherji, D. Comparison of all atom and united atom models for thermal transport calculations of amorphous polyethylene. Comput. Mater. Sci. 2022, 211, 111539. [Google Scholar] [CrossRef]
- Shenogin, S.; Bodapati, A.; Keblinski, P.; McGaughey, A.J.H. Predicting the thermal conductivity of inorganic and polymeric glasses: The role of anharmonicity. J. Appl. Phys. 2009, 105, 034906. [Google Scholar] [CrossRef] [Green Version]
- Simavilla, D.N.; Sgouros, A.P.; Vogiatzis, G.; Tzoumanekas, C.; Georgilas, V.; Verbeeten, W.M.H.; Theodorou, D.N. Molecular Dynamics Test of the Stress-Thermal Rule in Polyethylene and Polystyrene Entangled Melts. Macromolecules 2020, 53, 789–802. [Google Scholar] [CrossRef]
- Ruscher, C.; Rottler, J.; Boott, C.E.; MacLachlan, M.J.; Mukherji, D. Elasticity and thermal transport of commodity plastics. Phys. Rev. Mater. 2019, 3, 125604. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.C.; Blase, X.; Roche, S. Electronic and transport properties of nanotubes. Rev. Mod. Phys. 2007, 79, 677–732. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, S.; Pigard, L.; Ryu, Y.K.; Lorenzoni, M.; Evangelio, L.; Fernández-Regúlez, M.; Rawlings, C.D.; Spieser, M.; Perez-Murano, F.; Müller, M.; et al. Thermal Imaging of Block Copolymers with Sub-10 nm Resolution. ACS Nano 2021, 15, 9005–9016. [Google Scholar] [CrossRef]
- Pigard, L.; Mukherji, D.; Rottler, J.; Müller, M. Microscopic Model to Quantify the Difference of Energy-Transfer Rates between Bonded and Nonbonded Monomers in Polymers. Macromolecules 2021, 54, 10969–10983. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Phani, A.S.; Nojeh, A.; Mukherji, D. Thermal Transport in Molecular Forests. ACS Nano 