Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review
Abstract
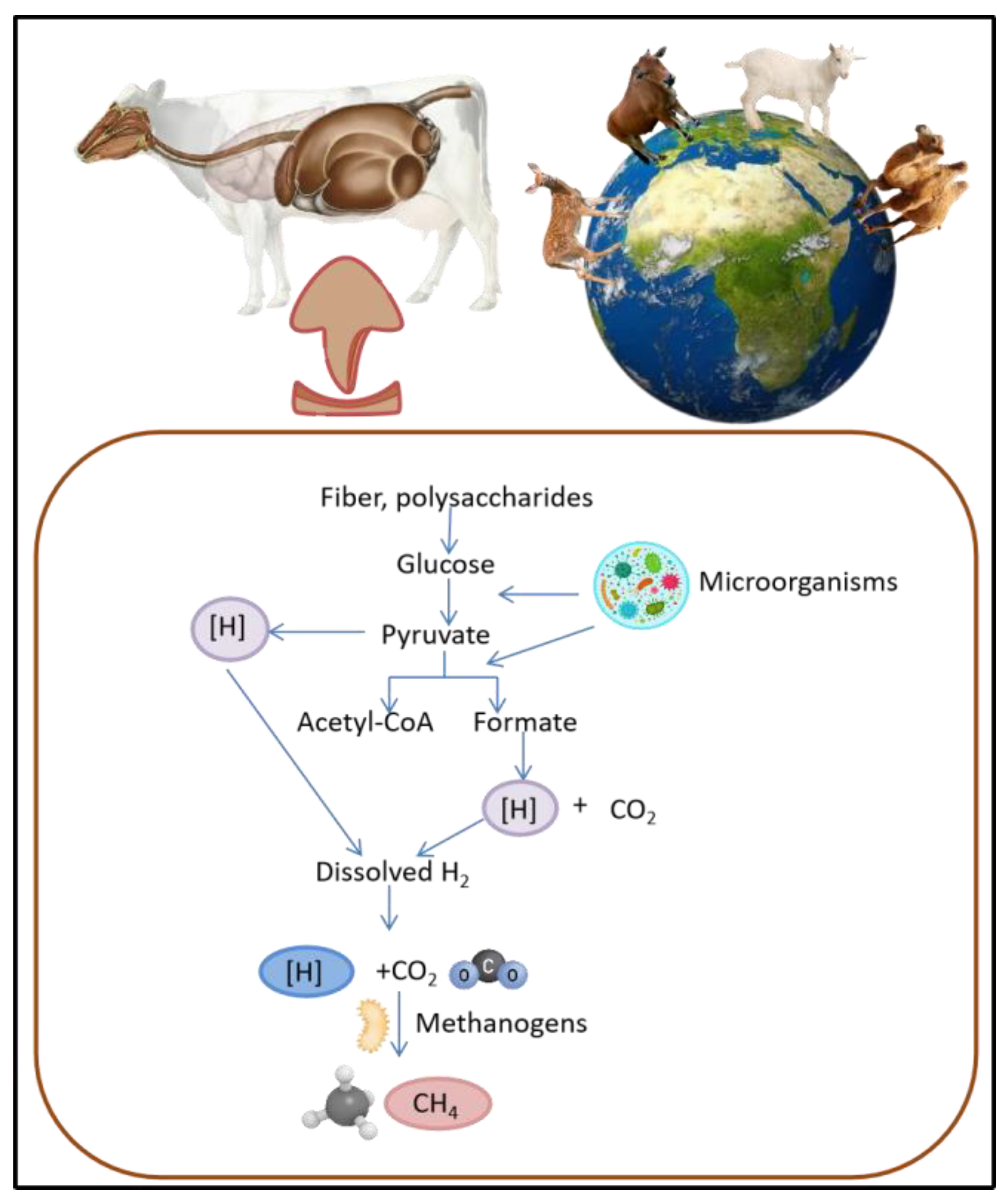
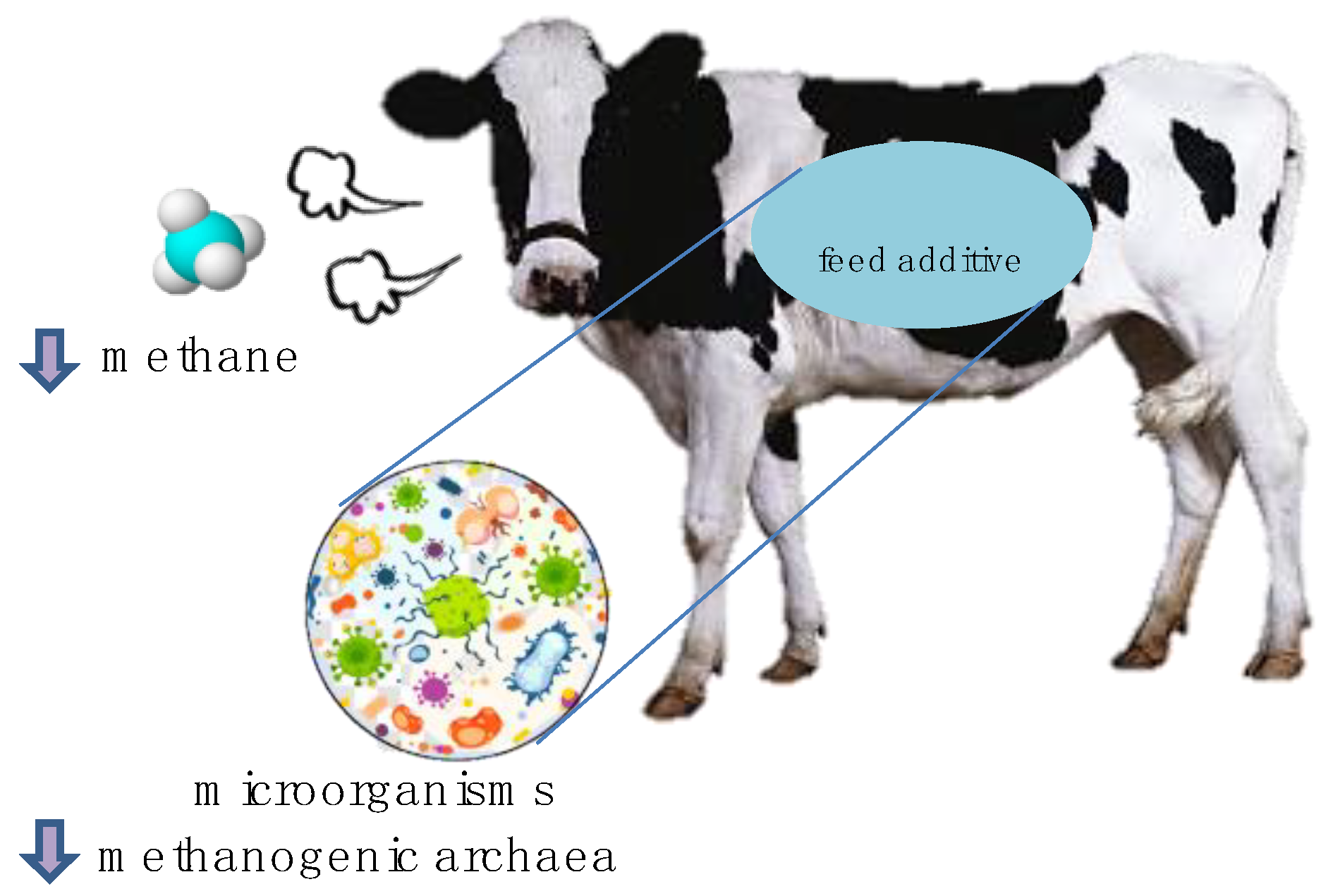
:1. Introduction
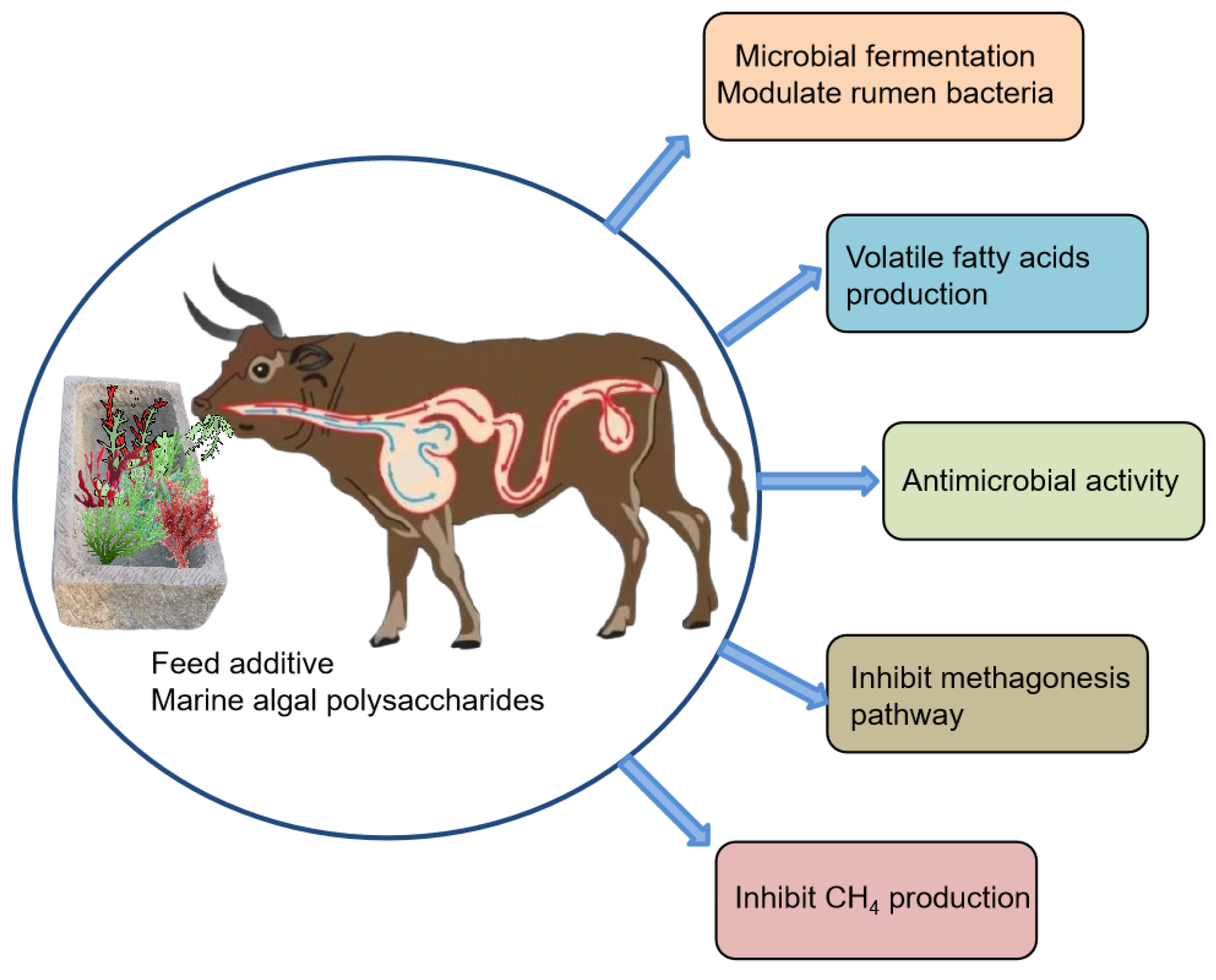
2. Marine Seaweed Polysaccharides
3. Effects of MAPs on Rumen Microbial Populations
4. Effect of MAPs and VFAs in Rumen Fermentation and Methane Production
5. Antimicrobial Activity of Polysaccharides and Related Mechanisms
6. Future Trends and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Balcombe, P.; Speirs, J.F.; Brandon, N.P.; Hawkes, A.D. Methane emissions: Choosing the right climate metric and time horizon. Environ. Sci.-Proc. Imp. 2018, 20, 1323–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global warming and dairy cattle: How to control and reduce methane emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Alex Thumba, D.; Lazarova-Molnar, S.; Niloofar, P. Comparative evaluation of data requirements and level of decision support provided by decision support tools for reducing livestock-related greenhouse gas emissions. J. Clean. Prod. 2022, 373, 133886. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef] [Green Version]
- Ramin, M.; Huhtanen, P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013, 96, 2476–2493. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Wang, L.Z.; Yan, T. Methane emission inventories for enteric fermentation and manure management of yak, buffalo and dairy and beef cattle in China from 1988 to 2009. Agric. Ecosyst. Environ. 2014, 195, 202–210. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.-Z.; Han, J.-L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Black, J.L.; Davison, T.M.; Box, I. Methane emissions from ruminants in Australia: Mitigation potential and applicability of mitigation strategies. Animals 2021, 11, 951. [Google Scholar] [CrossRef]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Barsanti, L.; Birindelli, L.; Gualtieri, P. Paramylon and other bioactive molecules in micro and macroalgae. Int. J. Mol. Sci. 2022, 23, 8301. [Google Scholar] [CrossRef]
- Cheong, K.L.; Li, J.K.; Zhong, S. Preparation and structure characterization of high-value Laminaria digitata oligosaccharides. Front. Nutr. 2022, 9, 945804. [Google Scholar] [CrossRef]
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.-L. Fucoidan-derived functional oligosaccharides: Recent developments, preparation, and potential applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine—A mini review. Carbohydr. Polym. 2021, 271, 118408. [Google Scholar] [CrossRef]
- Qiu, S.-M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Bioactive polysaccharides from red seaweed as potent food supplements: A systematic review of their extraction, purification, and biological activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef]
