Highly Porous Para-Aramid Aerogel as a Heterogeneous Catalyst for Selective Hydrogenation of Unsaturated Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
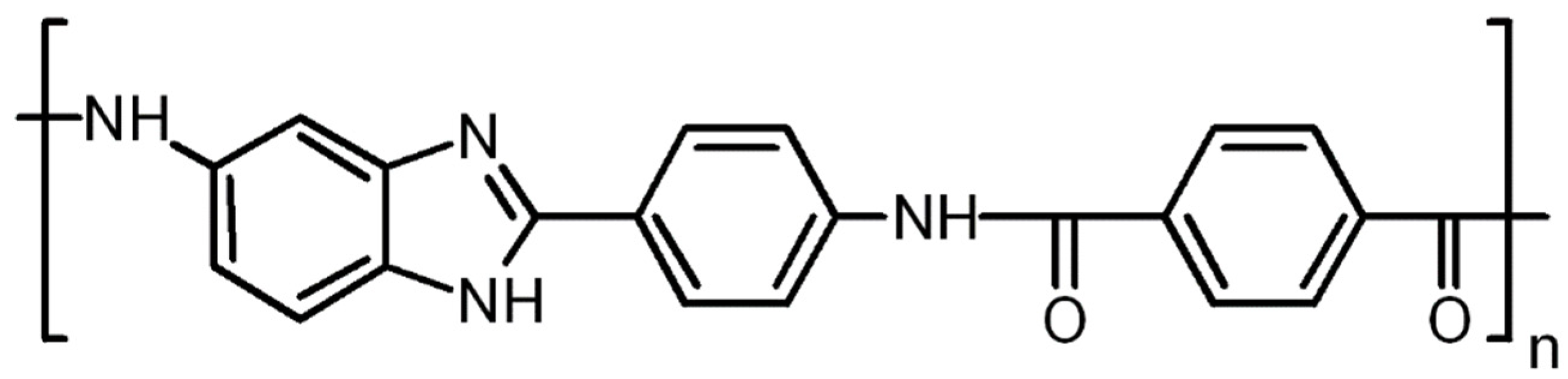
2.2. Preparation of PABI-Based Samples
2.2.1. Preparation of PABI-1 Solution
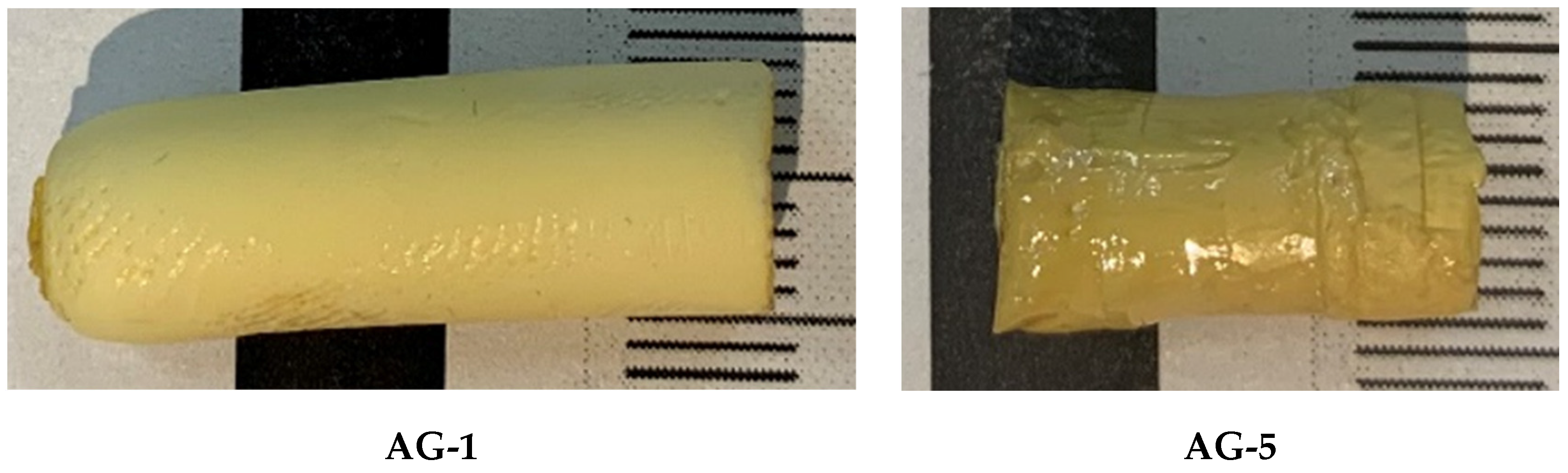
2.2.2. Preparation of Bulk Aerogels
