Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials
Abstract
1. Introduction
2. Lignin Structure and Properties
2.1. Analytics and Structure
2.2. Technical Lignins
2.3. Other Sources of Lignin
3. Biobased Products from Lignin
3.1. Aromatic Chemicals
3.2. Phenolic Compounds
3.3. Vanillin
3.4. Adipic Acid from Lignin
3.5. Carbon Fibers
3.6. Biochar
4. New Developments in Lignin Use as Raw Material for Some Polymeric Materials
4.1. Phenolic Resins
4.2. Polyurethanes
4.3. Epoxy Thermosets
5. New Developments in the Use of Lignin as a Bioactive Compound
5.1. Antioxidant, Antimicrobial, Antifungal, Antiviral, Antitumoral, and Drug Carrier Activities
5.2. Lignin Chemical Modification
5.3. Lignin Use in Agriculture
6. Lignin Nanoparticles
6.1. Green Approaches to Preparing LNPs and Their Properties
6.2. Lignin Nanoparticle Applications
6.3. Lignin Nanofibers
| LNPs and Other Components | Properties and Applications | Refs |
|---|---|---|
| Lignin nanoparticles (LNPs)/starch | Green nanofiller in starch-based biocomposites; strong interfacial hydrogen bonding; enhanced mechanical properties, thermal stability, antioxidant activity, and good (UV) irradiation shielding performance; applications such as delayed oxidative deterioration of soybean oil/advanced food packaging | [199] |
| Lignin nanoparticles obtained by a acidolysis process from corn lignin/polyurethane, polyethylene glycol and diisocyanates | LNPs as crosslinking agent in polyurethane nanocomposites. Composites with hydrophobic, UV resistant and dielectric properties, enhanced tensile strength; good reprocessability | [200] |
| 0.5 wt% lignin and nanolignin in poly(lactic acid) composites synthesized by in situ reactive processing as melt mixing and ring-opening polymerization (ROP) | Reactive processing produced nanolignin-containing composites with superior crystallization, mechanical, and antioxidant properties. LNPs act as a macroinitiator in the ROP of lactide, resulting in PLA-grafted nanolignin particles, improved dispersion and the formation of interfacial covalent bonds facilitated by LNPs | [201] |
| Lignin-based nano-micelles using the “grafting-from” method | pH-sensitive, biocompatible, suitable for oral drug delivery | [202] |
| Nanolignin/chitin nanofibrils and complexes loaded with active molecules, such as vitamin E, sodium ascorbyl phosphate, lutein, nicotinamide, and glycyrrhetinic acid | Spray-drying method; nanostructured chitin; Functional agents in skin regeneration and antimicrobial, anti-inflammatory, and antioxidant activities; cytocompatibility in skin regeneration. | [203] |
| PCL coated with chitin–lignin gel; shell/core fiber, coaxial electrospinning; electrospun PCL/lignin, initial blending | Methylene blue, penicillin/streptomycin; sustainable drug release; wound dressing; fibrous scaffolds; MTT tissue engineering | [197,204] |
| LNP/PVA/PVP electrospinning. Initial blending | Paclitaxel local anticancer therapy | [205] |
| AgNP-loaded electrospun PVA/lignin nanofibers | Membrane filtration, antimicrobial fabrics, and wound dressing | [206] |
| Lignin/cellulose acetate/N-vanillidene-phenylthiazole copper (Il) complexes, nanofibrous materials | Antimicrobial for hygienic applications | [207] |
7. Lignin-Based Hydrogels
8. Three-Dimensional-Printing-Based Lignin Biomaterials
9. Lignin Use in Green Functional/Complex Materials
| Components of Biocomposites | Lignin Role/Application of Product | References |
|---|---|---|
| Agriculture | ||
| Azide-modified cellulose/0.5–2 wt% covalently bonded lignin with UV-blocking properties; propargylated lignin | Biodegradable, flexible, and transparent UV protection films from renewable resources | [297] |
| Polyvinyl alcohol PVA/lignin, spherical organosolv, lignin particles, LNPs obtained by dialysis with THF or ethanol as solvents | UV-blocking lignin–PVA composite film | [328] |
| Alkali lignin/carbon nanoparticles (C-NPs) containing lignin nanoparticles (L-NPs) | Agro-nanotechnology - Cost-effective alternative compared to conventional commercial fungicides - Environmentally benign nanopesticides for long-term plant protection - Antifungal nanocomposite - Control agent against Fusarium verticillioides in maize | [329] |
| Alginate/polyvinyl alcohol/calcium chloride and boric as crosslinkers | Three-dimensional (3D) biocomposite adsorbent; multiporous architecture; excellent substitute for a commercial EDTA-Fe micronutrient; eco-friendly, highly efficient | [330] |
| Waste treatment | ||
| Hydrophilic sulfonated kraft lignin/polyethersulfone (PES) | Layer-by-layer (LbL) assembly; antifouling coating; oily wastewater treatment | [331] |
| Lignin-derived adsorption materials | Compounded lignin with other materials; high-value-added lignin adsorbent material wastewater treatment | [332] |
| Food Packaging | ||
| Enzymatic hydrolysis of dried solid from alkali treatment of wheat straw/dry corn starch, glycerol, solution casting method | Lignin particles act as reinforcements for green biodegradable starch films | [323] |
| Technical lignin/cellulose | Cellulose/technical lignin composites; antioxidant and UV barrier Advanced packaging | [333] |
| Lignosulfonate/alginate | Photoprotective and antioxidant properties; enhanced barrier properties of the blend films and antioxidant activity; active packaging applications | [334] |
| Lignin nanoparticles/chitosan/polyvinyl alcohol | Lignin increased mechanical strength and antioxidant and antibacterial activity. Active food packaging | [335] |
| Low molar mass alkali lignin/PLA | Increased water barrier properties (up to 73%) photodegradability; biocompatibility, antimicrobial activity; very good cellular response and very low cytotoxicity levels; food packaging | [336] |
| Hydroxypropyl lignin or lignosulfonate or alkaline lignin/soy protein | Enhanced mechanical performance, water resistivity of soy protein plastics and specific functional properties; biomedical uses; food packaging | [337] |
| Alkaline lignin (AL) and sodium lignosulfonate (LSS)/PEG/thermoplastic zein | Strong H-bonding modifying α-helix, β-sheet, and β-turn secondary structures; improved physical properties; due to the interaction of AL with zein molecules, enhanced strength and water resistivity of soy protein plastics. Food packaging Biomedical uses | [338] |
| Lignin/tannic acid/biodegradable PBAT composite film: epoxidized soya bean (ESO) oil as plasticizer and encapsulated lignin/tannic acid as filler | Reduced water vapor and oxygen transmission rate; improved properties and degradability. Extended shelf-life and preservation of food, including fresh potato and onion Packaging of dry food products | [339] |
| Kraft lignin/poly(3-hydroxybutyrate) nano-composite Pickering emulsion and hot compression | Hydrogen bonding interactions; improved mechanical properties, UV resistance/blocking and higher melt viscosity; Food packaging | [340] |
| Technical soda lignin (ethyl acetate extract/high-density polyethylene. Melt extrusion | Composite for food packaging. Antioxidant and insect repellent activities | [341] |
| Biomedical | ||
| Alkali lignin green synthesis of AgNP/carrageenan/calcium chloride, copper chloride magnesium chloride silver | Antibacterial against Staphylococcus aureus and Escherichia coli; biocompatible; Wound dressing and healing effect | [190] |
| Nanolignin (NL) and its composites | Cancer therapy drug and gene delivery, biosensing, bioimaging, and tissue engineering; therapeutic potency of chemotherapeutic drugs by decreasing their dose and reducing their adverse effects | [342] |
| Lignin as decoration for multi-walled nanotubes/PVA-lignin fiber nanocomposites | Antimicrobial properties Wound healing/tissue engineering | [343] |
| Lignosulfonate/PVA/chitosan | 70% reduction in free radicals; good antibacterial abilities at 10% (w/w) lignin; wound healing | [220] |
| Kraft lignin/PVA/poly(glycerol sebacate) (PGS) electrospinning | PVA-PGS-lignin fibers. Lignin incorporation promotes neural cell proliferation and differentiation. Tissue engineering/regeneration | [344] |
| Kraft lignin/electrospun PCL fibers embedding lignin nanoparticles | Peripheral nerve regeneration | [345] |
| Alkali lignin/PLA-lignin nanofiber silver-ion-containing lignin nanoparticles/poly(lactide)–lignin nanofibers. | Antioxidant activity; eco-friendly alternative to AgNPs in antimicrobial and antioxidative applications; biomedical application Tissue engineering | [193] |
| Lignins extracted from pine residue esterified with succinic anhydride/PLA | Enhanced mechanical and thermal properties of PLA Excellent antimicrobial and biocompatibility. | [346] |
| Lignosulfonate/polyoxazoline/triazoles (linked silver) | Antimicrobial against many strains such as Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi Klebsiella pneumonia, Staphylococcus aureus, Staphylococcus epidermidis, Candida albicans, and Candida tropicalis; Antioxidant, anti-inflammatory, preventing infection; promotion of healing; reduced inflammation on burn wound | [347] |
| Softwood kraft lignin/poly(butylene succinate) melt-mixing extrusion | Antimicrobial and antioxidant properties; resistance to adherence of the common nosocomial pathogen Staphylococcus aureus. Pharmaceutical/biomedical applications | [181] |
| New High-Performance Materials | ||
| Organosolv lignin/polylactide/PVAc poly(vinyl acetate)/GMAglycidyl methacrylate, reactive extrusion | Super-toughened bio-elastomer PLA composite; improved interfacial adhesion; PVAc and GMA as toughening agents; single glass transition temperature. High elongation at break and impact strength; replaces petroleum-based elastomers such as EPDM elastomer | [348] |
| Micro- or nano-Soda lignin (NL) as green filler; content between 0.5–5 wt%/PLA masterbatch prepared by solvent casting, then melt mixing. | Biobased and biodegradable PLA films; Interfacial interactions, slightly stronger in the case of NL acting as fillers. Competitive green alternative in the food packaging industry | [201] |
| Acetylated lignin/thermoplastic polyurethane mixing | Lignin-based thermoplastic polyurethane adhesive. Wood adhesive | [349] |
| 1–10 wt% grape seed lignin/highly crystalline (PHB)/amorphous (PHA). Polyhydroxyalkanoate modification | Improved mechanical and gas-barrier properties, high antioxidant capacity of lignin; biodegradability; active biodegradable packaging films; lignin can change the crystallinity of PHB in compost; nontoxicity of materials and its degradation products; positive effect on white mustard (Sinapis alba L.) seed germination | [350] |
| Methylated lignin, lignin esters, or lignin-containing epoxy groups/poly(butylene adipate-co-terephthalate) (PBAT) coextrusion; PBAT grafted with maleic anhydride as a stabilizer; poly-3-hydroxybutyrate PHB or poly-3-hydroxybutyrate-co-3-hydroxyvalerate | Economically competitive biodegradable PBAT/lignin and PHB/L composites Low-cost filler, 36% price reduction; good properties; degradability | [351] |
| Lignin-containing microfibrillated cellulose isolated from chemi-thermomechanical pulp or acetylated/PLA | Biocomposites with controlled biodegradation of polylactic acid (PLA). Biotic degradability | [352] |
| Unfunctionalized lignin and 3-aminopropyltriethoxy silane functionalized lignin/waterborne polyurethane. Chemical reaction | Lignin as natural reinforcing filler | [353] |
| Lignin/polyfurfuryl alcohol | Thermosets; Eco-friendly composite resins | [354] |
10. Energy Storage and Conversion Technologies
10.1. Sustainable Fuel from Lignin
10.2. Sustainable Aviation Fuel (SAF)
10.3. Electrochemical and Energy Storage Applications
10.4. Lignin-Based Organic Flow Batteries
11. Conclusions
11.1. Lignin as Promising Renewable Source
11.2. Challenges and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kakkalameli, S.; Daphedar, A.B.; Faniband, B.; Sharma, S.; Nadda, A.K.; Ferreira, L.F.R.; Bilal, M.; Américo-Pinheiro, J.H.P.; Mulla, S.I. Chapter 2 Biopolymers and Environment. In Biopolymers Recent. Updates, Challenges and Opportunities; Nadda, K.A., Sharma, S., Bhat, R., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 19–35. ISBN 978-3-030-98391-8. [Google Scholar]
- European Bioplastics. Available online: https://www.european-bioplastics.org (accessed on 12 May 2023).
- Thiruchelvi, R.; Das, A.; Sikdar, E. Bioplastics as better alternative to petro plastic. Mater. Today Proc. 2021, 37, 1634–1639. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Kvalvåg-Pettersen, M. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Abdul Khalil, H.P.S. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Amidon, T.E.; Liu, S. Water-based woody biorefinery. Biotechnol. Adv. 2009, 27, 542–550. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Review Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Renewables. Biofuels. IEA Report. 2021. Available online: https://www.iea.org/reports/renewables-2021/biofuels?mode=transport®ion=World&publication=2021&flow=Consumption&product=Ethanol (accessed on 12 May 2023).
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular Economy Aspects of Lignin: Towards a Lignocellulose Biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- NILE—New Improvements for Lignocellulosic Ethanol Project. Raporteur Alfred Abächerli. Evaluation by Computer Simulation of the Influence of Lignin Separation on Mass Flow and Cost for Lignocellulosic Bioethanol Plants Contract No.: 019882;/28 May 2010′/Granit Recherche & Dévelopement SA D3-3-11 DelivReport2010-Public V2-1.doc. Available online: https://cordis.europa.eu/project/id/19882 (accessed on 15 May 2023).