- Qiu, H.-M.; Veeraperumal, S.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Zeng, Q.-K.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Allen, V.G.; Pond, K.R.; Saker, K.E.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Schmidt, R.E.; Fike, J.H.; Zhang, X.; et al. Tasco: Influence of a brown seaweed on antioxidants in forages and livestock—A review. J. Anim. Sci. 2001, 79, E21–E31. [Google Scholar] [CrossRef]
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Coppa, M.; Fougère, H.; Bougouin, A.; Baumont, R.; Eugène, M.; Bernard, L. Diets supplemented with corn oil and wheat starch, marine algae, or hydrogenated palm oil modulate methane emissions similarly in dairy goats and cows, but not feeding behavior. Anim. Feed Sci. Technol. 2021, 272, 114783. [Google Scholar] [CrossRef]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef]
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows. Animals 2020, 10, 976. [Google Scholar] [CrossRef]
- Meehan, D.J.; Cabrita, A.R.J.; Silva, J.L.; Fonseca, A.J.M.; Maia, M.R.G. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 2021, 56, 102284. [Google Scholar] [CrossRef]
- Lee-Rangel, H.A.; Roque-Jiménez, J.A.; Cifuentes-López, R.O.; Álvarez-Fuentes, G.; Cruz-Gómez, A.D.l.; Martínez-García, J.A.; Arévalo-Villalobos, J.I.; Chay-Canul, A.J. Evaluation of three marine algae on degradability, in vitro gas production, and CH4 and CO2 emissions by ruminants. Fermentation 2022, 8, 511. [Google Scholar] [CrossRef]
- Pandey, D.; Hansen, H.H.; Dhakal, R.; Aryal, N.; Rai, S.P.; Sapkota, R.; Nielsen, M.O.; Novoa-Garrido, M.; Khanal, P. Interspecies and seasonal variations in macroalgae from the Nordic region: Chemical composition and impacts on rumen fermentation and microbiome assembly. J. Clean. Prod. 2022, 363, 132456. [Google Scholar] [CrossRef]
- Ahmed, E.; Batbekh, B.; Fukuma, N.; Hanada, M.; Nishida, T. Evaluation of different brown seaweeds as feed and feed additives regarding rumen fermentation and methane mitigation. Fermentation 2022, 8, 504. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Effect of sunflower and marine oils on ruminal microbiota, in vitro fermentation and digesta fatty acid profile. Front. Microbiol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Maia, M.R.G.; Fonseca, A.J.M.; Oliveira, H.M.; Mendonça, C.; Cabrita, A.R.J. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 2016, 6, 32321. [Google Scholar] [CrossRef]
- Brooke, C.G.; Roque, B.M.; Shaw, C.; Najafi, N.; Gonzalez, M.; Pfefferlen, A.; De Anda, V.; Ginsburg, D.W.; Harden, M.C.; Nuzhdin, S.V.; et al. Methane reduction potential of two pacific coast macroalgae during in vitro ruminant fermentation. Front. Mar. Sci. 2020, 7, 561. [Google Scholar] [CrossRef]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbø, R.; Lock, E.-J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Hu, J.-L.; Li, J.; Wang, J.; Zhang, X.-Y.; Wu, X.-Y.; Li, X.; Guo, Z.-B.; Zou, L.; Wu, D.-T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023, 163, 112166. [Google Scholar] [CrossRef]