2.2.3. Preparation of Aerogel Pellets
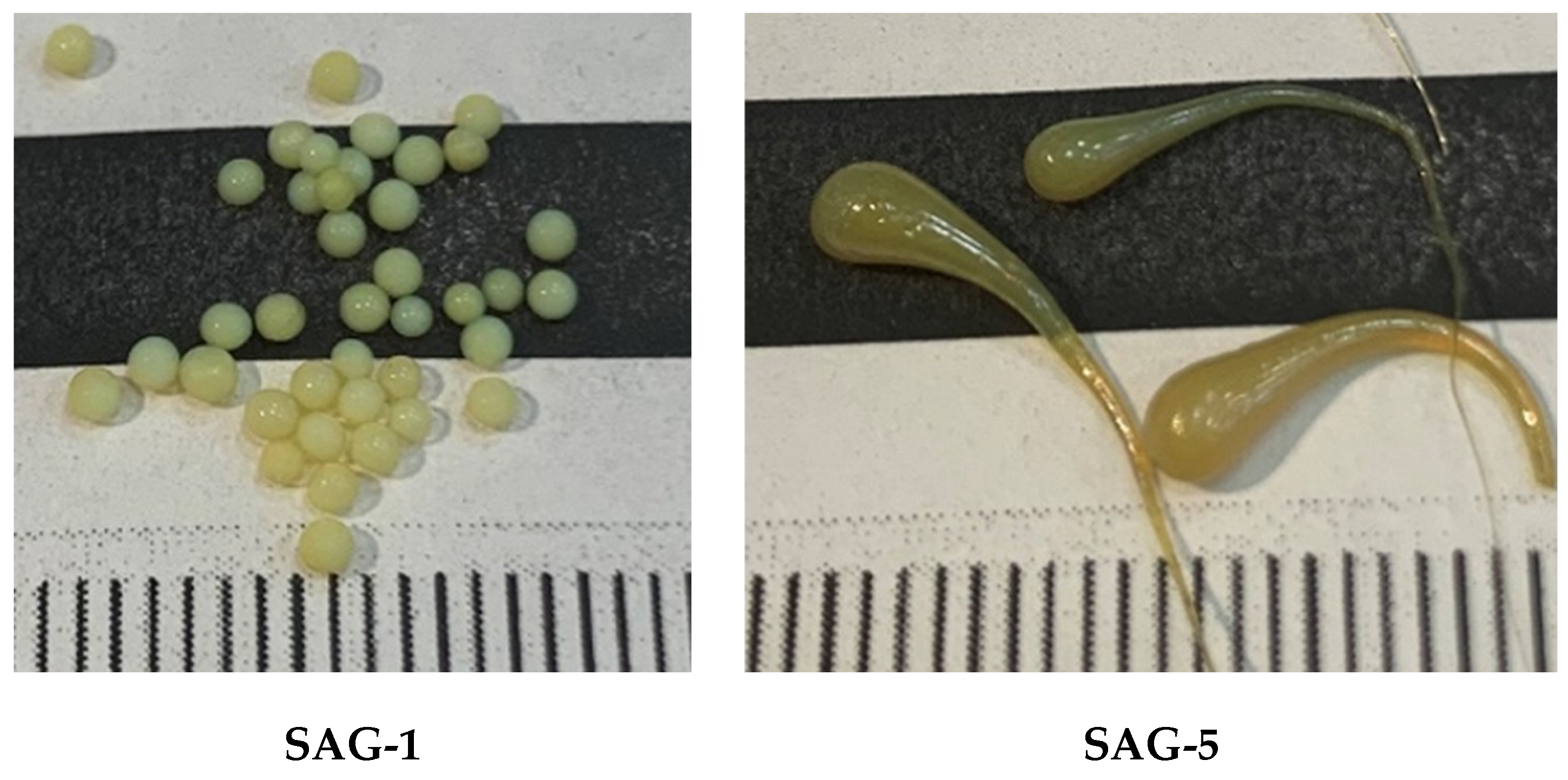
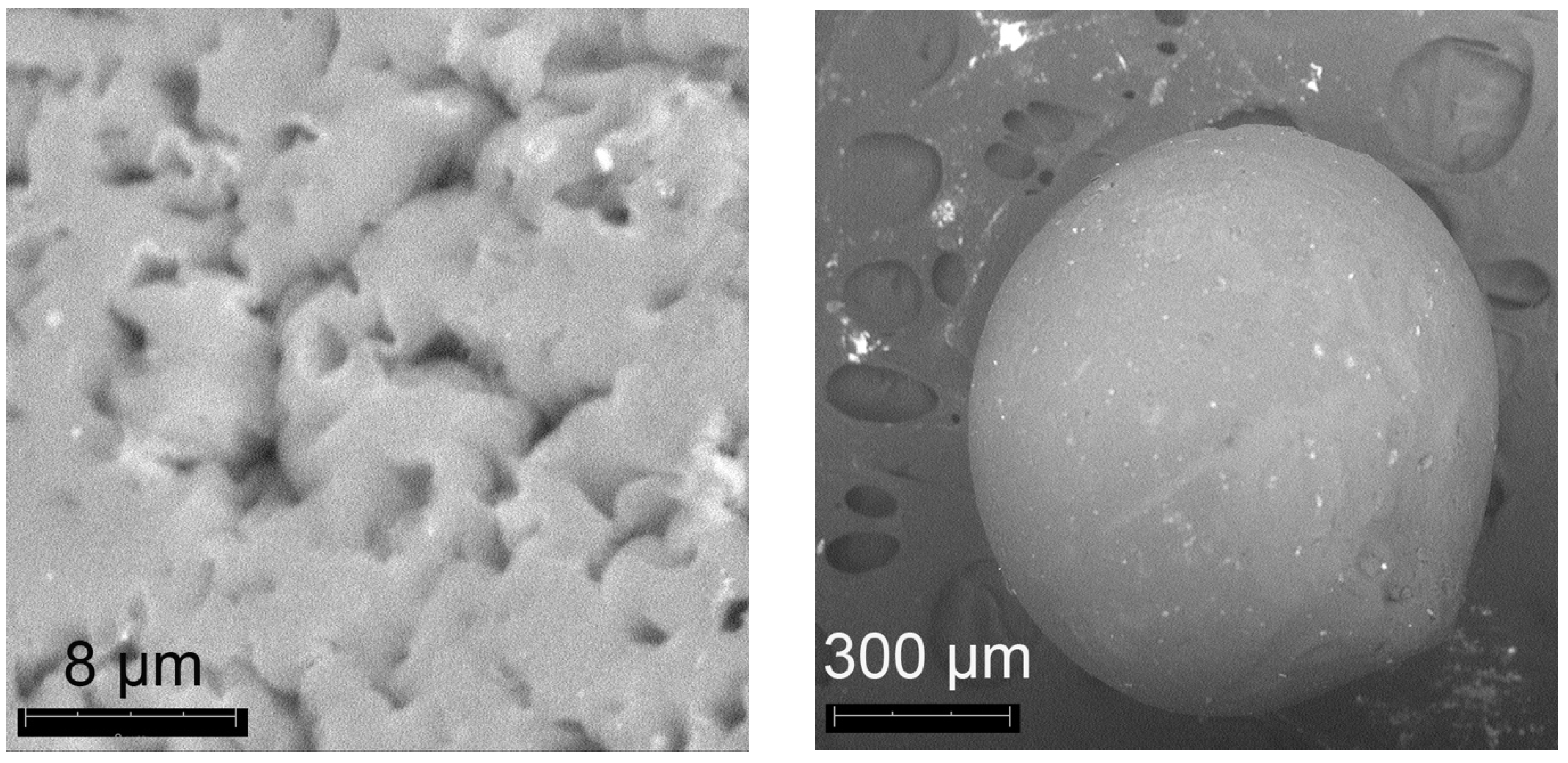
2.2.4. Preparation of Spherical Aerogel Pellets Doped with Pd
2.3. Supercritical Drying
2.4. Characterization of Aerogels
2.5. Hydrogenation of Unsaturated Compounds
3. Results
4. Conclusions
- Terminal C=C bond (hexene-1) is more active compared with the 1,2-disubstituted one (cyclohexene);
- Electron-withdrawing group (CN) decreases the C=C bond hydrogenation speed, leaving a CN group untouched;
- The C≡C group (hexyne-3) is much less active in comparison with the C=C group;
- The catalyst retains its activity together with its shape at least up to 150 °C in a hydrogen/organic vapor atmosphere.