- Li, W.; Sun, N.; Stoner, B.; Jiang, X.; Lu, X.; Rogers, R.D. Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin. Green Chem. 2011, 13, 2038–2047. [Google Scholar] [CrossRef]
- Nimz, H. Beech lignin proposal of a constitutional scheme. Angew. Chem. Int. Ed. Engl. 1974, 13, 313–321. [Google Scholar] [CrossRef]
- Beckham, G.T. Lignin Valorization: Emerging Approaches; OSTI.GOV United States: Oak Ridge, TN, USA, 2018; ECCC Environmental eBooks 1968–2022 Series: Energy and Environment; Publication date: 29 March 2018; 528p.
- Agrawal, R.; Kumar, A.; Singh, S.; Sharma, K. Recent advances and future perspectives of lignin biopolymers. J. Polym. Res. 2022, 29, 222. [Google Scholar] [CrossRef]
- Bass, G.F.; Epps, T.H. Recent developments towards performance-enhancing lignin-based polymers. From themed collection: Sustainable Polymers. Polym. Chem. 2021, 12, 4130–4158. [Google Scholar] [CrossRef]
- Sethupathya, S.; Morales, G.M.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin valorization: Status, challenges and opportunities. Review. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E. Potential synergies and challenges in refining cellulosic biomass to fuels, chemicals, and power. Biotechnol. Prog. 2003, 19, 254–262. [Google Scholar] [CrossRef]
- The Global Market for Lignin 2023–2033. Available online: https://www.giiresearch.com/report/fmi1223534-global-market-lignin.html (accessed on 21 July 2023).
- Shu, F.; Jiang, B.; Yuan, Y.; Li, M.; Wu, W.; Jin, Y.; Xiao, H. Biological Activities and Emerging Roles of Lignin and Lignin-Based Products—A Review. Biomacromolecules 2021, 22, 4905–4918. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Ye, F.; Zhou, Y.; Lei, L.; Zhao, G. Lignin—An Underutilized, Renewable and Valuable Material for Food Industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 2011–2033. [Google Scholar] [CrossRef]
- Ariyanta, H.A.; Sari, F.P.; Sohail, A.; Restu, W.K.; Septiyanti, M.; Aryana, N.; Fatriasari, W.; Kumar, A. Current roles of lignin for the agroindustry: Applications, challenges, and opportunities. Int. J. Biol. Macromol. 2023, 240, 124523. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Hasan, M.S.; Sardar, M.R.; Shafin, A.; Rahman, M.S.; Mahmud, M.; Hossen, M.M. A Brief Review on Applications of Lignin. J. Chem. Rev. 2023, 5, 56–82. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, B.; Wang, H.; Shahnawaz, M. Editorial: Lignin valorization: Recent trends and future perspective. Front. Bioeng. Biotechnol. 2023, 11, 1190128. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Y.; Yang, J.; Li, X.A. comprehensive review of bio-oil, bio-binder and bio-asphalt materials: Their source, composition, preparation and performance. J. Traffic Transp. Eng. (Engl. Ed.) 2022, 9, 151–166. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, D.; Qiu, X.; Yang, D.; Fan, L.; Zheng, J. A novel branched claw-shape lignin-based polycarboxylate superplasticizer: Preparation, performance and mechanism. Cem. Concr. Res. 2019, 119, 89–101. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez Maldonado, D.; Davis, V.A.; Peresin, M.S. A review on lignocellulose chemistry, nanostructure, and their impact on interfacial interactions for sustainable products development. J. Mater. Sci. 2022, 58, 685–706. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Landa, P. Lignin-Based Bio-Asphalt. Patent WO2019/092278, 13 November 2018. [Google Scholar]
- Malik, S.; Khan, A.; Ali, N.; Rahdar, A.; Yasin, G.; Hussain, S.; Bilal, M. Natural polymer-based nanostructures and their applications. In Smart Polymer Nanocomposites: Design, Synthesis, Functionalization, Properties, and Applications. Part III: Applications of Polymer Nanostructures and Polymer Nanocomposites; Ali, N., Khan, A., Gupta, R.K., Bilal, M., Nguyen, T.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Chapter 24; pp. 529–541. [Google Scholar]
- Mondal, S.; Jatrana, A.; Maan, S.; Sharma, P. Lignin modification and valorization in medicine, cosmetics, environmental remediation and agriculture: A review. Environ. Chem. Lett. 2023, 21, 2171–2197. [Google Scholar] [CrossRef]
- Boarino, A.; Klok, H.-A. Opportunities and Challenges for Lignin Valorization in Food Packaging, Antimicrobial, and Agricultural Applications. Biomacromolecules 2023, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Nan, N.; Hu, W.; Wang, J. Lignin-Based Porous Biomaterials for Medical and Pharmaceutical Applications. Biomedicines 2022, 10, 747. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.c.d.; Subramaniam, S.; Sanyal, U.; Rallo, R.; Zhanget, X. Molecular structural dataset of lignin macromolecule elucidating experimental structural compositions. Sci. Data 2022, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- International Lignin Institute, Analytical Protocol. Available online: https://www.ili-lignin.com/activities/analytical-protocols.html (accessed on 21 July 2023).
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Bartolome, M.J.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The Biomodified Lignin Platform: A Review. Polymers 2023, 15, 1694. [Google Scholar] [CrossRef]
- Mikame, K.; Ohashi, Y.; Naito, Y.; Nishimura, H.; Katahira, M.; Sugawara, S.; Koike, K.; Watanabe, T. Natural Organic Ultraviolet Absorbers from Lignin. ACS Sustain. Chem. Eng. 2021, 9, 16651–16658. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefin. 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Gao, Q.; You, J.; Liao, X.; Wang, Z. Identification and analysis of ancient ship wood excavated at Nantong hydraulic site. Bioresources 2023, 18, 5028–5040. [Google Scholar] [CrossRef]
- Blindheim, F.H.; Ruwoldt, J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers 2023, 15, 2901. [Google Scholar] [CrossRef]
- Vasile, C.; Gosselink, R.; Quintus, P.; Koukios, E.G.; Koullas, D.P.; Avgerinos, E.; Abacherli, A. Analytical methods for lignin characterization I. Thermogravimetry. Cell. Chem. Technol. 2006, 40, 421–429. [Google Scholar]
- Pokryshkin, S.; Sypalova, Y.; Ivahnov, A.; Kozhevnikov, A. Optimization of Approaches to Analysis of Lignin by Thermal Decomposition. Polymers 2023, 15, 2861. [Google Scholar] [CrossRef] [PubMed]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cell. Chem.Technol. 2010, 44, 353–363. [Google Scholar]
- Tham, D.; Gardner, C.; Haskell, W. Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. Challenges of lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Services Testing Laboratory, Technical Lignins: Physico-Chemical Characterisations. Specification of Technical and Industrial Lignins. Available online: https://www.webctp.com/en/technical-lignins-physico-chemical-characterisations (accessed on 25 July 2023).
- Mastrolitti, S.; Borsella, E.; Guliano, A.; Petrone, M.T.; De Bari, I.; Gosselink, R.; van Erven, G.; Annevelik, E.; Triantafyllidi, K.S.; Stichnothe, H. Sustainable-Lignin-Valorization. Technical Lignins, Processes and Market Development. IEA Bioenergy Task 42 & LignoCOST—195p. BBP Biorefinery & Sustainable Value Chains IAEA Bioenergy, LignoCOST Task 42: Biorefining in a Circular Economy. Edited by: IEA Bioenergy Task 42 Biorefining in a Circular Economy & LignoCOST. October 2021. Available online: https://task42.ieabioenergy.com/publications/sustainable-lignin-valorization/ (accessed on 6 June 2023).
- Jia, W.; Shi, H.; Sheng, X.; Guo, Y.; Fatehi, P.; Niu, M. Correlation between physico-chemical characteristics of lignin deposited on autohydrolyzed wood chips and their cellulase enzymatic hydrolysis. Bioresour. Technol. 2022, 350, 126941. [Google Scholar] [CrossRef]
- Rosado, M.J.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Structural Characterization of the Milled-Wood Lignin Isolated from Sweet Orange Tree (Citrus sinensis) Pruning Residue. Polymers 2023, 15, 1840. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Khan, A.S.; Ajab, H. Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic Fibre and Biomass-Based Composite Materials. Processing, Properties and Applications; Jawaid, M., Tahir, P.M., Saba, N., Eds.; In Composites Science and Engineering. Cambridge Series; Woodhead Publishing: Sawston, UK, 2017; Chapter 9; pp. 165–178. [Google Scholar]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Smit, A.T.; Verges, M.; Schulze, P.; van Zomeren, A.; Lorenz, H. Laboratory- to Pilot-Scale Fractionation of Lignocellulosic Biomass Using an Acetone Organosolv Process. ACS Sustain. Chem. Eng. 2022, 10, 10503–10513. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef]
- Mukundan, S.; Boffito, D.; Shrotri, A.; Atanda, L.; Beltramini, J.; Patience, G. Thermocatalytic Hydrodeoxygenation and Depolymerization of Waste Lignin to Oxygenates and Biofuels in a Continuous Flow Reactor at Atmospheric Pressure. ACS Sustain. Chem. Eng. 2020, 8, 13195–13205. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, J.; Xiao, R. Catalytic transformation of lignin to aromatic hydrocarbons over solid-acid catalyst: Effect of lignin sources and catalyst species. Energy Convers. Manag. 2016, 124, 61–72. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef]
- Montazeri, M.; Zaimes, G.G.; Khanna, V.; Eckelman, M.J. Meta-Analysis of Life Cycle Energy and Greenhouse Gas Emissions for Priority Biobased Chemicals. ACS Sustain. Chem. Eng. 2016, 4, 6443–6454. [Google Scholar] [CrossRef]
- Fadlallah, S.; Sinha Roy, P.; Garnier, G.; Saito, K.; Allais, F. Are lignin-derived monomers and polymers truly sustainable? An in-depth green metrics calculations approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Vural Gursel, I.; Dijkstra, J.W.; Huijgen, W.J.J.; Ramirez, A. Techno-economic comparative assessment of novel lignin depolymerization routes to bio-based aromatics. Biofuels Bioprod. Biorefin. 2019, 13, 1068–1084. [Google Scholar] [CrossRef]
- Funkenbusch, L.T.; Mullins, M.E.; Vamling, L.; Belkhieri, T.; Srettiwat, N.; Winjobi, O.; Shonnard, D.R.; Rogers, T.N. Techno-economic assessment of hydrothermal liquefaction oil from lignin with catalytic upgrading for renewable fuel and chemical production, Advanced Review. WIREs Energy Environ. 2019, 8, e319. [Google Scholar] [CrossRef]
- Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelić, S.; Vogel, F.; Konttinen, J.; Joronen, T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies 2020, 13, 3309. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Gonzalez Alriols, M.; Labidi, J. Techno-economic analysis of different integrated biorefinery scenarios using lignocellulosic waste streams as source for phenolic alcohols production. J. Clean. Prod. 2021, 285, 124829. [Google Scholar] [CrossRef]
- Tey, T.O.; Chen, S.; Cheong, Z.X.; Xian Choong, A.S.; Ng, L.Y.; Chemmangattuvalappil, N.G. Synthesis of a sustainable integrated biorefinery to produce value-added chemicals from palm-based biomass via mathematical optimisation. Sustain. Prod. Consum. 2021, 26, 288–315. [Google Scholar] [CrossRef]
- Serrano, L.; Cecilia, J.A.; García-Sancho, C.; García, A. Lignin Depolymerization to BTXs Review. Top. Curr. Chem. 2019, 377, 26. [Google Scholar] [CrossRef]
- Ramirez Brenes, R.G.; Alhadeff, E.M.; Bojorge, N.; Trales, L.E.M.; Pazos, G.A.D. Review. BTX production by breaking down lignin: Current status and future prospects. Biofuels Bioprod. Biorefin. 2023, 17, 3664–3681. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Hou, Q.; Mo, Z.; Liu, X.; Li, W. A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst. Fermentation 2023, 9, 386. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Sanchez-Silva, L.; Dorado, F. Enhancement of BTX production via catalytic fast pyrolysis of almond shell, olive pomace with polyvinyl chloride mixtures. Process Saf. Environ. Prot. 2022, 163, 218–226. [Google Scholar] [CrossRef]
- Nadányi, R.; Ház, A.; Lisý, A.; Jablonský, M.; Šurina, I.; Majová, V.; Baco, A. Lignin Modifications, Applications, and Possible Market Prices. Energies 2022, 15, 6520. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.; Fan, J.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Catalytic hydrothermal liquefaction of alkali lignin over activated bio-char supported bimetallic catalyst. Bioresour. Technol. 2021, 337, 125439. [Google Scholar] [CrossRef]
- Shu, R.; Long, J.; Yuan, Z.; Zhang, Q.; Wang, T.; Wang, C.; Ma, L. Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with Pd/C. Bioresour. Technol. 2015, 179, 84–90. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Kim, E.; Ban, C.; Kim, S.O.; Lim, S.; Choi, Y.J. Applications and perspectives of polyphenol-loaded solid lipid nanoparticles and nanostructured lipid carriers for foods. Food Sci. Biotechnol. 2022, 31, 1009–1026. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Phenols from Lignin. Chem. Eng. Technol. Spec. Issue Chang. Raw Mater. 2008, 31, 736–745. [Google Scholar] [CrossRef]
- Bbosa, D.; Mba-Wright, M.; Brown, R.C. Modeling and Analysis. More than ethanol: A techno-economic analysis of a corn stover-ethanol biorefinery integrated with a hydrothermal liquefaction process to convert lignin into biochemicals. Biofuels Bioprod. Biorefin. 2018, 12, 497–509. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Cui, X.; Scown, C.D.; Amezcua-Allieri, M.A.; Aburto, J.; Simmons, B.A. Techno-economic and greenhouse gas analyses of lignin valorization to eugenol and phenolic products in integrated ethanol biorefineries. Biofuels Bioprod. Biorefin. 2019, 13, 978–993. [Google Scholar] [CrossRef]
- Reshmy, R.; Balakumaran, P.A.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Awasthi, M.K.; Sindhu, R. Microbial valorization of lignin: Prospects and challenges. Bioresour. Technol. Part A 2022, 344, 126240. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and conversion of lignin to value added bioproducts by microbial and enzymatic catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Casimiro, F.M.; Vega-Aguilar, C.; Rodrigues, A.E. Lignin Valorization for Added-Value Chemicals: Kraft Lignin versus Lignin Fractions. Chem. Eng. Spec. Issue Green. Environ. Sustain. Chem. Process. 2023, 7, 42. [Google Scholar] [CrossRef]
- Unlu, S.; Niu, W.; Demirel, Y. Bio-based adipic acid production: Feasibility analysis using a multi-criteria decision matrix. Biofuels Bioprod. Biorefin. 2020, 14, 794–807. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Ni, Y. Current overview of carbon fiber: Toward green sustainable raw materials. Bioresources 2020, 15, 7234–7259. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Abdelwahab, M.A.; Misra, M.; Khalil, H.; Gharabaghi, B.; Mohanty, A.K. Processing, Carbonization, and Characterization of Lignin Based Electrospun Carbon Fibers: A Review. Energy Res. Sec. Bioenergy Biofuels 2020, 8, 208. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Bajpai, P. Carbon Fibre from Lignin; Springer Nature Singapore Pte Ltd.: Singapore, 2017; ISBN 978-981-10-4229-4. [Google Scholar]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials. In Green Chemistry and Sustainable Technology; Springer: Berlin, Germany, 2017; pp. 55–79. ISBN 978-3-662-54959-9. Available online: https://link.springer.com/book/10.1007/978-3-662-54959-9 (accessed on 21 July 2023).