- Wang, M.; Cheong, K.-L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef]
- Xie, X.-T.; Cheong, K.-L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 7703–7717. [Google Scholar] [CrossRef]
- Fauzi, M.A.R.D.; Pudjiastuti, P.; Wibowo, A.C.; Hendradi, E. Preparation, properties and potential of carrageenan-based hard capsules for replacing gelatine: A review. Polymers 2021, 13, 2666. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.d.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Mandal, S.; Nagi, G.K.; Corcoran, A.A.; Agrawal, R.; Dubey, M.; Hunt, R.W. Algal polysaccharides for 3D printing: A review. Carbohydr. Polym. 2023, 300, 120267. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Liu, D.; Ouyang, Y.; Chen, R.; Wang, M.; Ai, C.; El-Seedi, H.R.; Sarker, M.M.R.; Chen, X.; Zhao, C. Nutraceutical potentials of algal ulvan for healthy aging. Int. J. Biol. Macromol. 2022, 194, 422–434. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; Pimentel, T.C.; Sant’Ana, A.S.; Magnani, M. Microalgae in the meat processing chain: Feed for animal production or source of techno-functional ingredients. Curr. Opin. Food Sci. 2021, 37, 125–134. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Guo, G.; Yang, W.; Fan, C.; Lan, R.; Gao, Z.; Gan, S.; Yu, H.; Yin, F.; Wang, Z. The effects of fucoidan as a dairy substitute on diarrhea rate and intestinal barrier function of the large intestine in weaned lambs. Front. Vet. Sci. 2022, 9, 1007346. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Matloup, O.H.; Anele, U.Y.; Mohamed, A.G.; El-Sayed, A.B. Dietary Chlorella vulgaris microalgae improves feed utilization, milk production and concentrations of conjugated linoleic acids in the milk of Damascus goats. J. Agric. Sci. 2016, 155, 508–518. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Carpinelli, N.A.; Halfen, J.; Trevisi, E.; Chapman, J.D.; Sharman, E.D.; Anderson, J.L.; Osorio, J.S. Effects of peripartal yeast culture supplementation on lactation performance, blood biomarkers, rumen fermentation, and rumen bacteria species in dairy cows. J. Dairy Sci. 2021, 104, 10727–10743. [Google Scholar] [CrossRef]
- Grimm, P.; Philippeau, C.; Julliand, V. Faecal parameters as biomarkers of the equine hindgut microbial ecosystem under dietary change. Animal 2017, 11, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Abecia, L.; Angarita, E.; Aravena, P.; Nora Arenas, G.; Ariza, C.; et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Imlay, J.A. When anaerobes encounter oxygen: Mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef]