- The catalyst reveals a very sharp increase in activity with an increase in reaction temperature from the reduction of hexyne-3. The conversion of a triple bond turned from 1% to 99% within a narrow temperature interval of only 30 °C. The explanation may lie in the reaction mechanism. In the first step, hexyne-3 is converted to hexene-3, which is then quickly reduced to n-hexane. A higher reaction ability of alkenes over alkynes in hydrogenation reactions is known from a general course of organic chemistry [59]. In addition, we found traces of the olefinic bond (probably hexene-3) in the 1H NMR spectrum of the hexyne-3 reduction at 120 °C. Therefore, the speed-limiting step of hexyne-3 hydrogenation is the conversion of C≡C → C=C with a fast step of C=C → C-C.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, Y.; Zhu, J.; Yu, H.; Zhang, Q.; Zhu, M. Self-reinforcement of light, temperature-resistant silica nanofibrous aerogels with tunable mechanical properties. Adv. Fiber Mater. 2020, 2, 338–347. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, X.; Hu, H.; Meng, X.; Hou, L. Facile fabrication of biochar/Al2O3 adsorbent and its application for fluoride removal from aqueous solution. J. Chem. Eng. Data. 2018, 64, 83–89. [Google Scholar] [CrossRef]
- Anoshkin, I.V.; Campion, J.; Lioubtchenko, D.V.; Oberhammer, J. Freeze-dried carbon nanotube aerogels for high-frequency absorber applications. ACS Appl. Mater. Interfaces. 2018, 10, 19806–19811. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.C. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A Physicochem. Eng. Aspects 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Guarin-Romero, J.R.; Rodriguez-Estupinan, P.; Giraldo, L.; Moreno-Piraján, J.C. Study of adsorption of CO2 and CH4 on resorcinol–formaldehyde aerogels at high pressures. J. Chem. Eng. Data. 2019, 64, 5263–5274. [Google Scholar] [CrossRef]
- Cardea, S.; Gugliuzza, A.; Sessa, M.; Aceto, M.C.; Drioli, E.; Reverchon, E. Supercritical gel drying: A powerful tool for tailoring symmetric porous PVDF−HFP membranes. ACS Appl. Mater. Interfaces 2009, 1, 171–180. [Google Scholar] [CrossRef]
- Daniel, C.; Vitillo, J.G.; Fasano, G.; Guerra, G. Aerogels and polymorphism of isotactic poly (4-methyl-pentene-1). ACS Appl. Mater. Interfaces 2011, 3, 969–977. [Google Scholar] [CrossRef]
- Daniel, C.; Alfano, D.; Venditto, V.; Cardea, S.; Reverchon, E.; Larobina, D.; Mensitieri, G.; Guerra, G. Aerogels with a microporous crystalline host phase. Adv. Mater. 2005, 17, 1515–1518. [Google Scholar] [CrossRef]
- Daniel, C.; Sannino, D.; Guerra, G. Syndiotactic polystyrene aerogels: Adsorption in amorphous pores and absorption in crystalline nanocavities. Chem. Mater. 2008, 20, 577–582. [Google Scholar] [CrossRef]