- Porc, O.; Hark, N.; Carus, M.; Carrez, D. (BIC) European Bioeconomy in Figures 2008–2019. Commissioned by Biobased Industries Consortium. Brussel, Belgium. 2022. Available online: www.biconsortium.eu (accessed on 26 May 2023).
- Naydenova, I.; Radoykova, T.; Petrova, T.; Sandov, O.; Valchev, I. Utilization Perspectives of Lignin Biochar from Industrial Biomass Residue. Molecules 2023, 28, 4842. [Google Scholar] [CrossRef]
- Gul, E.; Al Bkoor Alrawashdeh, K.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; de Freitas, A.d.S.M.; Lopes, H.S.M.; Pires, A.A.F.; Lemes, A.P.; Ferreira, M.; Botaro, V.R. Improvement of UV stability of thermoplastic starch matrix by addition of selected lignin fraction—Photooxidative degradation. Int. J. Biol. Macromol. 2023, 230, 123142. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, S.K.; Chandna, S.; Kim, K.H.; Bhaumik, J. Lignin-based metal oxide nanocomposites for UV protection applications: A review. J. Clean. Prod. 2021, 317, 128300. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Xu, C. Synthesis of phenol–formaldehyde resol resins using organosolv pine lignins. Eur. Polym. J. 2009, 45, 3380–3388. [Google Scholar] [CrossRef]
- Qiao, W.; Li, S.; Guo, G.; Han, S.; Ren, S.; Ma, Y. Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin. J. Ind. Eng. Chem. 2015, 21, 1417–1422. [Google Scholar] [CrossRef]
- Pandey, M.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Vieira, F.R.; Magina, S.; Evtuguin, D.V.; Barros-Timmons, A. Lignin as a Renewable Building Block for Sustainable Polyurethanes. Materials 2022, 15, 6182. [Google Scholar] [CrossRef]
- Lettner, M.; Hesser, F.; Hedeler, B.; Tobias, S. Barriers and incentives for the use of lignin-based resins: Results of a comparative importance performance analysis. J. Clean. Prod. 2020, 256, 120520. [Google Scholar] [CrossRef]
- Fache, M.; Boutevina, B.; Caillol, S. Epoxy thermosets from model mixtures of the lignin-to-vanillin process. Lignin-Based Epoxy Resins. Green Chem. 2016, 18, 712–725. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Unravelling the Relationship between Structure and Material Properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef]
- Liu, G.; Jin, C.; Huo, S.; Kong, Z.; Chu, F. Preparation and properties of novel bio-based epoxy resin thermosets from lignin oligomers and cardanol. Int. J. Biol. Macromol. 2021, 193, 1400–1408. [Google Scholar] [CrossRef]
- Karagoz, P.; Khiawjan, S.; Marques, M.P.C.; Santzouk, S.; Bugg, T.D.H.; Lye, G.J. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: Linking lignin source and processing with clinical indication. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Shi, K.; Liu, G.; Sun, H.; Weng, Y. Polylactic Acid/Lignin Composites: A Review. Polymers 2023, 15, 2807. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, E.G.; Vasile, C.; Popescu, M.C. Effect of the lignin type on the morphology and thermal properties of the xanthan/lignin hydrogels. Int. J. Biol. Macromol. 2013, 54, 230–237. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A. Assessment of Osteogenic Differentiation Potential of Cytocompatible Rice Husk-Derived Lignin/Silica Nanohybrids for Bone Tissue Engineering. Silicon 2023. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.M.; Gu, L.B.; Sun, R.C.; Wang, X.D. Lignin as a Natural Antioxidant: Property-Structure Relationship and Potential Applications. In Reactive and Functional Polymers Volume One; Gutiérrez, T.J., Ed.; Springer: Cham, Switzerland, 2020; pp. 65–93. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, W.; Huang, J.; Lou, H.; Qiu, X. Study on the Antioxidant Activity of Lignin and Its Application Performance in SBS Elastomer. Ind. Eng. Chem. Res. 2021, 60, 790–797. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Chupka, É.I.; Vershal, V.V.; Burlakov, V.M.; Gvozdev, V.N. A comparison of the antioxidant properties of some natural antioxidants and lignin. Chem. Nat. Compd. 1980, 16, 513–516. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crop. Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, I.S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.P.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhães, W.L.E. Antioxidant, Antibacterial and Antitumoural Activities of Kraft Lignin from Hardwood Fractionated by Acid Precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef]
- Luzi, F.; Yang, W.; Ma, P.; Torre, L.; Puglia, D. Chapter 9-Lignin-Based Materials with Antioxidant and Antimicrobial Properties Lignin-Based Materials for Biomedical Applications Preparation, Characterization, and Implementation; Santos, H., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherland, 2021; pp. 291–326. [Google Scholar]
- Lee, E.; Song, Y.; Lee, S. Crosslinking of Lignin/Poly(Vinyl Alcohol) Nanocomposite Fiber Webs and Their Antimicrobial and Ultraviolet-Protective Properties. Text. Res. J. 2019, 89, 3–12. [Google Scholar] [CrossRef]
- de Melo, C.M.L.; da Cruz Filho, I.J.; de Sousa, G.F.; de Souza Silva, G.A.; do Nascimento Santos, D.K.D.; da Silva, R.S.; de Sousa, B.R.; de Lima Neto, R.G.; de Lima, C.A.M.; de Moraes Rocha, G.J. Lignin Isolated from Caesalpinia Pulcherrima Leaves has Antioxidant, Antifungal and Immunostimulatory Activities. Int. J. Biol. Macromol. 2020, 162, 1725–1733. [Google Scholar] [CrossRef]
- Li, R.; Ouda, R.; Kimura, C.; Narita, R.; Nishimura, H.; Fujita, T.; Watanabe, T. Conversion of Beech Wood into Antiviral Lignin–Carbohydrate Complexes by Microwave Acidolysis. ACS Sustain. Chem. Eng. 2021, 9, 9248–9256. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Saif Ur Rehman, M.; Li, H.; Xu, X.; Xu, J. Lignin-Carbohydrate Complexes (LCCs) and Its Role in Biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Fukuchi, K.; Koshikawa, T.; Asai, D.; Inomata, M.; Sakagami, H.; Takemura, H.; Kanamoto, T.; Aimi, H.; Kikkawa, Y. Lignosulfonate Rapidly Inactivates Human Immunodeficiency and Herpes Simplex Viruses. Medicines 2021, 8, 56. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
- Zongo, L.; Lange, H. Lignins and their potential for use as biopolymers in pharmaceutical engineering—A review. J. Afr. Technol. Pharm. Biopharm. (JATPB) 2022, 1, 37–62. [Google Scholar] [CrossRef]
- .Chandna, S.; Paul, S.; Kaur, R.; Gogde, K.; Bhaumik, J. Photodynamic Lignin Hydrogels: A Versatile Self-Healing Platform for Sustained Release of Photosensitizer Nanoconjugates. ACS Appl. Polym. Mater. 2022, 4, 8962–8976. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Baier, G.; Thines, E.; Landfester, K.; Wurm, F.R. Biodegradable lignin nanocontainers. RSC Adv. 2014, 4, 11661–11663. [Google Scholar] [CrossRef]
- Jiang, S.; Kai, D.; Dou, Q.Q.; Loh, X.J. Multi-arm carriers composed of an antioxidant lignin core and poly (glycidyl methacrylate-co-poly (ethylene glycol) methacrylate) derivative arms for highly efficient gene delivery. J. Mater. Chem. B 2015, 3, 6897–6904. [Google Scholar] [CrossRef]
- Zheng, Q.; Chai, L.; Du, B.; Li, W.; Fu, L.-H.; Chen, X. A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline. Polymers 2023, 15, 1867. [Google Scholar] [CrossRef]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can lignin be transformed into agrochemicals? Recent advances in the agricultural applications of lignin. Ind. Crop. Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, Z.; Zhang, Y.; Peng, P.; She, D. Lignin-based controlled release fertilizers. A review. Int. J. Biol. Macromol. Part B 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting Strategies for Hydroxy Groups of Lignin for Producing Materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of Lignin in Preparation of Slow-Release Fertilizer: Current Status and Future Perspectives. Ind. Crop. Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V. Novel Fertilising Products from Lignin and Its Derivatives to Enhance Plant Development and Increase the Sustainability of Crop Production. J. Clean. Prod. 2022, 366, 132832. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Zhang, L.; Chen, X.; Sun, S.; Sun, R. Research Progress in Lignin-Based Slow/Controlled Release Fertilizer. ChemSusChem 2020, 13, 4356–4366. [Google Scholar] [CrossRef]
- Sutradhar, S.; Fatehi, P. Latest development in the fabrication and use of lignin-derived humic acid. Biotechnol. Biofuels Bioprod. 2023, 16, 38. [Google Scholar] [CrossRef]
- Gazzurelli, C.; Migliori, A.; Mazzeo, P.P.; Carcelli, M.; Pietarinen, S.; Leonardi, G.; Pandolfi, A.; Rogolino, D. Making agriculture more sustainable: An environmentally friendly approach to the synthesis of lignin@Cu pesticides. ACS Sustain. Chem. Eng. 2020, 8, 14886–14895. [Google Scholar] [CrossRef]
- Machado, T.O.; Beckers, S.; Fischer, J.; Müller, B.; Sayer, C.; Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Bio-based Lignin Nanocarriers Loaded with Fungicides as Versatile Platform for Drug Delivery in Plants. Biomacromolecules 2020, 21, 2755–2763. [Google Scholar] [CrossRef]
- Machado, T.O.; Grabow, J.; Sayer, C.; de Araújo, P.H.H.; Ehrenhard, M.L.; Wurm, F.R. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of agri- and horticulture. Adv. Colloid. Interface Sci. 2022, 303, 102645. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted drug delivery in plants: Enzyme-responsive lignin nanocarriers for the curative treatment of the worldwide grapevine trunk disease esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Ortega-Sanhueza, I.; Teruel-Juanes, R.; Henríquez-Gallegos, S.; Ribes-Greus, A.; Pereira, M. Understanding the effect of lignin on the production process and characteristics of lignocellulose nanofibrils from Eucalyptus nitens. Cellulose 2023, 30, 6811–6831. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. Chapter 16—Progress in the synthesis and applications of polymeric nanomaterials derived from waste lignocellulosic biomass. In Advanced Materials for Sustainable Environmental Remediation; Terrestrial and Aquatic Environments; Giannakoudakis, D., Meili, L., Anastopoulos, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 419–433. [Google Scholar]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef]
- Prasadini Perera, U.; Foo, M.L.; Leng Chew, I.M. Synthesis and characterization of lignin nanoparticles isolated from oil palm empty fruit bunch and application in biocomposites. Sustain. Chem. Clim. Action. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. Electrospinning of Softwood Organosolv Lignin without Polymer Addition. ACS Sustain. Chem. Eng. 2022, 11, 607–616. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Wang, Z.; Zhou, L.; Meng, Z.; Wang, X.; Chen, G.; Wang, S.; Jiang, Y. In Situ Lignin Modification Enabling Enhanced Interfibrillar Interactions in Lignocellulosic Nanomaterials toward Structural Applications. ACS Sustain. Chem. Eng. 2023, 11, 7705–7718. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Chu, F. Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin. Nanomaterials 2019, 9, 997. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.H.; Qiu, X.Q.; Li, H.; Yang, D.J. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Beckers, S.J.; Lu, H.; Landfester, K.; Wurm, F.R. Morphology-Controlled Synthesis of Lignin Nanocarriers for Drug Delivery and Carbon Materials. ACS Biomater. Sci. Eng. 2017, 3, 2375–2383. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In Vitro Evaluation of Biodegradable Lignin-Based Nanoparticles for Drug Delivery and Enhanced Antiproliferation Effect in Cancer Cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Xiong, F.; Chu, F. Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials 2019, 9, 188. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-Responsive Lignin-Based Nanocapsules for Controlled Release of Hydrophobic Molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevicius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. Chemphyschem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent Advances in the Fabrication and Application of Biopolymer-Based Micro and Nanostructures: A Comprehensive Review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Li, B.Q.; You, S.P.; Qi, W.; Wang, Y.F.; Su, R.X.; He, Z.M. Structure-tunable assembly of lignin sub-micro spheres by modifying the amphiphilic interfaces of lignin via n-alkane. Eur. Polym. J. 2020, 126, 109539. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Liao, G.F.; Zou, X.X.; Zhao, M.K.; Wu, T.; Chen, Y.H.; Fang, G.G. Size-Controlled and super long-term stable lignin nanospheres through a facile self-assembly strategy from kraft lignin. J. Agric. Food Chem. 2020, 68, 8341–8349. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crop. Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Yadav, K.V.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Choudhary, S.I.N.; Algahtani, A.; Bera, S.P.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953. [Google Scholar] [CrossRef]