2021, 15, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Crist, B.; Hereña, P.G. Molecular orbital studies of polyethylene deformation. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 449–457. [Google Scholar] [CrossRef]
- Hsu, H.P.; Singh, M.K.; Cang, Y.; Thérien-Aubin, H.; Mezger, M.; Berger, R.; Lieberwirth, I.; Fytas, G.; Kremer, K. Free Standing Dry and Stable Nanoporous Polymer Films Made through Mechanical Deformation. Adv. Sci. 2023, 10, 2207472. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Jensen, E.; Shen, C.; Yang, K.; Evans, C.M.; Cahill, D.G. Effect of Amine Hardener Molecular Structure on the Thermal Conductivity of Epoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 259–267. [Google Scholar] [CrossRef]
- Lv, G.; Soman, B.; Shan, N.; Evans, C.M.; Cahill, D.G. Effect of Linker Length and Temperature on the Thermal Conductivity of Ethylene Dynamic Networks. ACS Macro Lett. 2021, 10, 1088–1093. [Google Scholar] [CrossRef]
- Wei, X.; Luo, T. Role of Ionization in Thermal Transport of Solid Polyelectrolytes. J. Phys. Chem. C 2019, 123, 12659–12665. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, W.P.; Losego, M.D.; Braun, P.V.; Shenogin, S.; Keblinski, P.; Cahill, D.G. Testing the minimum thermal conductivity model for amorphous polymers using high pressure. Phys. Rev. B 2011, 83, 174205. [Google Scholar] [CrossRef] [Green Version]
- Lv, G.; Shen, C.; Shan, N.; Jensen, E.; Li, X.; Evans, C.M.; Cahill, D.G. Odd–even effect on the thermal conductivity of liquidcrystalline epoxy resins. Proc. Natl. Acad. Sci. USA 2022, 119, e2211151119. [Google Scholar] [CrossRef]