- Daniel, C.; Giudice, S.; Guerra, G. Syndiotatic Polystyrene Aerogels with β, γ, and ε Crystalline Phases. Chem. Mater. 2009, 21, 1028–1034. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Maksimkin, A.V.; Sipyagina, N.A.; Malkova, A.N.; Kolesnikov, E.A.; Zadorozhnyy, M.Y.; Dayyoub, T. Ultra-high molecular weight polyethylene with hybrid porous structure. Polymer 2020, 202, 122744. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Sipyagina, N.A.; Straumal, E.A.; Maksimkin, A.V.; Kolesnikov, E.A.; Senatov, F.S. Properties of highly porous aerogels prepared from ultra-high molecular weight polyethylene. Polymer 2019, 182, 121824. [Google Scholar] [CrossRef]
- Bai, Y.; Yi, X.; Li, B.; Chen, S.; Fan, Z. Constructing porous polyimide/carbon quantum dots aerogel with efficient photocatalytic property under visible light. Appl. Surf. Sci. 2022, 578, 151993. [Google Scholar] [CrossRef]
- Shi, B.; Ma, B.; Wang, C.; He, H.; Qu, L.; Xu, B.; Chen, Y. Fabrication and applications of polyimide nano-aerogels. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106283. [Google Scholar] [CrossRef]
- Hou, X.; Mao, Y.; Zhang, R.; Fang, D. Super-flexible polyimide nanofiber cross-linked polyimide aerogel membranes for high efficient flexible thermal protection. Chem. Eng. J. 2021, 417, 129341. [Google Scholar] [CrossRef]
- Li, M.; Gan, F.; Dong, J.; Fang, Y.; Zhao, X.; Zhang, Q. Facile preparation of continuous and porous polyimide aerogel fibers for multifunctional applications. ACS Appl. Mater. 2021, 13, 10416–10427. [Google Scholar] [CrossRef]
- Lv, L.; Han, X.; Zong, L.; Li, M.; You, J.; Wu, X.; Li, C. Biomimetic hybridization of kevlar into silk fibroin: Nanofibrous strategy for improved mechanic properties of flexible composites and filtration membranes. ACS Nano 2017, 11, 8178–8184. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhang, M.; Luo, J.; Lu, Z.; Ding, X. Fabrication, applications, and prospects of aramid nanofiber. Adv. Funct. Mater. 2020, 30, 2000186. [Google Scholar] [CrossRef]
- Kwon, S.R.; Harris, J.; Zhou, T.; Loufakis, D.; Boyd, J.G.; Lutkenhaus, J.L. Mechanically strong graphene/aramid nanofiber composite electrodes for structural energy and power. ACS Nano 2017, 11, 6682–6690. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Di, J.; Li, H.; Wang, J. Highly thermally conductive, ductile biomimetic boron nitride/aramid nanofiber composite film. Compos. Sci. Technol. 2020, 189, 108021. [Google Scholar] [CrossRef]
- Shao, Z.; Okubayashi, S. Preparation of p-aramid aerogels using supercritical CO2. J. Fiber Sci. Technol. 2014, 70, 233–239. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous kevlar aerogel threads for thermal insulation in harsh environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, Z.; Wu, X.; Li, G.; Fang, D.; Zhang, X. Nanofibrous kevlar aerogel films and their phase-change composites for highly efficient infrared stealth. ACS Nano 2019, 13, 2236–2245. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Shen, J.; Gao, C.; Van der Bruggen, B. The potential of Kevlar aramid nanofiber composite membranes. J. Mater. Chem. A 2020, 8, 7548–7568. [Google Scholar] [CrossRef]