- Williams, A.G.; Withers, S.; Sutherland, A.D. The potential of bacteria isolated from ruminal contents of seaweed-eating North Ronaldsay sheep to hydrolyse seaweed components and produce methane by anaerobic digestion in vitro. Microb. Biotechnol. 2013, 6, 45–52. [Google Scholar] [CrossRef]
- Tong, J.-j.; Zhang, H.; Wang, J.; Liu, Y.; Mao, S.-y.; Xiong, B.-h.; Jiang, L.-s. Effects of different molecular weights of chitosan on methane production and bacterial community structure in vitro. J. Integr. Agric. 2020, 19, 1644–1655. [Google Scholar] [CrossRef]
- Zanferari, F.; Vendramini, T.H.A.; Rentas, M.F.; Gardinal, R.; Calomeni, G.D.; Mesquita, L.G.; Takiya, C.S.; Rennó, F.P. Effects of chitosan and whole raw soybeans on ruminal fermentation and bacterial populations, and milk fatty acid profile in dairy cows. J. Dairy Sci. 2018, 101, 10939–10952. [Google Scholar] [CrossRef] [Green Version]
- Skillman, L.C.; Evans, P.N.; Strömpl, C.; Joblin, K.N. 16S rDNA directed PCR primers and detection of methanogens in the bovine rumen. Lett. Appl. Microbiol. 2006, 42, 222–228. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea are interactive components of complex microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef]
- Welander, P.V.; Metcalf, W.W. Loss of the mtr operon in Methanosarcina blocks growth on methanol, but not methanogenesis, and reveals an unknown methanogenic pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 10664–10669. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Shin, N.H.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Lee, S.S.; Kim, E.T.; Lee, S.S. In vitro five brown algae extracts for efficiency of ruminal fermentation and methane yield. J. Appl. Phycol. 2021, 33, 1253–1262. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Dijkstra, J. Production and absorption of volatile fatty acids in the rumen. Livest. Prod. Sci. 1994, 39, 61–69. [Google Scholar] [CrossRef]
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Monroy, J.C. Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 2018, 124, 106–115. [Google Scholar] [CrossRef]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total Environ. 2021, 798, 149292. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, M.; Chen, X.; Li, D.; Qin, L.; Li, Z.; Yao, J.; Liang, X. Mixed culture of Saccharomyces cerevisiae and Acetobacter pasteurianus for acetic acid production. Biochem. Eng. J. 2013, 79, 41–45. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [Green Version]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Im, J.A.; Choi, S.Y.; Lee, J.I.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab. Eng. 2014, 23, 165–174. [Google Scholar] [CrossRef]