- Pathania, K.; Sah, S.P.; Salunke, D.B.; Jain, M.; Yadav, A.K.; Yadav, V.G.; Pawar, S.V. Green synthesis of lignin-based nanoparticles as a bio-carrier for targeted delivery in cancer therapy. Int. J. Biol. Macromol. 2023, 229, 684–695. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Shi, Y.; Gao, B.; Wu, J.; Kirk, T.B.; Xu, J.; Xue, W. Green synthesis of lignin nanoparticle in aqueous hydrotropic solution toward broadening the window for its processing and application. Chem. Eng. J. 2018, 346, 217–225. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lahtinen, M.H.; Agustin, M.B.; Morais de Carvalho, D.; Hirvonen, S.P.; Penttilä, P.A.; Mikkonen, K.S. Green Fabrication Approaches of Lignin Nanoparticles from Different Technical Lignins: A Comparison Study. ChemSusChem 2021, 14, 4718–4730. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jiang, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin Nanoparticles: Green Synthesis in a γ-Valerolactone/Water Binary Solvent and Application to Enhance Antimicrobial Activity of Essential Oils. ACS Sustain. Chem. Eng. 2020, 8, 714–722. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-Based Micro- and Nanomaterials and their Composites in Biomedical Applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cárcamo-Martínez, Á.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for Pharmaceutical and Biomedical Applications—Could This Become a Reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel Lignin Nanoparticles for Oral Drug Delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Garg, J.; Chiu, M.N.; Krishnan, S.; Tripathi, L.K.; Pandit, S.; Far, B.F.; Jha, N.K.; Kesari, K.K.; Tripathi, V.; Pandey, S. Applications of lignin nanoparticles for cancer drug delivery: An update. Mater. Lett. 2022, 311, 131573. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol /chitosan films for active packaging. Ind. Crop. Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Far, B.F.; Isfahani, A.A.; Nasiriyan, E.; Pourmolaei, A.; Mahmoudvand, G.; Rouzbahani, A.K.; Amin, M.N.; Jamal, M.R.N. An Updated Review on Advances in Hydrogel-Based Nanoparticles for Liver Cancer Treatment. Livers 2023, 3, 161–189. [Google Scholar] [CrossRef]
- Agustin, M.; Lehtonen, M.; Kemell, M.; Lahtinen, P.; Oliaei, E.; Mikkonen, K.S. Lignin nanoparticle-decorated nanocellulose cryogels as adsorbents for pharmaceutical pollutants. J. Environ. Manag. 2023, 330, 117210. [Google Scholar] [CrossRef] [PubMed]
- Zikeli, F.; Vettraino, A.M.; Biscontri, M.; Bergamasco, S.; Palocci, C.; Humar, M.; Romagnoli, M. Lignin Nanoparticles with Entrapped Thymus spp. Essential Oils for the Control of Wood-Rot Fungi. Polymers 2023, 15, 2713. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Banpean, A.; Hararak, B.; Winotapun, C.; Kitchaicharoenporn, S.; Naimlang, S. Lignin nanoparticles as sustainable biobased nucleating agents of poly(L-lactic acid): Crystallization behavior and effect of particle sizes. J. Mater. Sci. 2023, 58, 6823–6838. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-Based Electrospun Carbon Nanofibers Frontiers in Materials: Rising Stars mini review. Front. Mater. 2019, 6, 62. [Google Scholar] [CrossRef]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering poly (lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Wang, D.; Jang, J.; Kim, K.; Kim, J.; Park, C.B. Tree to Bone: Lignin/Polycaprolactone Nanofibers for Hydroxyapatite Biomineralization. Biomacromolecules 2019, 20, 2684–2693. [Google Scholar] [CrossRef]
- An, L.; Heo, J.W.; Chen, J.; Kim, Y.S. Water-soluble lignin quaternary ammonium salt for electrospun morphology-controllable antibacterial polyvinyl alcohol/lignin quaternary ammonium salt nanofibers. J. Clean. Prod. 2022, 368, 133219. [Google Scholar] [CrossRef]
- Kai, D.; Chong, H.M.; Chow, L.P.; Jiang, L.; Lin, Q.; Zhang, K.; Zhang, H.; Zhang, Z.; Loh, X.J. Strong and biocompatible lignin/poly (3-hydroxybutyrate) composite nanofibers. Compos. Sci. Technol. 2018, 158, 26–33. [Google Scholar] [CrossRef]
- Abudulah, T.; Gauthaman, K.; Mostafavi, A.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Tamayol, A.; Memic, A. Sustainable Drug Release from Polycaprolactone Coated Chitin-Lignin Gel Fibrous Scaffolds. Sci. Rep. 2020, 10, 20428. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Yang, X.; Yew, P.Y.M.; Sugiarto, S.; Zhu, Q.; Zhao, J.; Loh, X.J.; Zheng, L.; Kai, D. PLA-lignin nanofibers as antioxidant biomaterials for cartilage regeneration and osteoarthritis treatment. J. Nanobiotechnol. 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- . Ni, S.; Bian, H.; Zhang, Y.; Fu, Y.; Liu, W.; Qin, M.; Xiao, H. Starch-Based Composite Films with Enhanced Hydrophobicity, Thermal Stability, and UV-Shielding Efficacy Induced by Lignin Nanoparticles. Biomacromolecules 2022, 23, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yang, W.; Puglia, D.; Wang, H.; Xu, P.; Dong, W.; Zheng, T.; Ma, P. Hydrophobic, UV resistant and dielectric polyurethane-nanolignin composites with good reprocessability. Mater. Des. 2020, 196, 109150. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Valera, M.A.; Mangas, A.; Marra, G.; Ruiz, V.; Koltsakidis, S.; Tzetzis, D.; Zoikis Karathanasis, A.; Deligkiozi, I.; et al. Poly(Lactic Acid) Composites with Lignin and Nanolignin Synthesized by In Situ Reactive Processing. Polymers 2023, 15, 2386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Deng, B.; Luo, W.; Nie, S.; Liu, X.; Yin, Y.; Liu, S.; Wu, Z.; Zhan, P.; Zhang, L.; et al. pH-Responsive Lignin-Based Nanomicelles for Oral Drug Delivery. J. Agric. Food Chem. 2020, 68, 5249–5258. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, J.X.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Chen, J.; Xia, D.; Xu, R.; Zou, X.; Wang, H.; Liang, C. Paclitaxel-loaded lignin particle encapsulated into electrospun PVA/PVP composite nanofiber for effective cervical cancer cell inhibition. Nanotechnology 2020, 32, 015101. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Mussatto, S.I.; Jha, H. Synthesis and characterization of silver nanoparticles loaded poly(vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 120, 763–767. [Google Scholar] [CrossRef]
- Elsherbiny, D.A.; Abdelgawad, A.M.; El-Naggar, M.E.; Hemdan, B.A.; Ghazanfari, S.; Jockenhövel, S.; Rojas, O.J. Bioactive tri-component nanofibers from cellulose acetate/lignin//N-vanillidene-phenylthiazole copper-(II) complex for potential diaper dermatitis control. Int. J. Biol. Macromol. 2022, 205, 703–718. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid electrospinning for multi-drug delivery systems: Pros and cons, challenges, and future directions. Biomater. Sci. 2023, 11, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-L.; Faraz, F.; Wang, J.-X.; Radacsi, N. Combination of 3D Printing and Electrospinning Techniques for Biofabrication. Adv. Mater. Technol. 2022, 7, 2101309. [Google Scholar] [CrossRef]
- Gujjala, L.K.S.; Kim, J.; Won, W. Technical lignin to hydrogels: An Eclectic review on suitability, synthesis, applications, challenges and future prospects. J. Clean. Prod. 2022, 363, 132585. [Google Scholar] [CrossRef]
- Abdullah, T.; Ilyasoglu, G.; Memi, A. Designing Lignin-Based Biomaterials as Carriers of Bioactive Molecules. Pharmaceutics 2023, 15, 1114. [Google Scholar] [CrossRef]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Altuntas, E.; Özkan, B.; Güngör, S.; Özsoy, Y. Biopolymer-Based Nanogel Approach in Drug Delivery: Basic Concept and Current Developments. Pharmaceutics 2023, 15, 1644. [Google Scholar] [CrossRef]
- Daassi, R.; Durand, K.; Rodrigue, D.; Stevanovic, T. Optimization of the Electrospray Process to Produce Lignin Nanoparticles for PLA-Based Food Packaging. Polymers 2023, 15, 2973. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Memic, A.; Rezaeeyazdi, M.; Villard, P.; Rogers, Z.J.; Abudulah, T.; Colombani, T.; Bencherif, S.A. Effect of Polymer Concentration on Autoclaved Cryogel Properties. Macromol. Mater. Eng. 2020, 305, 1900824. [Google Scholar] [CrossRef]
- Abdullah, T.; Colombani, T.; Alade, T.; Bencherif, S.A.; Memic, A. Injectable Lignin-co-Gelatin Cryogels with Antioxidant and Antibacterial Properties for Biomedical Applications. Biomacromolecules 2021, 22, 4110–4121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef]
- Tian, R.; Liu, Q.; Zhang, W.; Zhang, Y. Preparation of lignin-based hydrogel and its adsorption on Cu2+ ions and Co2+ ions in wastewaters. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2545–2553. [Google Scholar] [CrossRef]
- Larraneta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and characterization of lignin hydrogels for potential applications as drug eluting antimicrobial coatings for medical materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, X.; Xie, J.; Feng, B.; Han, Q. Synthesis of a novel tunable lignin-based star copolymer and its flocculation performance in the treatment of kaolin suspension. Sep. Purif. Technol. 2019, 210, 355–363. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and assessment of biodegradable macroporous cryogels as advanced tissue engineering and drug carrying materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Sanz-Santos, E.; García, J. Chapter 22—Activated carbons derived from biomass for the removal by adsorption of several pesticides from water. In Advanced Materials for Sustainable Environmental Remediation. Terrestrial and Aquatic Environments; e-book; Elsevier: Amsterdam, The Netherlands, 2022; pp. 565–583. [Google Scholar]
- Gizli, N.; Çok, S.S.; Koç, F. Chapter 7, Aerogel, xerogel, and cryogel: Synthesis, surface chemistry, and properties—Practical environmental applications and the future developments. In Advanced Materials for Sustainable Environmental. Remediation Terrestrial and Aquatic Environments; Giannakoudakis, D., Meili, L., Anastopoulos, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–229. [Google Scholar]
- .Lyu, Z.; Zheng, Y.; Zhou, H.; Dai, L. Lignin-based Hydrogels for Biological Application. Pap. Biomater. 2023, 8, 37–52. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Mazloom, N.; Khorassani, R.; Zohury, G.H.; Emami, H.; Whalen, J. Lignin-based hydrogel alleviates drought stress in maize. Environ. Exp. Bot. 2020, 175, 104055. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullon, P. Assessment of green approaches for the synthesis of physically crosslinked lignin hydrogels. J. Ind. Eng. Chem. 2020, 81, 475–487. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, Y.; Fang, G.; Ren, S.; Fu, Y. Controlled pesticides release from porous composite hydrogels based on lignin and polyacrylic acid. BioResources 2016, 11, 2361–2371. [Google Scholar] [CrossRef]