- Zhang, J.M.; Cortés-Ballesteros, B.; Peijs, T. All-aramid composites by partial fiber dissolution in mixed solvents. Polym. Compos. 2018, 39, 3013–3021. [Google Scholar] [CrossRef]
- Williams, J.C.; Nguyen, B.N.; McCorkle, L.; Scheiman, D.; Griffin, J.S.; Steiner, S.A.; Meador, M.A.B. Highly porous, rigid-rod polyamide aerogels with superior mechanical properties and unusually high thermal conductivity. ACS Appl. Mater. 2017, 9, 1801–1809. [Google Scholar] [CrossRef]
- Wu, W.; Song, Q.; Yu, J.; Li, N.; Hu, Z.; Wang, Y.; Zhu, J. High-performance heterocyclic para-aramid aerogels for selective dye adsorption and thermal insulation applications. J. Appl. Polym. Sci. 2023, 140, e53301. [Google Scholar] [CrossRef]
- Dai, Y.; Meng, C.; Tang, S.; Qin, J.; Liu, X. Construction of dendritic structure by nano-SiO2 derivate grafted with hyperbranched polyamide in aramid fiber to simultaneously improve its mechanical and compressive properties. Eur. Polym. J. 2019, 119, 367–375. [Google Scholar] [CrossRef]
- Yang, S.; Xie, C.; Qiu, T.; Tuo, X. The aramid-coating-on-aramid strategy toward strong, tough, and foldable polymer aerogel films. ACS Nano 2022, 16, 14334–14343. [Google Scholar] [CrossRef] [PubMed]
- Motahari, S.; Nodeh, M.; Maghsoudi, K. Absorption of heavy metals using resorcinol formaldehyde aerogel modified with amine groups. Desalination Water Treat. 2016, 57, 16886–16897. [Google Scholar] [CrossRef]
- Yorov, K.E.; Baranchikov, A.E.; Kiskin, M.A.; Sidorov, A.A.; Ivanov, V.K. Functionalization of aerogels with coordination compounds. Russ. J. Coord. Chem. 2022, 48, 89–117. [Google Scholar] [CrossRef]
- Grau, A.; Baeza, A.; Serrano, E.; García-Martínez, J.; Nájera, C. Mesoporous metal complex–silica aerogels for environmentally friendly amination of allylic alcohols. Chem. Cat. Chem 2015, 7, 87–93. [Google Scholar] [CrossRef]
- Elbaz, L.; Korin, E.; Soifer, L.; Bettelheim, A. Evidence for the formation of cobalt porphyrin-quinone complexes stabilized at carbon-based surfaces toward the design of efficient non-noble-metal oxygen reduction catalysts. J. Phys. Chem. 2010, 1, 398–401. [Google Scholar] [CrossRef]
- Martínez, S.; Moreno-Mañas, M.; Vallribera, A.; Schubert, U.; Roig, A.; Molins, E. Highly dispersed nickel and palladium nanoparticle silica aerogels: Sol–gel processing of tethered metal complexes and application as catalysts in the Mizoroki–Heck reaction. New J. Chem. 2006, 30, 1093–1097. [Google Scholar] [CrossRef]
- Heinrichs, B.; Noville, F.; Pirard, J.P. Pd/SiO2-cogelled aerogel catalysts and impregnated aerogel and xerogel catalysts: Synthesis and characterization. J. Catal. 1997, 170, 366–376. [Google Scholar] [CrossRef]