- Lim, M.; Rak, Z.; Braun, J.L.; Rost, C.M.; Kotsonis, G.N.; Hopkins, P.E.; Maria, J.P.; Brenner, D.W. Influence of mass and charge disorder on the phonon thermal conductivity of entropy stabilized oxides determined by molecular dynamics simulations. J. Appl. Phys. 2019, 125, 055105. [Google Scholar] [CrossRef]
- Ahmed, J.; Wang, Q.J.; Balogun, O.; Ren, N.; England, R.; Lockwood, F. Molecular Dynamics Modeling of Thermal Conductivity of Several Hydrocarbon Base Oils. Tribol. Lett. 2023, 71, 70. [Google Scholar] [CrossRef]
- Gao, H.; Menzel, T.P.W.; Müser, M.H.; Mukherji, D. Comparing simulated specific heat of liquid polymers and oligomers to experiments. Phys. Rev. Mater. 2021, 5, 065605. [Google Scholar] [CrossRef]
- Bhowmik, R.; Sihn, S.; Varshney, V.; Roy, A.K.; Vernon, J.P. Calculation of specific heat of polymers using molecular dynamics simulations. Polymer 2019, 167, 176–181. [Google Scholar] [CrossRef]
- Demydiuk, F.; Solar, M.; Meyer, H.; Benzerara, O.; Paul, W.; Baschnagel, J. Role of torsional potential in chain conformation, thermodynamics, and glass formation of simulated polybutadiene melts. J. Chem. Phys. 2022, 156, 234902. [Google Scholar] [CrossRef] [PubMed]
- Horbach, J.; Kob, W.; Binder, K. Specific Heat of Amorphous Silica within the Harmonic Approximation. J. Phys. Chem. B 1999, 103, 4104–4108. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Brió Pérez, M.; Cao, C.; de Beer, S. Switching (bio-) adhesion and friction in liquid by stimulus responsive polymer coatings. Eur. Polym. J. 2021, 147, 110298. [Google Scholar] [CrossRef]


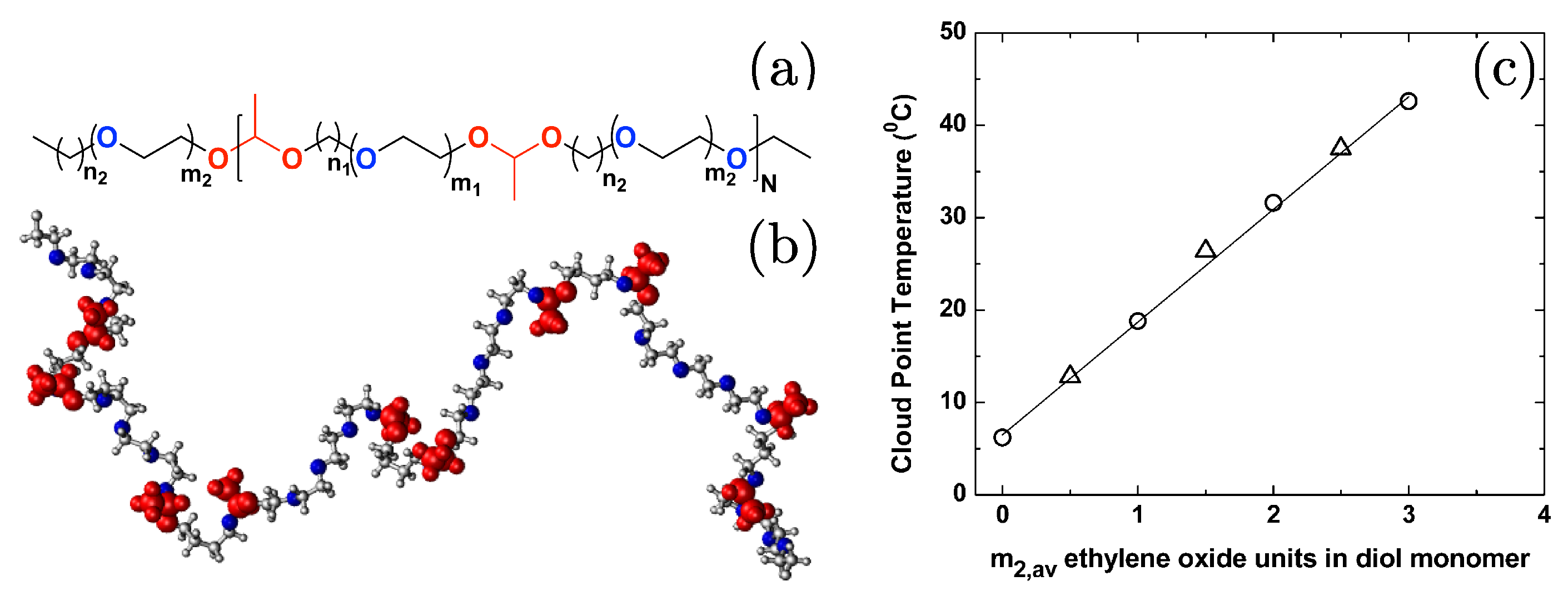
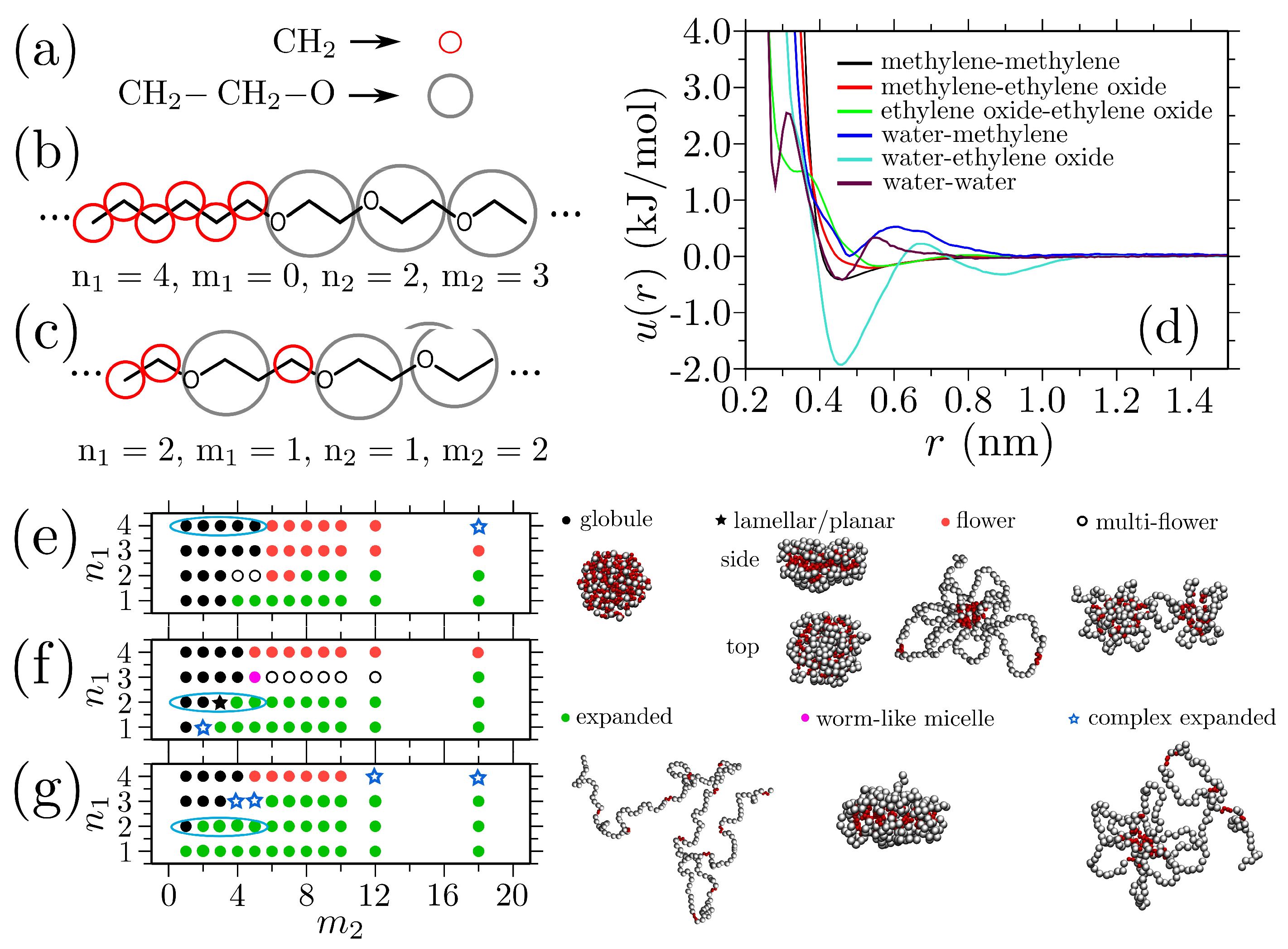
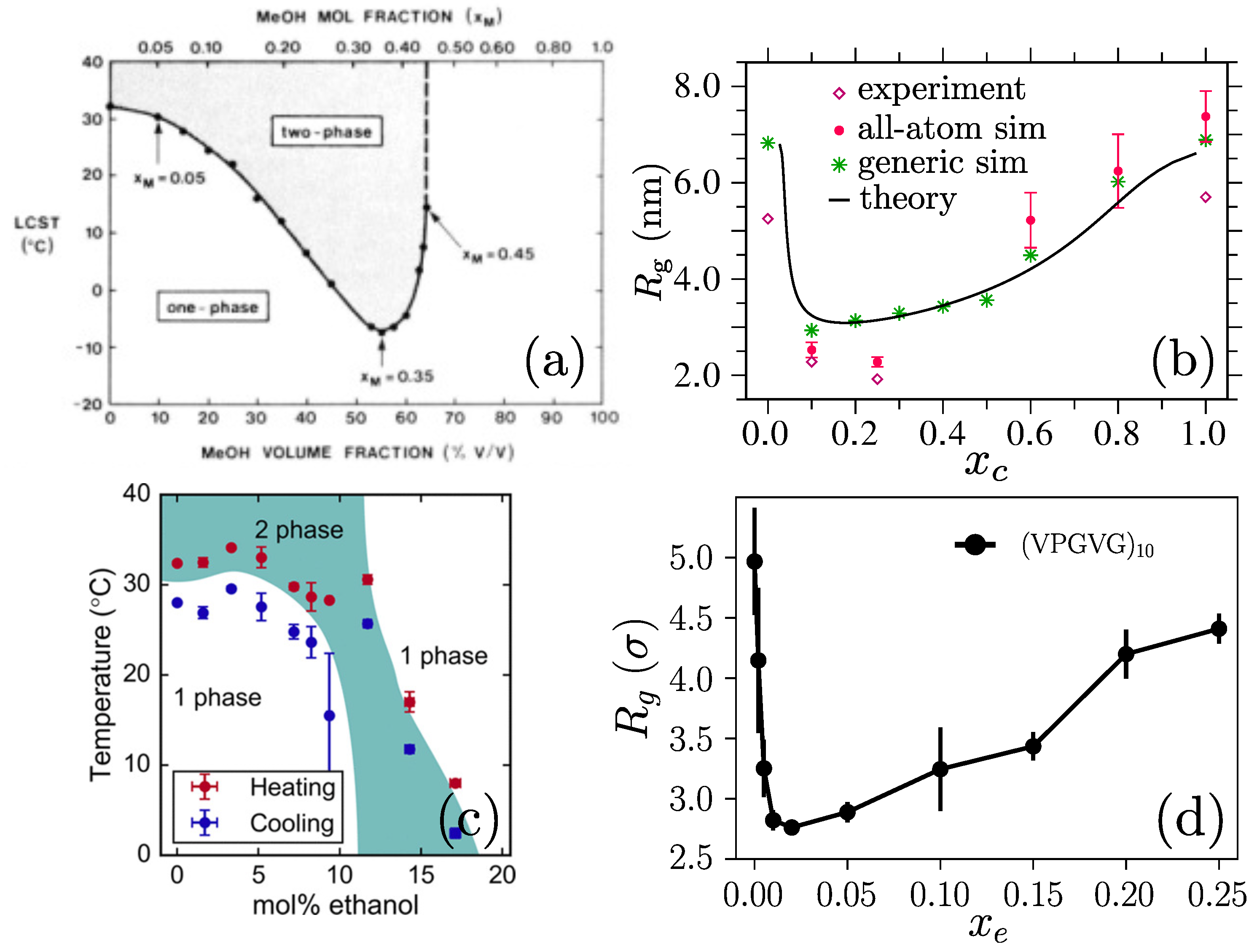

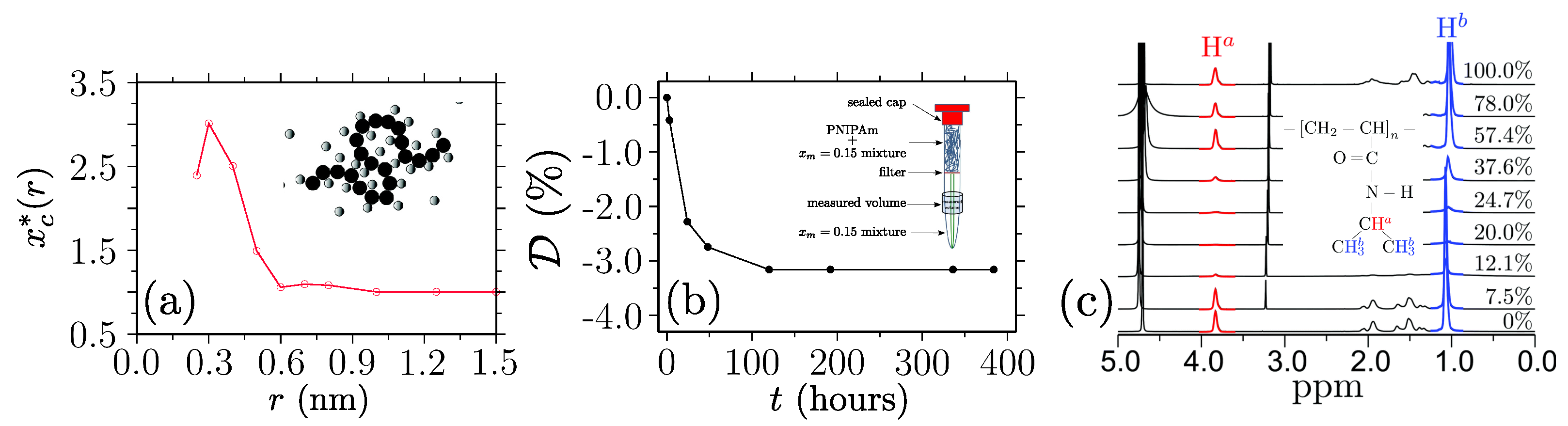

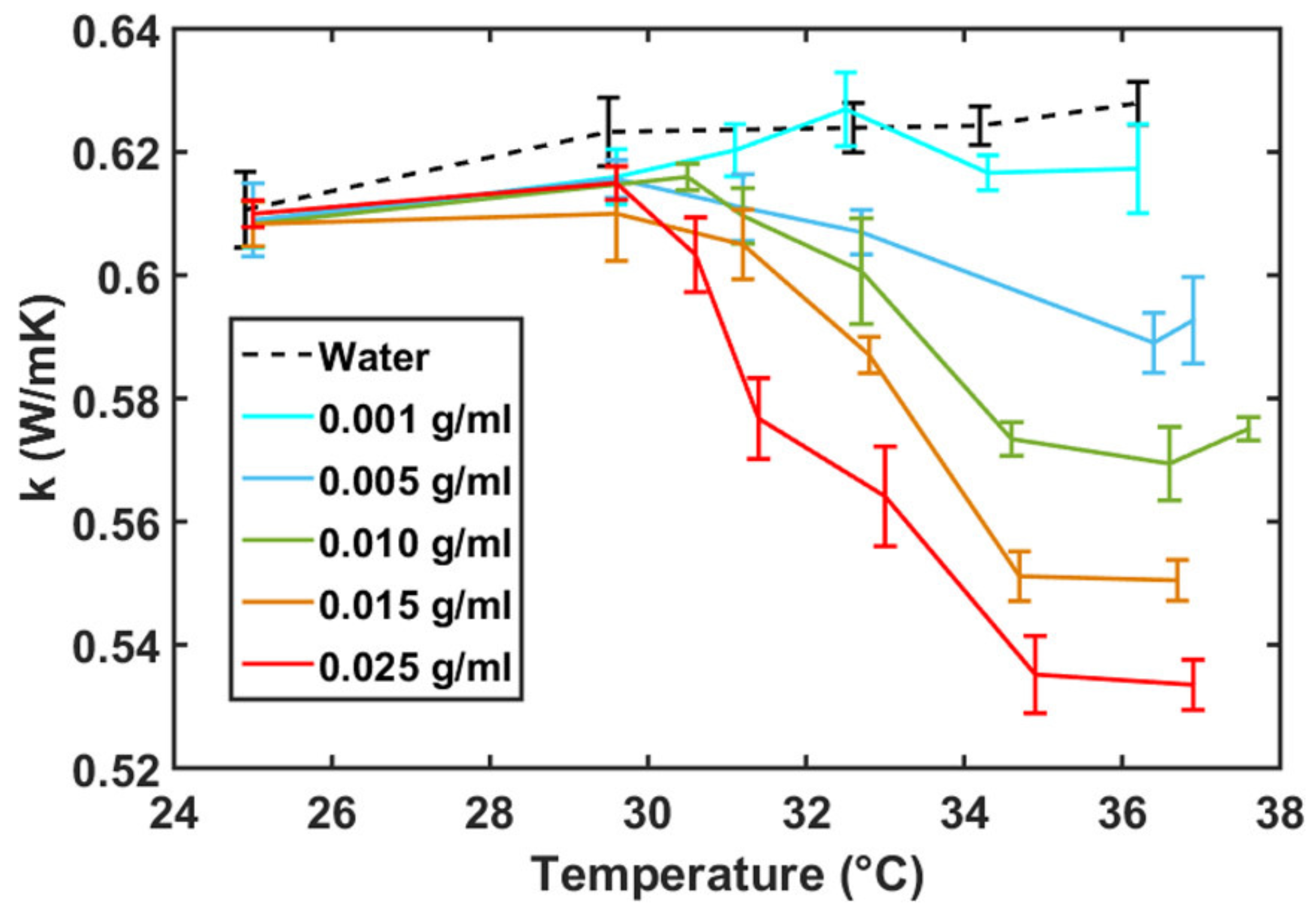