- Baroi, G.N.; Baumann, I.; Westermann, P.; Gavala, H.N. Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted Clostridium tyrobutyricum strain. Microb. Biotechnol. 2015, 8, 874–882. [Google Scholar] [CrossRef]
- Lang, K.; Zierow, J.; Buehler, K.; Schmid, A. Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites. Microb. Cell Fact. 2014, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.; McSweeney, C.; Wright, A.-D.G.; Bishop-Hurley, G.; Kalantar-zadeh, K. Measuring methane production from ruminants. Trends Biotechnol. 2016, 34, 26–35. [Google Scholar] [CrossRef]
- González, L.A.; Kyriazakis, I.; Tedeschi, L.O. Review: Precision nutrition of ruminants: Approaches, challenges and potential gains. Animal 2018, 12, s246–s261. [Google Scholar] [CrossRef] [Green Version]
- Pragna, P.; Chauhan, S.S.; Sejian, V.; Leury, B.J.; Dunshea, F.R. Climate change and goat production: Enteric methane emission and its mitigation. Animals 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef]
- Menon, A.; Lyng, J.; Giannis, A. Higher bacterial diversity in two-phase thermophilic anaerobic digestion of food waste after micronutrient supplementation. Biomass Convers. Bior. 2023, 13, 5187–5195. [Google Scholar] [CrossRef]
- Xu, F.; Shi, J.; Lv, W.; Yu, Z.; Li, Y. Comparison of different liquid anaerobic digestion effluents as inocula and nitrogen sources for solid-state batch anaerobic digestion of corn stover. Waste Manag. 2013, 33, 26–32. [Google Scholar] [CrossRef]
- Hong, Z.S.; Kim, E.J.; Jin, Y.C.; Lee, J.S.; Choi, Y.J.; Lee, H.G. Effects of supplementing brown seaweed by-products in the diet of Holstein cows during transition on ruminal fermentation, growth performance and endocrine responses. Asian-Australas J. Anim. Sci. 2015, 28, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Jo, Y.H.; Ghassemi Nejad, J.; Lee, J.C.; Lee, H.G. Evaluation of nutritional value of Ulva sp. and Sargassum horneri as potential eco-friendly ruminants feed. Algal Res. 2022, 65, 102706. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Vickers, C.; Liu, F.; Abe, K.; Salama-Alber, O.; Jenkins, M.; Springate, C.M.K.; Burke, J.E.; Withers, S.G.; Boraston, A.B. Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to α-l-fucosidases from GH29. J. Biol. Chem. 2018, 293, 18296–18308. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, D.C.; Reichenbach, T.; Aspeborg, H.; Divne, C. A homodimeric bacterial exo-β-1,3-glucanase derived from moose rumen microbiome shows a structural framework similar to yeast exo-β-1,3-glucanases. Enzyme Microb. Technol. 2021, 143, 109723. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, M.; Teng, B.; Veeraperumal, S.; Cheung, P.C.-K.; Zhong, S.; Cheong, K.-L. Partially acid-hydrolyzed porphyran improved dextran sulfate sodium-induced acute colitis by modulation of gut microbiota and enhancing the mucosal barrier. J. Agric. Food. Chem. 2023, 71, 7299–7311. [Google Scholar] [CrossRef]