- Murphy, E.F.; Schmid, L.; Bürgi, T.; Maciejewski, M.; Baiker, A.; Günther, D.; Schneider, M. Nondestructive sol-gel immobilization of metal (salen) catalysts in silica aerogels and xerogels. Chem. Mater. 2001, 13, 1296–1304. [Google Scholar] [CrossRef]
- Seçkin, T.; Çetinkaya, B.; Özdemir, I. Sol-gel synthesis of Ru (II) complex of 3-4, 5-dihydroimidazol-1-yl-propyltriethoxysilane aerogels and xerogels. Polym. Bull. 2000, 44, 47–53. [Google Scholar] [CrossRef]
- Linares, N.; Sepulveda, A.E.; Pacheco, M.C.; Berenguer, J.R.; Lalinde, E.; Nájera, C.; Garcia-Martinez, J. Synthesis of mesoporous metal complex-silica materials and their use as solvent-free catalysts. New J. Chem. 2011, 35, 225–234. [Google Scholar] [CrossRef]
- Sipyagina, N.A.; Malkova, A.N.; Straumal, E.A.; Yurkova, L.L.; Baranchikov, A.E.; Ivanov, V.K.; Lermontov, S.A. Novel aminophosphonate ligand for the preparation of catalytically active silica aerogels with finely dispersed palladium. J. Porous Mater. 2023, 30, 449–457. [Google Scholar] [CrossRef]
- Uozumi, Y. Recent progress in polymeric palladium catalysts for organic synthesis. In Immobilized Catalysts: Solid Phases, Immobilization and Applications; Springer: Berlin/Heidelberg, Germany, 2004; pp. 77–112. [Google Scholar] [CrossRef]
- Michalska, Z.M.; Ostaszewski, B.; Zientarska, J.; Sobczak, J.W. Catalytic hydrogenation of alkadienes and alkynes by palladium catalysts supported on heterocyclic polyamides. J. Mol. Catal. A Chem. 1998, 129, 207–218. [Google Scholar] [CrossRef]
- Poltarzewski, Z.; Galvagno, S.; Pietropaolo, R.; Staiti, P. Hydrogenation of α,β-unsaturated aldehydes over Pt Sn/Nylon. J. Catal. 1986, 102, 190–198. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Hopkins, B.T.; Köbberling, J. ROMPgel reagents in parallel synthesis. Chem. Rev. 2002, 102, 3301–3324. [Google Scholar] [CrossRef]
- Tabuani, D.; Monticelli, O.; Chincarini, A.; Bianchini, C.; Vizza, F.; Moneti, S.; Russo, S. Palladium nanoparticles supported on hyperbranched aramids: Synthesis, characterization, and some applications in the hydrogenation of unsaturated substrates. Macromolecules 2003, 36, 4294–4301. [Google Scholar] [CrossRef]
- Monticelli, O.; Chincarini, A. On the use of hyperbranched aramids as support of Pt nanoparticles. e-Polymers 2007, 7, 276–282. [Google Scholar] [CrossRef][Green Version]
- Cum, G.; Gallo, R.; Ipsale, S.; Spadaro, A. Selective synthesis of alkynes by catalytic dehydrogenation of alkenes over polymer-supported palladium acetate in the liquid phase. J. Chem. Soc. Chem. Commun. 1985, 22, 1571–1573. [Google Scholar] [CrossRef]
- Capannelli, G.; Cum, G.; Gallo, R.; Spadaro, A.; Costa, G.; Piaggio, P. Polymer-supported catalysts: Preparation and characterisation of complexes between palladium derivatives and oligomeric aramides. J. Mol. Catal. 1990, 59, 39–52. [Google Scholar] [CrossRef]