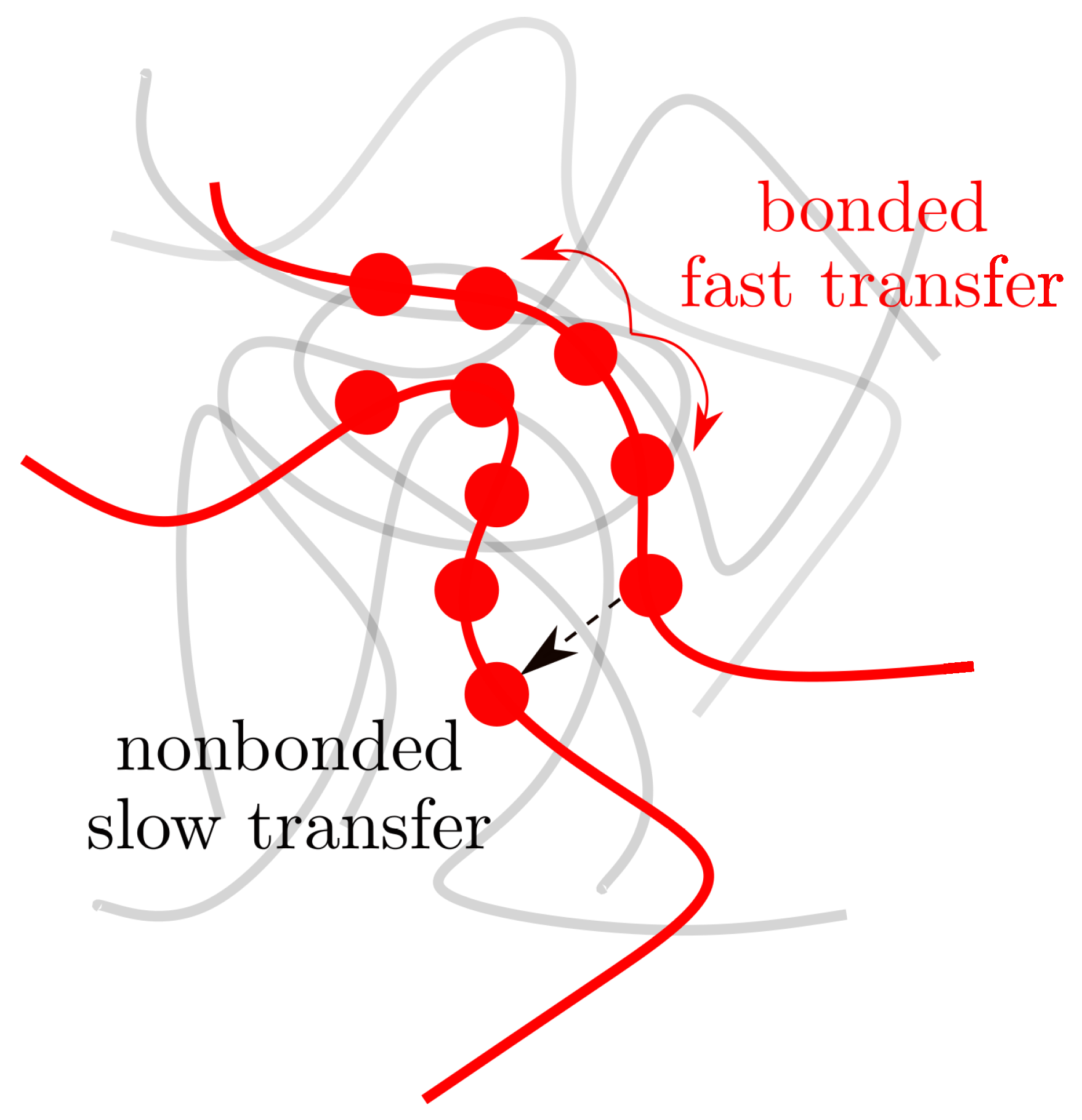


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherji, D.; Kremer, K. Smart Polymers for Soft Materials: From Solution Processing to Organic Solids. Polymers 2023, 15, 3229. https://doi.org/10.3390/polym15153229
Mukherji D, Kremer K. Smart Polymers for Soft Materials: From Solution Processing to Organic Solids. Polymers. 2023; 15(15):3229. https://doi.org/10.3390/polym15153229
Chicago/Turabian StyleMukherji, Debashish, and Kurt Kremer. 2023. "Smart Polymers for Soft Materials: From Solution Processing to Organic Solids" Polymers 15, no. 15: 3229. https://doi.org/10.3390/polym15153229
APA StyleMukherji, D., & Kremer, K. (2023). Smart Polymers for Soft Materials: From Solution Processing to Organic Solids. Polymers, 15(15), 3229. https://doi.org/10.3390/polym15153229