- Gäbel, G.; Aschenbach, J.R.; Müller, F. Transfer of energy substrates across the ruminal epithelium: Implications and limitations. Anim. Health Res. Rev. 2007, 3, 15–30. [Google Scholar] [CrossRef]
- Hao, L.-P.; Lü, F.; He, P.-J.; Li, L.; Shao, L.-M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations. Environ. Sci. Technol. 2011, 45, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Abdalkareem Jasim, S.; Jade Catalan Opulencia, M.; Alexis Ramírez-Coronel, A.; Kamal Abdelbasset, W.; Hasan Abed, M.; Markov, A.; Raheem Lateef Al-Awsi, G.; Azamatovich Shamsiev, J.; Thaeer Hammid, A.; Nader Shalaby, M.; et al. The emerging role of microbiota-derived short-chain fatty acids in immunometabolism. Int. Immunopharmacol. 2022, 110, 108983. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Marques, E.C.; Gilbert, R.O.; Bicalho, R.C. The association of plasma glucose, BHBA, and NEFA with postpartum uterine diseases, fertility, and milk production of Holstein dairy cows. Theriogenology 2017, 88, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiong, B.; Zhao, X. Could propionate formation be used to reduce enteric methane emission in ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Kim, S.C.; Kim, E.T.; Lee, S.S. New challenges for efficient usage of Sargassum fusiforme for ruminant production. Sci. Rep. 2020, 10, 19655. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Yu, B.; Chen, J.; Zhong, S. A comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeitz, J.O.; Meile, L.; Kreuzer, M.; Schwarm, A. Influence of pH and the degree of protonation on the inhibitory effect of fatty acids in the ruminal methanogen Methanobrevibacter ruminantium strain M1. J. Appl. Microbiol. 2015, 119, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2016, 28, 1443–1452. [Google Scholar] [CrossRef]
- Chojnacka, A.; Szczęsny, P.; Błaszczyk, M.K.; Zielenkiewicz, U.; Detman, A.; Salamon, A.; Sikora, A. Noteworthy facts about a methane-producing microbial community processing acidic effluent from sugar beet molasses fermentation. PLoS ONE 2015, 10, e0128008. [Google Scholar] [CrossRef] [Green Version]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, G.; Yañez-Ruiz, D.R.; Seradj, A.R.; Balcells, J.; Belanche, A. Methanogenesis in animals with foregut and hindgut fermentation: A review. Anim. Prod. Sci. 2019, 59, 2109–2122. [Google Scholar] [CrossRef]