- Qu, R.; Sun, X.; Sun, C.; Ji, C.; Wang, C. Adsorption properties for metal ions of waste poly (p-phenylene terephthalamide) fiber after chemical modification. Polym. Adv. Technol. 2012, 23, 21–30. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, C.; Huang, J.; Chen, T.; Liu, Y.; Liu, X. Nondestructive grafting of PEI on aramid fiber surface through the coordination of Fe (III) to enhance composite interfacial properties. Appl. Surf. Sci. 2017, 401, 323–332. [Google Scholar] [CrossRef]
- Shi, Y.; Tuo, X. Synthesis of heterocyclic aramid nanofibers and high performance nanopaper. Mater. Adv. 2020, 1, 595–598. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size, and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- de Ruijter, C.; Jager, W.F.; Groenewold, J.; Picken, S.J. Synthesis and characterization of rod-coil poly(amide-block-aramid) alternating block copolymers. Macromolecules 2006, 39, 3824–3829. [Google Scholar] [CrossRef]
- Baker, A.D.; Engel, R. Organic Chemistry; West Publishing Company: St. Paul, MN, USA, 1992. [Google Scholar]
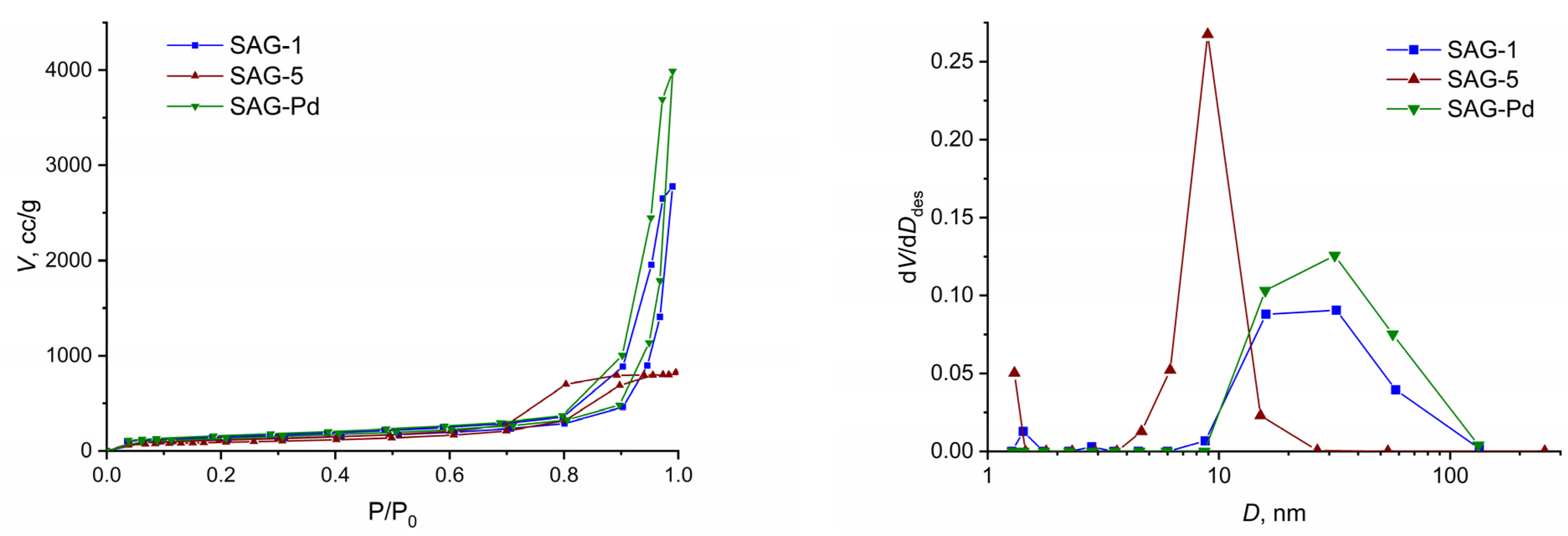


| Sample | ρ, g/cm3 | Ssp, m2/g | Vp, cm3/g |
|---|---|---|---|
| AG-1 | 0.057 | ||
| AG-5 | 0.375 | ||
| SAG-1 | 410 | 4.3 | |
| SAG-Pd | 470/430 * | 6.2 | |
| SAG-5 | 320 | 1.3 |
| T, °C | 20 °C | 150 °C | 200 °C SAG-1/SAG-Pd | 250 °C |
|---|---|---|---|---|
| Ssp, m2/g | 410 | 403 | 390/430 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lermontov, S.A.; Vlasenko, N.E.; Sipyagina, N.A.; Malkova, A.N.; Gozhikova, I.O.; Baranchikov, A.E.; Knerelman, E.I. Highly Porous Para-Aramid Aerogel as a Heterogeneous Catalyst for Selective Hydrogenation of Unsaturated Organic Compounds. Polymers 2023, 15, 3206. https://doi.org/10.3390/polym15153206
Lermontov SA, Vlasenko NE, Sipyagina NA, Malkova AN, Gozhikova IO, Baranchikov AE, Knerelman EI. Highly Porous Para-Aramid Aerogel as a Heterogeneous Catalyst for Selective Hydrogenation of Unsaturated Organic Compounds. Polymers. 2023; 15(15):3206. https://doi.org/10.3390/polym15153206
Chicago/Turabian StyleLermontov, Sergey A., Nikita E. Vlasenko, Nataliya A. Sipyagina, Alena N. Malkova, Inna O. Gozhikova, Alexander E. Baranchikov, and Evgeniya I. Knerelman. 2023. "Highly Porous Para-Aramid Aerogel as a Heterogeneous Catalyst for Selective Hydrogenation of Unsaturated Organic Compounds" Polymers 15, no. 15: 3206. https://doi.org/10.3390/polym15153206
APA StyleLermontov, S. A., Vlasenko, N. E., Sipyagina, N. A., Malkova, A. N., Gozhikova, I. O., Baranchikov, A. E., & Knerelman, E. I. (2023). Highly Porous Para-Aramid Aerogel as a Heterogeneous Catalyst for Selective Hydrogenation of Unsaturated Organic Compounds. Polymers, 15(15), 3206. https://doi.org/10.3390/polym15153206