- Huang, S.; Wu, L.; Li, T.; Xu, D.; Lin, X.; Wu, C. Facile preparation of biomass lignin-based hydroxyethyl cellulose super-absorbent hydrogel for dye pollutant removal. Int. J. Biol. Macromol. 2019, 137, 939–947. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Shen, J.; Zhang, S.; Liu, X.; Chen, X.; Ma, Y.; Ren, S.; Fang, G.; Li, S.; et al. Simultaneous removal of Pb2+, Cu2+ and Cd2+ ions from wastewater using hierarchical porous polyacrylic acid grafted with lignin. J. Hazard. Mater. 2020, 392, 122208. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, Ł.; Kołodynska, D.; Jesionowski, T. Development of functional lignin-based spherical particles for the removal of vanadium (V) from an aqueous system. Int. J. Biol. Macromol. 2021, 186, 181–193. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- . Ivanov, A.E.; Halthur, T.; Ljunggren, L. Flow permeable composites of lignin and poly(vinyl alcohol): Towards removal of bisphenol A and erythromycin from water. J. Environ. Chem. Eng. 2016, 4, 1432–1441. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr(VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Influence of lignin modifications on physically crosslinked lignin hydrogels for drug delivery applications. Sustain. Mater. Technol. 2022, 33, e00474. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Aliabadi, H.A.M.; Radinekiyan, F.; Sobhani, M.; Khalili, F.; Maleki, A.; Madanchi, H.; Mahdavi, M.; Shalan, A.E. Investigation of the Biological Activity, Mechanical Properties and Wound Healing Application of a Novel Scaffold Based on Lignin–Agarose Hydrogel and Silk Fibroin Embedded Zinc Chromite Nanoparticles. RSC Adv. 2021, 11, 17914–17923. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.; Quraishi, S.; Martins, M.; Gurikov, P.; Subrahmanyam, R.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Hybrid Alginate Based Cryogels for Life Science Applications. Chem. Ing. Tech. 2016, 88, 1770–1778. [Google Scholar] [CrossRef]
- Memic, A.; Abudulah, T. Antioxidant, Antibacterial, Injectable Lignin-Gelatin Composite Cryogels for Wound Healing and Tissue Engineering. U.S. Patent US10881760B1, 5 January 2021. [Google Scholar]
- Chiani, E.; Beaucamp, A.; Hamzeh, Y.; Azadfallah, M.; Thanusha, A.; Collins, M.N. Synthesis and characterization of gelatin/lignin hydrogels as quick release drug carriers for Ribavirin. Int. J. Biol. Macromol. 2023, 224, 1196–1205. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.; Lee, Y.; Oh, J.; Jung, Y.; Koh, W.G.; Chung, J.J. Reactive Oxygen Species Suppressive Kraft Lignin-Gelatin Antioxidant Hydrogels for Chronic Wound Repair. Macromol Biosci. 2022, 22, 2200234. [Google Scholar] [CrossRef]
- . Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Culebras, M.; Pishnamazi, M.; Walker, G.M.; Collins, M.N. Facile Tailoring of Structures for Controlled Release of Paracetamol from Sustainable Lignin Derived Platforms. Molecules 2021, 26, 1593. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, J.J.; Lin, Q.; Jiang, L.; Zhang, D.; Li, Z.; Ma, B.; Zhang, C.; Li, L.; Kai, D.; et al. Lignin-incorporated nanogel serving as an antioxidant biomaterial for wound healing. ACS Appl. Bio Mater. 2020, 4, 3–13. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, Q.; Xie, Y.; Li, J.; Chen, X. Preparation of biocompatible hydrogel from lignin-carbohydrate complex (LCC) as cell carriers. BioResources 2017, 12, 8490–8504. [Google Scholar] [CrossRef]
- Li, M.; Jiang, X.; Wang, D.; Xu, Z.; Yang, M. In situ reduction of silver nanoparticles in the lignin based hydrogel for enhanced antibacterial application. Colloids Surf. B Biointerfaces 2019, 177, 370–376. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Effect of the formulation parameters on the absorption capacity of smart lignin-hydrogels. Eur. Polym. J. 2020, 129, 109631. [Google Scholar] [CrossRef]
- Musilová, L.; Mráček, A.; Kovalcik, A.; Smolka, P.; Minařík, A.; Humpolíček, P.; Vícha, R.; Ponížil, P. Hyaluronan hydrogels modified by glycinated Kraft lignin: Morphology, swelling, viscoelastic properties and biocompatibility. Carbohydr. Polym. 2018, 181, 394–403. [Google Scholar] [CrossRef]
- Chandna, S.; Thakur, N.S.; Kaur, R.; Bhaumik, J. Lignin–bimetallic nanoconjugate doped pH-responsive hydrogels for laser-assisted antimicrobial photodynamic therapy. Biomacromolecules 2020, 21, 3216–3230. [Google Scholar] [CrossRef]
- Raschip, I.; Panainte, A.; Daniela, P.; Profire, L.; Vasile, C. In Vitro Testing of Xanthan/Lignin Hydrogels as Carriers for Controlled Delivery of Bisoprolol Fumarate. Rev. Med. Chir. Soc. Med. Nat. Iasi 2015, 119, 1189–1194. [Google Scholar] [PubMed]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldana, M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason Sonochem. 2020, 64, 104759. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, L.; Renneckar, S.; Jiang, F. Chemically and physically crosslinked lignin hydrogels with antifouling and antimicrobial properties. Ind. Crop. Prod. 2021, 170, 113759. [Google Scholar] [CrossRef]
- El-Nemr, K.F.; Mohamed, H.R.; Ali, M.A.; Fathy, R.M.; Dhmees, A.S. Polyvinyl alcohol/gelatin irradiated blends filled by lignin as green filler for antimicrobial packaging materials. Int. J. Environ. Anal. Chem. 2020, 100, 1578–1602. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaun, F.; Guan, J. Valorization of industrial lignin as biobased carbon source in fire retardant system for Polyamide 11 blends. Polymers 2019, 11, 180. [Google Scholar] [CrossRef]
- Wu, K.; Xu, S.; Tian, X.-Y.; Zeng, H.-Y.; Hu, J.; Guo, Y.-H.; Jian, J. Renewable lignin-based surfactant modified layered double hydroxide and its application in polypropylene as flame retardant and smoke suppression. Int. J. Biol. Macromol. 2021, 178, 580–590. [Google Scholar] [CrossRef]
- Xu, Q.; Bai, Y.; Zhao, X.; Ren, M.; Wang, S.; Kong, F. Synthesis and characterization of an amphiphilic lignin-based cationic surfactant. Ind. Crop. Prod. 2021, 164, 113376. [Google Scholar] [CrossRef]
- Soares, A.K.; Gatto, D.A.; Magalhaes, W.L.E.; Erdocia, X.; Gutierrez, M.A.U.; Delucis, R.A.; Missio, A.L. Kraft lignin as a partial replacement for a traditional surfactant in thymol-based biocidal suspensions: Physical and chemical features. J. Wood Chem. Technol. 2021, 41, 199–209. [Google Scholar] [CrossRef]
- Moreno, A.; Morsali, M.; Liu, J.; Sipponen, M.H. Access to tough and transparent nanocomposites of PS/PBMA (poly(butylmethacrylate) nanocomposites via Pickering emulsion polymerization using biocatalytic hybrid lignin nanoparticles as functional surfactants. Green Chem. 2021, 23, 3001–3014. [Google Scholar] [CrossRef]
- Ghavidel, N.; Fatehi, P. Interfacial and Emulsion Characteristics of Oil-Water Systems in the Presence of Polymeric Lignin Surfactant. Langmuir 2021, 37, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.; Qurban, R.O.; Bolarinwa, S.O.; Mirza, A.A.; Pasovic, M.; Memic, A. 3D Printing of Metal/Metal Oxide Incorporated Thermoplastic Nanocomposites With Antimicrobial Properties. Front. Bioeng. Biotechnol. 2020, 8, 568186. [Google Scholar] [CrossRef]
- Karakurt, I.; Lin, L. 3D printing technologies: Techniques, materials, and post-processing. Curr. Opin. Chem. Eng. 2020, 28, 134–143. [Google Scholar] [CrossRef]
- Abdullah, T.; Okay, O. 4D Printing of Body Temperature-Responsive Hydrogels Based on Poly (acrylic acid) with Shape-Memory and Self-Healing Abilities. ACS Appl. Bio Mater. 2023, 6, 703–711. [Google Scholar] [CrossRef]
- Sharma, K.S. Artificial Intelligence Assisted Fabrication of 3D, 4D and 5D Printed Formulations or Devices for Drug Delivery. Curr. Drug Deliv. 2023, 20, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, A.F.; Zhang, M.; Mujumdar, A.S.; Ghamry, M. Progress in 4D/5D/6D printing of foods: Applications and R&D opportunities. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhu, S.; Wei, X.; Zhou, N.; Liao, X.; Peng, Y.; Tang, Y.; Zhang, L.; Yang, X. Cryoprinting of nanoparticle enhanced injectable hydrogel with shape-memory properties. Mater. Des. 2022, 223, 111120. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.N.; Yong, W.P.; Low, H.R.; Kochhar, J.S.; Khanolkar, J.; Lim, T.S.E.; Sun, Y.; Wong, J.Z.E.; Soh, S. Customizable drug tablets with constant release profiles via 3D printing technology. Int. J. Pharm. 2021, 598, 120370. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Samandari, M.; Mostafavi, A.; Quint, J.; Memic, A.; Tamayol, A. In situ bioprinting: Intraoperative implementation of regenerative medicine. Trends Biotechnol. 2022, 40, 1229–1247. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; An, X.; Liu, L.; Tang, S.; Cao, H.; Xu, Q.; Liu, H. Cellulose, Hemicellulose, Lignin, and Their Derivatives as MultiComponents of Bio-Based Feedstocks for 3D Printing. Carbohydr. Polym. 2020, 250, 116881. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, Z.; Chmely, S.; Wang, Q.; Wang, S.; Xie, Y. Lignin-Coated Cellulose Nanocrystal Filled Methacrylate Composites Prepared via 3D Stereolithography Printing: Mechanical Reinforcement and Thermal Stabilization. Carbohydr. Polym. 2017, 169, 272–281. [Google Scholar] [CrossRef]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. Sec. Colloid. Mater. Interfaces 2020, 7, 76. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 160–163. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ebers, L.S.; Arya, A.; Bowland, C.C.; Glasser, W.G.; Chmely, S.C.; Naskar, A.K.; Laborie, M.P. 3D printing of lignin: Challenges, opportunities and roads onward. Biopolymers 2021, 112, e23431. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again. Some Lessons from the Past. Front. Chem. Sec. Green. Sustain. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Sachdeva, I.; Ramesh, S.; Chadha, U.; Punugoti, H.; Selvaraj, S.K. Computational AI models in VAT photopolymerization: A review, current trends, open issues, and future opportunities. Neural Comput. Appl. 2022, 34, 17207–17229. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Abdullah, T.; Qurban, R.O.; Abdel-Wahab, M.S.; Salah, N.A.; Melaibari, A.A.; Zamzami, M.A.; Memic, A. Development of Nanocoated Filaments for 3D Fused Deposition Modeling of Antibacterial and Antioxidant Materials. Polymers 2022, 14, 2645. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Slita, A.; Backman, O.; Gounani, Z.; Rosqvist, E.; Peltonen, J.; Willför, S.; Xu, C.; Rosenholm, J.M.; et al. Digital light processing (DLP) 3D-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres. Green Chem. 2022, 24, 2129–2145. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cuartas-Gómez, E.; Dynes, S.; Utomo, E.; Anjani, Q.K.; Detamornrat, U.; Donnelly, R.F.; MorenoCastellanos, N.; Larrañeta, E. Poly(caprolactone)/lignin-based 3D-printed dressings loaded with a novel combination of bioactive agents for wound-healing applications. Sustain. Mater. Technol. 2023, 35, e00581. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, Z.; Sun, H.; Bi, H.; Gu, T.; Xu, M. 3D printing with high content of lignin enabled by introducing polyurethane. Int. J. Biol. Macromol. 2022, 221, 1209–1217. [Google Scholar] [CrossRef]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-Containing Photoactive Resins for 3D Printing by Stereolithography. Available online: https://chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c73f19ee301c3697c7882e/original/lignin-containing-photoactive-resins-for-3d-printing-by-stereolithography.pdf (accessed on 19 May 2023).
- Ebers, L.-S.; Laborie, M.-P. Direct Ink Writing of Fully Bio-Based Liquid Crystalline Lignin/Hydroxypropyl Cellulose Aqueous Inks: Optimization of Formulations and Printing Parameters. ACS Appl. Bio Mater. 2020, 3, 6897–6907. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W.; Hahm, D.-H. Lignin-Mediated Green Synthesis of AgNPs in Carrageenan Matrix for Wound Dressing Applications. Int. J. Biol. Macromol. 2020, 159, 859–869. [Google Scholar] [CrossRef]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef]
- Nuutinen, E.-M.; Valle-Delgado, J.J.; Kellock, M.; Farooq, M.; Österberg, M. Affinity of Keratin Peptides for Cellulose and Lignin: A Fundamental Study toward Advanced Bio-Based Materials. Langmuir 2022, 38, 9917–9927. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Scott, S.M.; Plowman-Holmes, M.I.; Middlewood, P.G.; Recabar, K. Combination and processing keratin with lignin as biocomposite materials for additive manufacturing technology. Acta Biomater. 2020, 104, 95–103. [Google Scholar] [CrossRef]
- Lee, J.G.; Guo, Y.; Belgodere, J.A.; Al Harraq, A.; Hymel, A.A.; Pete, A.J.; Valsaraj, K.T.; Benton, M.G.; Miller, M.G.; Jung, J.P.; et al. Lignin-Zein Composite: Synthesis, Three-Dimensional Printing, and Microbial Degradation. ACS Sustain. Chem. Eng. 2021, 9, 1781–1789. [Google Scholar] [CrossRef]
- Hong, S.H.; Park, J.H.; Kim, O.Y.; Hwang, S.H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Researchers Upcycle Lignin for Sustainable 3D Printing with Economics in Mind Hayley Everett January 21st 2022—2:55pm. Available online: https://3dprintingindustry.com/news/researchers-upcycle-lignin-for-sustainable-3d-printing-with-economics-in-mind-202718/ (accessed on 28 May 2023).