- Saengkerdsub, S.; Ricke, S.C. Ecology and characteristics of methanogenic archaea in animals and humans. Crit. Rev. Microbiol. 2014, 40, 97–116. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, S.; Spillane, C.; Claffey, N.; Smith, P.E.; O’Rourke, T.; Diskin, M.G.; Waters, S.M. Rumen microbiome composition is altered in sheep divergent in feed efficiency. Front. Microbiol. 2020, 11, 1981. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, T.; Wu, Z.; Zheng, W.; Zhang, T.; Howe, S.; Chai, J.; Deng, F.; Li, Y.; Zhao, J. Archaea: An under-estimated kingdom in livestock animals. Front. Vet. Sci. 2022, 9, 973508. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K.; Trivedi, S.; Mohapatra, A.; Kolte, A.P.; Sejian, V.; Bhatta, R.; Rahman, H. Comparison of enteric methane yield and diversity of ruminal methanogens in cattle and buffaloes fed on the same diet. PLoS ONE 2021, 16, e0256048. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, H. Effects of nitrogen gas flushing in comparison with argon on rumen fermentation characteristics in in vitro studies. J. Anim. Sci. Technol. 2020, 62, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Dietvorst, J.; Vilaplana, L.; Uria, N.; Marco, M.-P.; Muñoz-Berbel, X. Current and near-future technologies for antibiotic susceptibility testing and resistant bacteria detection. TrAC Trends Anal. Chem. 2020, 127, 115891. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Elghandour, M.M.M.Y.; Ikpeazu, V.O.; Buendía, G.R.; Molina, O.M.; Arunsi, U.O.; Emmanuel, O.; Salem, A.Z.M. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. J. Clean. Prod. 2019, 213, 915–925. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Félix, R.; Dias, P.; Félix, C.; Cerqueira, T.; Andrade, P.B.; Valentão, P.; Lemos, M.F.L. The biotechnological potential of Asparagopsis armata: What is known of its chemical composition, bioactivities and current market? Algal Res. 2021, 60, 102534. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Chen, Z.; Li, D.; Liu, W.; Huang, L.; Zou, C.; Cao, M.-J.; Liu, G.-M.; Wang, Y. Antibacterial activity of sulfated galactans from Eucheuma serra and Gracilari verrucosa against diarrheagenic Escherichia coli via the disruption of the cell membrane structure. Mar. Drugs 2020, 18, 397. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Paulert, R.; Jr, A.; Stadnik, M.; Pizzolatti, M. Antifungal and antibacterial properties of the ulvan and crude extracts from the green seaweed Ulva fasciata delile. Algol. Stud. 2007, 123, 123–129. [Google Scholar] [CrossRef]
- Vitor, H.P. Antimicrobial sulfated glycans: Structure and function. Curr. Top. Med. Chem. 2017, 17, 319–330. [Google Scholar]
- Chua, E.-G.; Verbrugghe, P.; Perkins, T.T.; Tay, C.-Y. Fucoidans disrupt adherence of Helicobacter pylori to AGS cells in vitro. Evid.-Based Compl. Alt. 2015, 2015, 120981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, W.; Wang, Y.; Ma, Y.; Huang, L.; Zou, C.; Li, D.; Cao, M.-J.; Liu, G.