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Solihat, N.N.; Sari, F.P.; Falah, F.; Ismayati, M.; Lubis, M.A.R.; Fatriasari, W. Lignin as an active biomaterial: A review. J. Sylva Lestari 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Polat, Y.; Stojanovska, E.; Negawo, T.A.; Doner, E.; Kilic, A. Lignin as an additive for advanced composites. In Green Biocomposites: Manufacturing and Properties; Jawaid, M., Sapuan, S.M., Alothman, O.Y., Eds.; Springer International Publishing: Manhattan, NY, USA, 2017; pp. 71–89. [Google Scholar]
- Taleb, F.; Ammar, M.; Mosbah, M.; Salem, R.B.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef]
- Komisarz, K.; Majka, T.M.; Pielichowski, K. Chemical and Physical Modification of Lignin for Green Polymeric Composite Materials. Materials 2022, 16, 16. [Google Scholar] [CrossRef]
- Cazacu, G.; Chirilă, O.; Totolin, M.I.; Ciolacu, D.; Niţă, L.; Drobotă, M.; Vasile, C. Chemical Treatment of Lignosulfonates Under DBD Plasma Conditions. I. Spectral Characterization. J. Polym. Environ. 2021, 29, 900–921. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Review on Lignin Modifications toward Natural UV Protection Ingredient for Lignin-Based Sunscreens. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Mariana, M.; Alfatah, T.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.G.; Nuryawan, A.; Mistar, E.M.; Abdullah, C.K.; Abdulmadjid, S.N.; Ismail, H. A current advancement on the role of lignin as sustainable reinforcement material in biopolymeric blends. J. Mater. Resear. Technol. 2021, 15, 2287–2316. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Martínez, Á.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this: Action mechanisms of ligninolytic peroxidases. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Nägele, H.; Pfitzer, J.; Näegele, E.; Inone, E.R.; Eisenreich, N.; Eckl, W.; Eyerer, P. Arboform: A thermoplastic, processable material from lignin and natural fibers. In Chemical Modification, Properties, and Usage of Lignin; Hu, T.Q., Ed.; Kluwer Academic/Plenum: New York, NY, USA, 2002; pp. 101–120. [Google Scholar]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Vasile, C.; Cazacu, G. Biocomposites and Nanocomposites Containing Lignin. Chapter 23. In Biopolymer Nanocomposites: Processing, Properties, and Applications; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Wang, J.; He, Y.; Xie, H.; Zheng, Q. The Influence of Compatibility on the Structure and Properties of PLA/Lignin Biocomposites by Chemical Modification. Polymers 2020, 12, 56. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, W.; Liu, Y.; Hu, H.; Cochran, E.; Bai, X. Agricultural Residue-derived Lignin as the Filler of Polylactic Acid Composites and the Effect of Lignin Purity on the Composite Performance. J. Appl. Polym. Sci. 2019, 136, 47915. [Google Scholar] [CrossRef]
- Anugwom, I.; Lahtela, V.; Kallioinen, M.; Kärki, T. Lignin as a Functional Additive in a Biocomposite: Influence on Mechanical Properties of Polylactic Acid Composites. Ind. Crop. Prod. 2019, 140, 111704. [Google Scholar] [CrossRef]
- Kumar, A.; Tumu, V.R.; Ray Chowdhury, S.; Ramana Reddy, S.V.S. A Green Physical Approach to Compatibilize a Bio-Based Poly (Lactic Acid)/Lignin Blend for Better Mechanical, Thermal and Degradation Properties. Int. J. Biol. Macromol. 2019, 121, 588–600. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Investigation on Two Modification Strategies for the Reinforcement of Biodegradable Lignin/Poly(Lactic Acid) Blends. J. Appl. Polym. Sci. 2020, 137, 49354. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C.; Weng, Y. Enhancing Gas Barrier Performance of Polylactic Acid/Lignin Composite Films through Cooperative Effect of Compatibilization and Nucleation. J. Appl. Polym. Sci. 2021, 138, 50199. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, Antioxidant and Swelling Behaviour of Transparent Polyvinyl (Alcohol) Films in Presence of Hydrophobic Citric Acid-Modified Lignin Nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef]
- Boarino, A.; Schreier, A.; Leterrier, Y.; Klok, H.-A. Uniformly Dispersed Poly(Lactic Acid)-Grafted Lignin Nanoparticles Enhance Antioxidant Activity and UV-Barrier Properties of Poly (Lactic Acid) Packaging Films. ACS Appl. Polym. Mater. 2022, 4, 4808–4817. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Blindheim, F.H.; Chinga-Carrasco, G. Functional surfaces, films, and coatings with lignin—A critical review. RSC Adv. 2023, 13, 12529–12553. [Google Scholar] [CrossRef]
- Aqlil, M.; Nzenguet, A.M.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene Oxide Filled Lignin/Starch Polymer Bionanocomposite: Structural, Physical, and Mechanical Studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef]
- Bhat, R.; Abdullah, N.; Din, R.H.; Tay, G.S. Producing novel sago starch based food packaging films by incorporating lignin isolated from oil palm black liquor waste. J. Food Eng. 2013, 119, 707–713. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef]
- Stevens, E.S. Starch-lignin foams. Express Polym. Lett. 2010, 4, 311–320. [Google Scholar] [CrossRef]
- Roostazadeh, R.; Behzad, T.; Karimi, K. Isolation and characterization of lignin-rich particles as byproducts of bioethanol production from wheat straw to reinforce starch composite films. Ind. Crop. Prod. 2022, 186, 115175. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Wu, H.; Zhou, Z.; Feng, S.; Deng, P.; Zou, H.; Tian, D.; Lu, C. Sustainable Starch/Lignin Nanoparticle Composites Biofilms for Food Packaging Applications. Polymers 2023, 15, 1959. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Z.; Zeng, Y.; Yang, N.; Liu, M.; Zhao, Y.; Zheng, Y. Lignin Biodegradation and Its Valorization. Fermentation 2022, 8, 366. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.B.; et al. Electrosprayed chitin nanofibril/electrospun polyhydroxyalkanoate fiber mesh as functional nonwoven for skin application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef]
- Posoknistakul, P.; Tangkrakul, C.; Chaosuanphae, P.; Deepentham, S.; Techasawong, W.; Phonphirunrot, N.; Bairak, S.; Sakdaronnarong, C.; Laosiripojana, N. Fabrication and Characterization of Lignin Particles and Their Ultraviolet Protection Ability in PVA Composite Film. ACS Omega 2020, 5, 20976–20982. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Mosa, M.A.; Ismail, A.M.; Khalil, A.E. Lignin-Loaded Carbon Nanoparticles as a Promising Control Agent against Fusarium verticillioides in Maize: Physiological and Biochemical Analyses. Polymers 2023, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, H.; Yuncong, C.; Li, Y.C.; Li, T.; Liu, G.; Zhang, S.; Tong, Z. Porous 3D-Biocomposite Adsorbent for Iron Reclamation and Use in Agricultural Applications. ACS Appl. Polym. Mater. 2022, 4, 8277–8289. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; AmirulIslam, M.; Sadrzadeh, M. Industrial waste lignin as an antifouling coating for the treatment of oily wastewater: Creating wealth from waste. J. Clean. Prod. 2020, 256, 120304. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Stoica, I.; Vasilescu, D.S.; Cazacu, G.; Vasile, C. Alginate/Lignosulfonate Blends with Photoprotective and Antioxidant Properties for Active Packaging Applications. J. Polym. Environ. 2018, 26, 1100–1112. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Li, F.; Wang, X.; Man, J.; Li, J.; Zhang, C.; Peng, S. A Biodegradable Chitosan-Based Composite Film Reinforced by Ramie Fibre and Lignin for Food Packaging. Carbohydr. Polym. 2022, 281, 119078. [Google Scholar] [CrossRef]
- Buzarovska, A.; Blazevska-Gilev, J.; Perez-Martnez, B.T.; Balahura, L.R.; Pircalabioru, G.G.; Dinescu, S.; Costache, M. Poly(l-lactic acid)/alkali lignin composites: Properties, biocompatibility, cytotoxicity and antimicrobial behavior. J. Mater. Sci. 2021, 56, 13785–13800. [Google Scholar] [CrossRef]
- Wei, M.; Fan, L.; Huang, J.; Chen, Y. Role of star-like hydroxylpropyl lignin in soy-protein plastics. Macromol. Mater. Eng. 2006, 291, 524–530. [Google Scholar] [CrossRef]
- Oliviero, M.; Verdolotti, L.; Nedi, I.; Docimo, F.; Di Maio, E.; Iannance, S. Effect of two kinds of lignins, alkaline lignin and sodium lignosulfonate, on the foamibility of thermoplastic zein-based biocomposites. J. Cell. Plast. 2012, 48, 516–525. [Google Scholar] [CrossRef]
- Olonisakin, K.; Wen, A.; He, S.; Lin, H.; Tao, W.; Chen, S.; Yang, W. The Development of Biodegradable PBAT-Lignin-Tannic Acid Composite Film: Properties, Biodegradability, and Potential Barrier Application in Food Packaging. Food Bioprocess Technol. 2023, 16, 1525–1540. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Li, X.; Zhang, Y.; Mao, Z.; Wang, B.; Sui, X.; Feng, X. Fabrication of lignin/poly(3-hydroxybutyrate) nanocomposites with enhanced properties via a Pickering emulsion approach. Int. J. Biol. Macromol. 2020, 165 Pt B, 3078–3087. [Google Scholar] [CrossRef]
- Vachon, J.; Assad-Alkhateb, D.; Baumberger, S.; van Haveren, J.; Gosselink, R.J.A.; Monedero, M.; Bermudez, J.M. Use of lignin as additive in polyethylene for food protection: Insect repelling effect of an ethyl acetate phenolic extract. Compos. Part C 2020, 2, 100044. [Google Scholar] [CrossRef]
- Sathasivam, T.; Kai, J.; Sugiarto, S.; Yu, Y.; Soo, D.X.Y.; Zhu, Q.; Merzaban, J.; Kai, D. Review. Nano-Strategies for Lignin Biomaterials toward Cancer Therapy. Adv. Healthc. Mater. 2023, 2300024. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-S.; Kim, Y.-O.; Ha, Y.-M.; Lim, D.; Hwang, J.Y.; Kim, J.; Park, M.; Cho, J.W.; Jung, Y.C. Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly (vinyl alcohol) nanocomposites. Eur. Polym. J. 2018, 105, 79–84. [Google Scholar] [CrossRef]
- Saudi, A.; Amini, S.; Amirpour, N.; Kazemi, M.; Kharazi, A.Z.; Salehi, H. Promoting neural cell proliferation and differentiation by incorporating lignin into electrospun poly (vinyl alcohol) and poly (glycerol sebacate) fibers. Mater. Sci. Eng. C. 2019, 104, 110005. [Google Scholar] [CrossRef]
- Amini, S.; Saudi, A.; Amirpour, N.; Jahromi, M.; Najafabadi, S.S.; Kazemi, M.; Rafienia, M.; Salehi, H. Application of electrospun poly-caprolactone fibers embedding lignin nanoparticle for peripheral nerve regeneration: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 159, 154–173. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Zhao, X.; Zhang, X. Utilization of lignin upon successive fractionation and esterification in polylactic acid (PLA)/lignin biocomposite. Int. J. Biol. Macromol. 2022, 203, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-graft-polyoxazoline conjugated triazole a novel anti-infective ointment to control persistent inflammation. Sci. Rep. 2017, 7, 46412. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.A.; Jacob, S.; Misra, M.; Mohanty, A.K. Super-tough sustainable biobased composites from polylactide bioplastic and lignin for bio-elastomer application. Polymer 2021, 212, 123153. [Google Scholar] [CrossRef]
- Gouveia, J.R.; de Sousa Júnior, R.R.; Ribeiro, A.O.; Saraiva, S.A.; dos Santos, D.J. Effect of soft segment molecular weight and NCO: OH ratio on thermomechanical properties of lignin-based thermoplastic polyurethane adhesive. Eur. Polym. J. 2020, 131, 109690. [Google Scholar] [CrossRef]
- Vostrejs, P.; Adamcová, D.; Vaverková, M.D.; Enev, V.; Kalina, M.; Machovsky, M.; Šourková, M.; Marova, I.; Kovalcik, A. Active Biodegradable Packaging Films Modified with Grape Seeds Lignin. RSC Adv. 2020, 10, 29202–29213. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.J.; Pang, B.; Zhou, S.-J.; Li, M.-K.; Yang, S.; Wang, Y.-Y.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.-C. Economically competitive biodegradable PBAT/lignin composites: Effect of lignin methylation and compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Yetiş, F.; Liu, X.; Sampson, W.W.; Gong, R.H. Biodegradation of Composites of Polylactic Acid and Microfibrillated Lignocellulose. J. Polym. Environ. 2023, 31, 698–708. [Google Scholar] [CrossRef]
- Ng, Q.Y.; Low, J.H.; Pang, M.M.; Idumah, C.I. Properties Enhancement of Waterborne Polyurethane Bio-composite Films with 3-aminopropyltriethoxy Silane Functionalized Lignin. J. Polym. Environ. 2023, 31, 688–697. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
- Rasyidur, M.R.; Agustiany, E.A.; Dn, M.R.; Madyaratri, E.W.; Ghozali, M.; Restu, W.K.; Falah, F.; Lubis, M.A.R.; Syamani, F.A.; Nurhamiyah, Y.; et al. Lignin as Green Filler in Polymer Composites: Development Methods, Characteristics, and Potential Applications. Hindawi Adv. Mater. Sci. Eng. 2022, 10, 1–33. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional lignin-based nanocomposites and nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Shen, R.; Tao, L.; Yang, B. Modeling and Analysis. Techno-economic analysis of jet-fuel production from biorefinery waste lignin. Biofuels Bioprod. Biorefin. 2019, 13, 486–501. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Bacovsky, D. IEA-Energy a Report to IEA Bioenergy Task 39. Available online: https://www.osti.gov/etdeweb/servlets/purl/22110325 (accessed on 22 July 2023).
- Stone, M.L.; Webber, M.S.; Mounfield, W.P.; Bell, D.C.; Christensen, E.; Morais, A.R.C.; Li, Y.; Anderson, E.M.; Heyne, J.S.; Beckham, G.T.; et al. Continuous hydrodeoxygenation of lignin to jet-range aromatic hydrocarbons. Joule 2022, 6, 2324–2337. [Google Scholar] [CrossRef]
- 100% Sustainable Aviation Fuel Developed from Lignin. Energy. 28 September 2022. Available online: https://www.innovationnewsnetwork.com/100-sustainable-aviation-fuel-developed-from-lignin/25767/ (accessed on 22 July 2023).
- Mukhopadhyay, A.; Hamel, J.; Katahira, R.; Zhu, H. Metal-Free Aqueous Flow Battery with Novel Ultrafiltered Lignin as Electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 5394–5400. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crop. Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Wang, H.; Fu, F.; Huang, M.; Feng, Y.; Han, D.; Xi, Y.; Xiong, W.; Yang, D.; Niu, L. Lignin-based materials for electrochemical energy storage devices. Nano Mater. Sci. 2023, 5, 141–160. [Google Scholar] [CrossRef]
- Li, W.; Shi, J. Lignin-derived carbon material for electrochemical energy storage applications: Insight into the process-structure-properties-performance correlations. Front. Bioeng. Sec. Biomater. Biotechnol. 2023, 11, 21027. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Ren, S.; Dong, L.; Ai, Y.; Lei, T.; Wu, Q. Lignin containing cellulose nanofiber based nanopapers with ultrahigh optical transmittance and haze. Cellulose 2023, 30, 5967–5985. [Google Scholar] [CrossRef]
- Yeasmin, F.; Masud, R.A.; Chisty, A.H.; Hossain, M.A.; Mallik, A.K.; Rahman, M.M. Lignin-metal oxide composite for photocatalysis and photovoltaics. In Renewable Polymers and Polymer-Metal. Oxide Composites. Synthesis, Properties, and Applications. A Volume in Metal. Oxides; Haider, S., Haider, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 14; pp. 447–476. [Google Scholar] [CrossRef]
- Meraj, A.; Singh, S.P.; Jawaid, M.; Nasef, M.M.; Alomar, T.S.; AlMasoud, N. A Review on Eco-friendly Isolation of Lignin by Natural Deep Eutectic Solvents from Agricultural Wastes. J. Polym. Environ. 2023, 31, 3283–3316. [Google Scholar] [CrossRef]
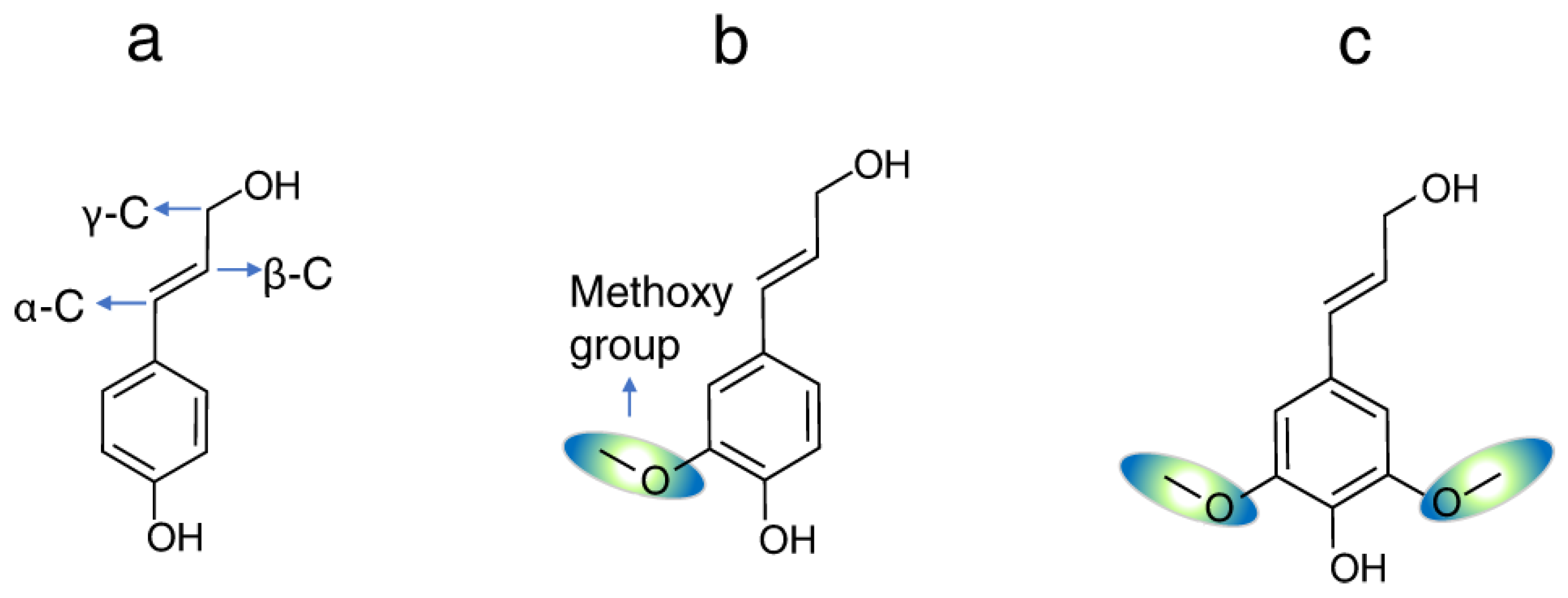
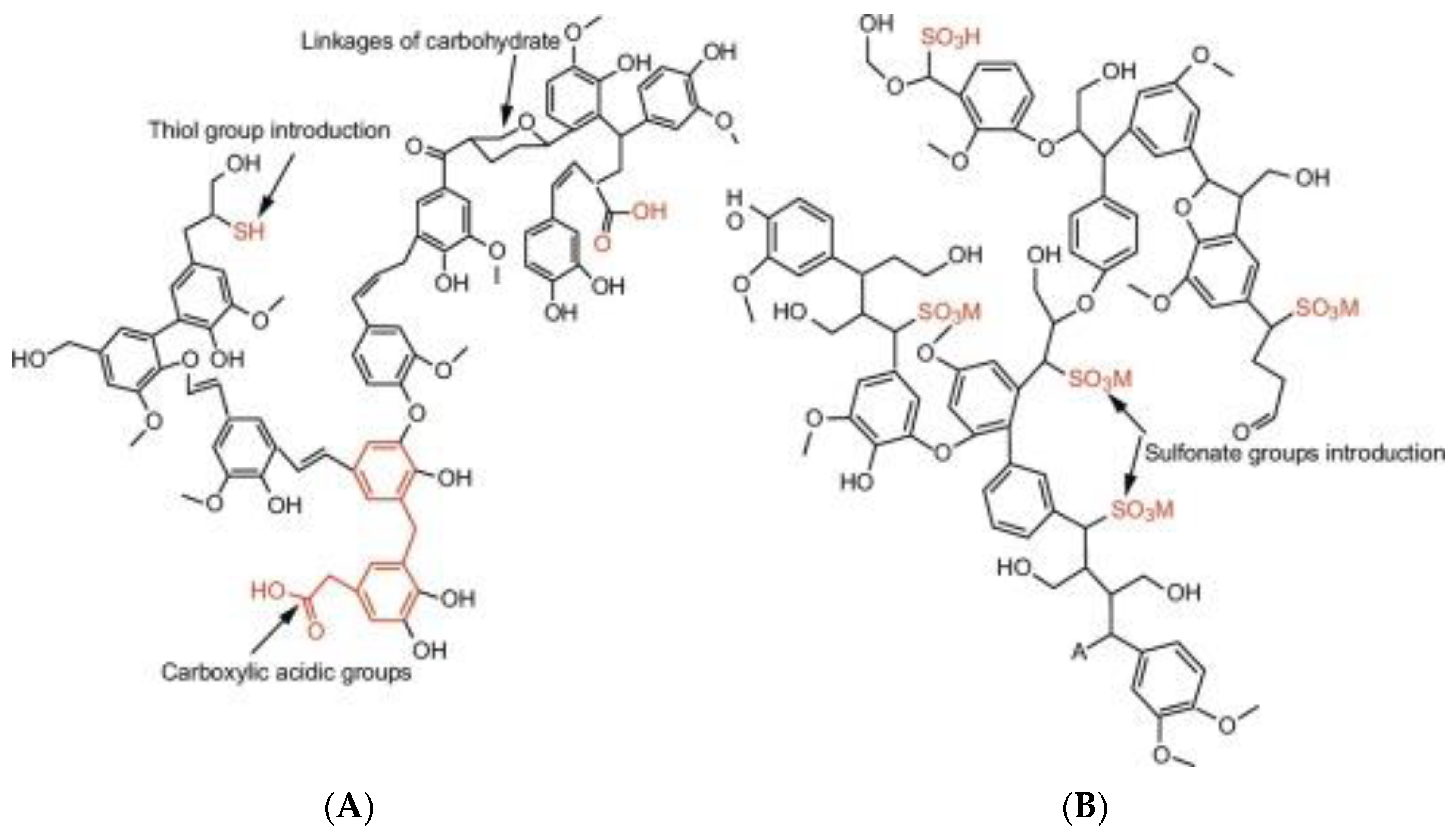
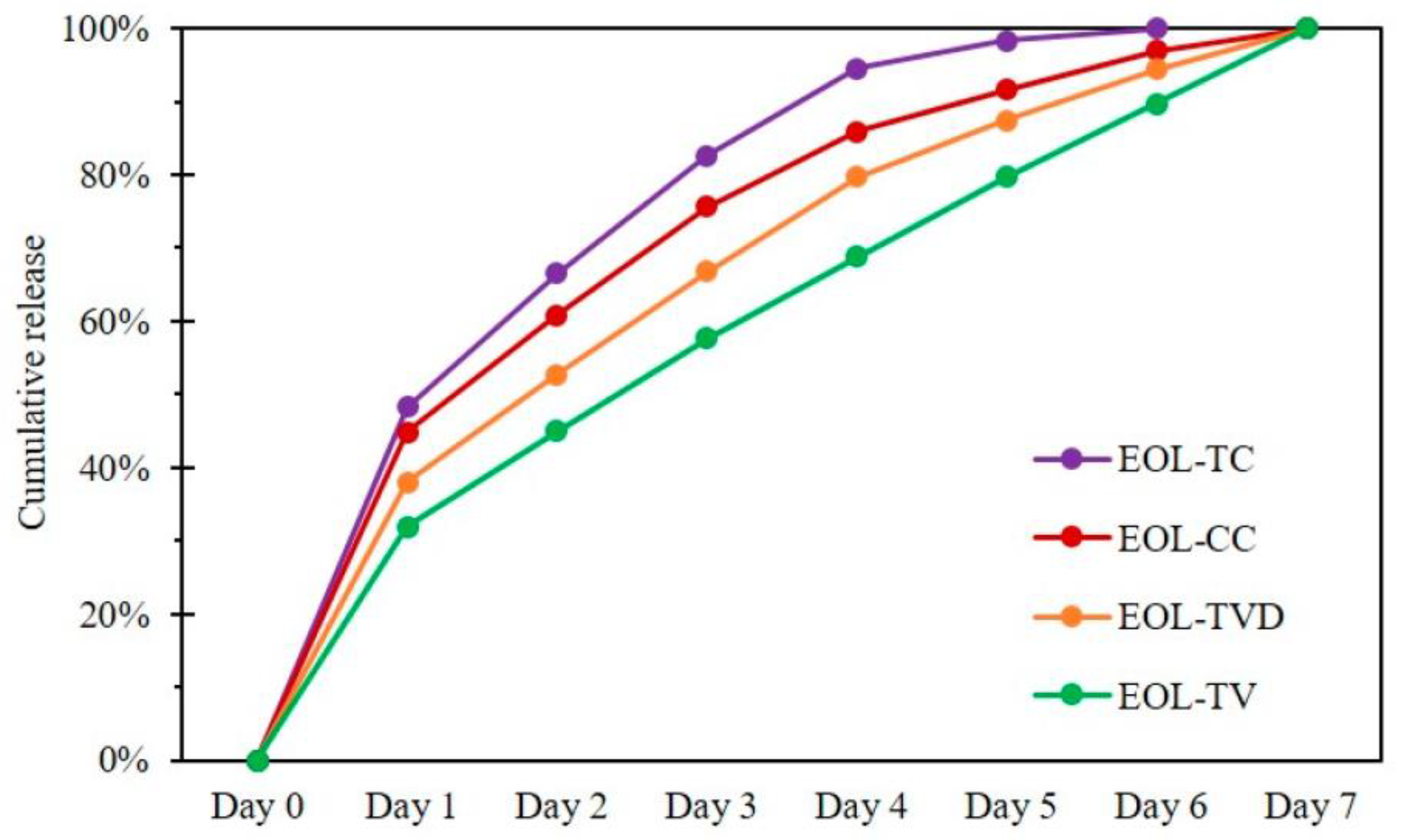
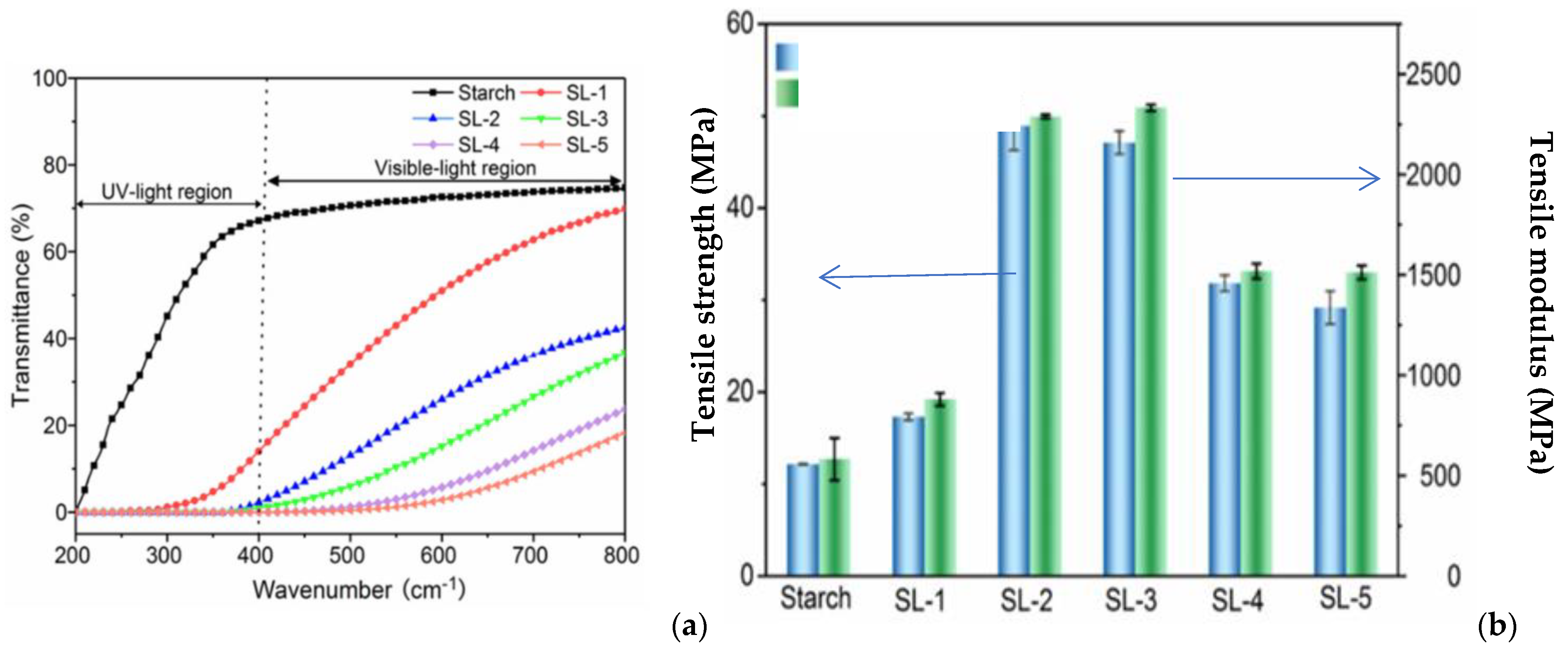
| Characteristic/ Technical Lignin | Soda and Alkali Lignin | Kraft Lignin | Acid Hydrolysis/ Enzymatic Lignin | Organosolv Lignin | Lignosulfonates | Ionic Liquid Lignin | Steam Explosion Lignin |
|---|---|---|---|---|---|---|---|
| Lignin separation | For non-woody biomass; 13–16% NaOH 140–170 °C | Na2S and NaOH 150–180 °C Thermochemical conversion of black liquor-small alkali soluble fragments | Diluted or concentrated acids as H2SO4, HCl, HNO3, H3PO4, maleic acid | Hydrothermal treatment of biomass with water and organic solvent | SO2 and water at 140–160 °C and carbonates and hydroxide salts | Ionic liquid | Mild hydrolysis; steam at high T temperature and pressure |
| Ash, % | 0.7–2.3 | 0.5–3.0 | 1.0–3.0 | 1.7 | 4.0–8.0 | 0.6–2.0 | 5–8 |
| Moisture content, % | 2.5–5.0 | 3.0–6.0 | 4.0–9.0 | 7.5 | 5.8 | - | |
| Carbohydrates, % | 1.5–3.0 | 1.0–2.3 | 10.0–22.4 | 1–3 | - | 0.1 | 2.5–4 |
| Acid soluble lignin, % | 1.0–11 | 1–4.9 | 2.9 | 1.9 | - | - | |
| Nitrogen, % | 0.2–1.0 | 0.05 | 0.5–1.4 | 0–0.3 | 0.02 | - | |
| Sulphur, % | 0 | 1.0–3.0 | 0–1.0 | 0 | 3.5–8.0 | 1.5/ | 0–0.5 |
| Phenolic hydroxyls, % | 2.9–5.1 | 2.6–5 | 3–9 | 3.7–3.4 | 2–2.5 | 4.5–7 | |
| Acids, % | 5.4–13.6 | 4.1–6 | 6–10 | 7–8 | 4.6 | 1.0–5 | |
| Methoxy, % | 10–19 | 11–14 | −19 | −15 | 9 | −13 | |
| Purity | Moderate–High | High | Moderate–Low | High | Low–moderate | Moderate–Low | |
| Molecular weight, Mw | 1000–3000 (up to 15,000) | 1500–5000 (up to 25,000) | 5000–10,000 | 500–5000 | 1000–50,000 (up to 150,000) | ≈~2000 | 3500–15,000 |
| Polydispersity | 2.5–3.5 | 2.5–3.5 | 4.0–11.0 | 1.5–4.2 | 7.0 | - |
| Hydrogel Components | Characteristics and Applications | Refs |
|---|---|---|
| Agriculture | ||
| Alkali lignin and -based poly(ethylene glycol) diglycidyl ether | Increased availability and retention of soil water, which is beneficial for plant growth; high efficiency in severe dryness conditions; alleviated drought stress in maize | [231] |
| Physically crosslinked alkaline and organosolv lignins or lignins/poly(vinyl alcohol) hydrogels | Significant swelling and water holding capacity; addition of lignin enhanced the swelling/water retention ability of hydrogels by 800% | [232] |
| Lignin/alginate/konjaku flour-based hydrogel | Increased water holding/retention and of the nutrient retention capacity of the soil, increase in the mass of tobacco plant under dryness conditions | [233] |
| Lignin/polyacrylic acid-based hydrogels | Controlled release of some pesticides (i.e., paraquat, cyfluthrin, and cyhalofop-butyl) | [234] |
| Wastewater treatment and removal of pollutants | ||
| Black liquor lignin-hydroxyethyl cellulose hydrogel | Super-absorbent hydrogel; removal of dye pollutants | [235] |
| Polyacrylic acid-g-pretreated alkali lignin porous hydrogel | Removal of Pb2+, Cu2+, and Cd2+ | [236] |
| Lignin functionalized with hexadecyltrimethylammonium-bromide-based spherical particles | Lignin-based biosorbents; removal of vanadium (V) ions from aqueous solution | [237] |
| Lignosulfonate/lysine hydrogel | Adsorption of heavy metal ions | [238] |
| Lignin/PVA or LNP/nanocellulose (cryogels); anchoring lignin nanoparticles (LNPs) to the nanocellulose network via electrostatic attraction | Environmental engineering. Adsorbents for pharmaceutical pollutants (e.g., diclofenac, metoprolol, tramadol, carbamazepine) and Bisphenol A | [186,239] |
| Hydrogel was obtained by free-radical polymerization in the presence of methylenebisacrylamide (MBA), alkali lignin (AL), and potassium persulfate (PPS). Carboxylated cellulose nanofibrils (CCNs) and carbon dots (CDs) as fluorescent probes were prepared via a condensation reaction | Fluorescent hydrogel. Adsorption of Cr (VI), excellent optical properties, biocompatibility, and nontoxicity significant efficiency in adsorption and detection of Cr (VI) | [240] |
| Biomedical [206] | ||
| Alkaline and organosolv lignins, hydoxymethylated lignins, peroxidated lignins/PVA | Onion peels were valorized for quercetin (natural drug) microwave extraction; controlled drug delivery, and quercetin-controlled delivery applications | [241] |
| Lignin-agarose hydrogel/silk fibroin (SF) embedded zinc chromite nanoparticles | Hemocompatibility and antibacterial activity; complete healing of the wounds in mice treated with the scaffold of crosslinked lignin–agarose/SF/ZnCr2O4 nano-biocomposite after five days; wound healing application; | [242] |
| Enzymatic hydrolysis lignin (wheat straw)–alginate cryogels and cryogels obtained from the freezing technique; wet and dry alginate–lignin aerogels | Porous structure; lignin reduced hydrophilicity of alginate; wound healing and tissue repairing/tissue engineering; regenerative medicine | [243] |
| Lignin-co-gelatin (cryogels) and chemically crosslinking and Ag2O/CuO NPs | Antioxidant, antibacterial, injectable lignin/gelatin composite cryogels; wound healing and tissue engineering; good mechanical properties and microporous structure; very good free scavenging activity; inhibited growth of both Gram-positive and Gram-negative bacteria | [244] |
| Lignin/gelatin hydrogels with different concentrations of lignin | Fast-release drug carriers used to deliver Ribavirin in COVID-19 treatment | [245,246] |
| Organosolv lignin/gelatin composite cryogels obtained by chemically crosslinking at −20 °C. Organosolv lignin and gelatin; multifunctional, bioactive lignin-co-gelatin composite cryogels obtained by chemically crosslinking lignin together with gelatin at −20 °C. | Multifunctional, bioactive cryogel with antimicrobial (reduced E. coli and S. aureus viability) and antioxidant activities; in vitro cyto-compatibility (after 72 h of incubation, viability of 3T3 cells was 97%); improved mechanical properties and syringe injectability | [219,242] |
| Ag/lignin NPs/PAA-pectin hydrogel | Good mechanical, antibacterial, and wound healing properties; epidermal growth factor loaded in the Ag-lignin NPs-PAA-pectin hydrogel increases the wound healing activity | [188,247] |
| Lignin/poly(ethylene) glycol diglycidyl ether hydrogel | Drug delivery; controlled release of paracetamol | [248] |
| Organosolv lignin extracted from coconut husks/polyethylene glycol (PEG), polypropylene glycol (PPG), polydimethylsiloxane (PDS) nanogel | Thermo-responsive polyurethane-based copolymer nanogel; lignin-incorporated nanogel; acts as an antioxidant biomaterial for wound healing; human L-02 hepatocyte cell viability > 90%; rapid and complete wound healing after 25 days; wound-dressing application | [249] |
| Lignin-carbohydrate complex/PEG diglycidyl ether | Cell carriers; positive hepatocyte adhesion; biocompatibility | [250] |
| Alkali lignin/chitosan hydrogels | Wound healing applications; low cytotoxicity, biocompatibility, 99 ± 3% cell viability | [143] |
| Lignosulfonate/poly(vinyl alcohol)/chitosan composites hydrogel; lignin mixing an aqueous acidic solution of chitosan and solutions of lignin and PVA | Composite hydrogel; 10 wt% lignin had enhanced hydrophilicity, antimicrobial behavior, and antioxidant capability; wound dressing | [220] |
| Lignosulfonate; in situ reduction of AgNPs then crosslinked in the lignin/PVA hydrogel | Improved antibacterial activity against Staphylococcus aureus and Escherichia coli, non-cytotoxicity, biocompatibility, wound healing | [251] |
| Lignin/PVA cryogel; freeze-drying crosslinking and curing methods | “Smart” biomaterial scaffolds elaborated by using a factorial design scaffold fabrication model; 800% water retention capacity; controlled drug loading and delivery; pH and temperature responsiveness; antifungal activity against Aspergillus niger strain | [232,252] |
| 40–24% lignin-containing alcohol groups/Gantrez S-97 (GAN) (methylvinylether and maleic acid copolymer)/PEG by esterification reaction accelerated by microwave radiation, crosslinking time was reduced from 24 to 1 h | Reductions in Staphylococcus aureus and Proteus mirabilis adherence (two common pathogens responsible for medical-device-associated infections); curcumin delivery | [222] |
| Glycinated kraft lignin/hyaluronic acid hydrogels | Non-cytotoxicity (>90% cell viability); positive cell migration and cell growth | [253] |
| Kraft lignin/PLA/Rose Bengal, gold, silver | pH-responsive hydrogels; antifungal and antimicrobial properties at very low IC50 values (0.1 μg/mL) | [254] |
| Lignin epoxy-modified resin/xanthan crosslinking with epichlorohydrin | Superabsorbent hydrogel; high swelling rate in aqueous medium; release of hydrophilic bisoprolol fumarate drug for high blood pressure and heart failure treatments | [255] |
| Low and high lignin content/cellulose nanofiber; ultrasound-assisted | Tissue engineering; cytocompatibility with gingival fibroblast cells | [256] |
| Methacrylated lignin (lignin-MA)/sulfobetaine methacrylate (SBMA) double network structure consisting of chemical and physical crosslinking between SBMA and lignin-MA | Lignin-MA as hydrogel skeleton and antibacterial agent; 94.8% reduction rate against E. coli and 95.7% against S. aureus; high hydrophilicity because of zwitterionic SBMA. Antifouling and antimicrobial properties, potential applications in medical devices and as biological material | [257] |
| Food packaging | ||
| Lignin (black liquor of bagasse) into PVA/gelatin blends | Antimicrobial and antibiofilm activities against food-borne contaminants as Gram-positive bacteria Bacillus subtilis and Staphylococcus aureus and Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa | [258] |
| Components | Characteristics and Applications | Refs |
|---|---|---|
| 15% by weight modified lignin-containing photoactive resins | These materials generate new products for additive manufacturing applications, with a four-fold increase in ductility. Excellent print quality was obtained with modified lignin resins with a commercial stereolithography system | [288] |
| Organosolv lignin OSL/hydroxypropyl cellulose (HPC), 40/60 and 50/50 mixtures | Lignin/HPC biobased aqueous inks; direct-write lyotropic blends; high performance | [289] |
| Lignin–carrageenan/AgNP-MgCl2 one-pot synthesis of AgNPs | Lignin as a reducing agent for AgNP green synthesis and capping agent in the carrageenan matrix crosslinked with divalent cations; wound healing; fast wound dressing | [290] |
| Spherical colloidal lignin particles/cellulose nanofibril-alginate | Three-dimensional printed cell culture model; soft-tissue engineering | [291] |
| Keratin/lignin hydrogels | Smooth films and nanoparticles; 4D functional biocomposite materials. Protein complexation by lignin was applied to form copolymers and reinforce keratin crosslinking networks on aqueous and solid state processing 3D and 4D printing | [292] |
| Kraft lignin/keratin | Fully biodegradable keratin hydrogels through ”greener” processes. Lignin binder to deliver 3D-printability; keratin/lignin biocomposite materials. Lignin as material additive manufacturing technology: 3D and 4D printed responsive materials without the need for synthetic chemical modifications | [293] |
| Granular dealkaline lignin/corn-derived zein protein by extrusion | 3D-printed insoluble lignin granules act as a binder for enhanced printability and degradation in soil. Suitable biocomposites for 3D printing manufacturing biodegradable circuit boards are used, along with inexpensive industrial byproducts lignin and zein as precursors and benign solvents, such as ethanol and water | [294] |
| Poly(caprolactone)/lignin/loaded curcumin | 3D-printed dressings; antioxidant and antimicrobial activities | [286] |
| Carboxylated lignin/polylactic acid melt mixing; | Suitable biocomposites for 3D printing via fused deposition modeling (FDM); improved interfacial adhesion between the COOH-lignin surface and PLA matrix; reduced cost of printing PLA 3D filaments without changing their thermal and mechanical properties | [295] |
| Lignin-coated cellulose nanocrystal/methacrylate composites | 3D stereolithography printing; mechanical reinforcement and thermal stabilization | [276] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. https://doi.org/10.3390/polym15153177
Vasile C, Baican M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers. 2023; 15(15):3177. https://doi.org/10.3390/polym15153177
Chicago/Turabian StyleVasile, Cornelia, and Mihaela Baican. 2023. "Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials" Polymers 15, no. 15: 3177. https://doi.org/10.3390/polym15153177
APA StyleVasile, C., & Baican, M. (2023). Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers, 15(15), 3177. https://doi.org/10.3390/polym15153177