-M. Inhibitory effect of depolymerized sulfated galactans from marine red algae on the growth and adhesion of diarrheagenic Escherichia coli. Mar. Drugs 2019, 17, 694. [Google Scholar] [CrossRef] [Green Version]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of rumen bacterial community and feed utilization in camel and sheep using combined supplementation of live yeast and microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, G.M.; Raghavankutty, M.; Kurup, G.M. Sulfated polysaccharides from Padina tetrastromatica induce apoptosis in HeLa cells through ROS triggered mitochondrial pathway. Process Biochem. 2018, 68, 197–204. [Google Scholar] [CrossRef]

| Feed Additive | Animal/In Vitro | Treatment | Methane Reduction (%) | Reference |
|---|---|---|---|---|
| 3-Nitrooxypropanol | Cattle | 10 mg/kg dry matter | 39 | [20] |
| Corn oil, wheat starch, marine algae | Dairy cows and goats | 1.5% inclusion | 28 | [21] |
| Asparagopsis armata | Cows | 1% inclusion level | 47.2 | [22] |
| origanum oil, hydrolysable tannins, and tea saponin | Sheep | 40 mL/kg origanum oil | 30 | [23] |
| Grape marc | Dairy cows | 5.0 kg dry matter of grape marc and 10.0 kg dry matter of ryegrass | 15 | [24] |
| Nannochloropsis oceanica (polysaccharide) | In vitro | 2.5% incubation | 10 | [25] |
| Macrocystis pyrifera (polysaccharide) | In vitro | 0.25 g of each diet | 47.3 | [26] |
| Fucus vesiculosus (polyphenol and polysaccharides) | In vitro | inclusion rate of 20% in dry matter | 62.6 | [27] |
| Laminaria japonica | In vitro | inclusion rate of 20% in dry matter | 18.3 | [28] |
| Sunflower and marine oils | In vitro | 2.0% inclusion | 16 | [29] |
| Ulva sp. (ulvan) | In vitro | 25% incubation | 55 | [30] |
| Zonaria farlowii (high starch and protein) | In vitro | 5% inclusion | 11 | [31] |
| VFA | Microorganisms | Ref. |
|---|---|---|
| Acetic acid | Acetobacter pasteurianus, A. aceti, Acetobacterium wieringae, Acetomicrobium flavidum, Acetobacterium woodii, Clostridium formicaceticum, C. aceticum, C. thermoaceticum, Gluconobacter strains, Moorella thermoacetica, Streptococcus lactis, Thermoanaerobacter kivui | [67,68,69] |
| Propionic acid | Propionibacterium freudenreichii, P. shermanii, P. acidipropionici, P. thoenii, P. jensenii | [70] |
| Butyric acid | Clostridium barkeri, C. thermobutyricum, C. butyricum, C. acetobutylicum, C. beijerinckii, Butyribacterium sp., Butyrivibrio fibrisolvens, Eubacterium, Fusobacterium nucleatum, Sarcinalimosum, Clostridium tyrobutyricum | [71,72,73] |
| Isovaleric acid | Propionibacterium freudenreichii, Pseudomonas sp. strain VLB120 | [74] |
| Feed Additive | Substrate | Reaction | Reference |
|---|---|---|---|
| Methanobrevibacter gottschalkii | Acetate Formate Pyruvate Methylamine Methanol Dimethylsulfide Acetate H2 CO CO2 | H2 + CO2 → CH4 + 2H2O H2 + CH3OH → CH4 + H2O 4HCOO− + 4H+ → CH4 + 3CO2 + 2H2O 4CO + 5H2O → CH4 + 3HCO3− + 3H+ 4CH3OH → 3CH4 + HCO3− + H2O + H+ 2(CH3)2S + 3H2O → 3CH4 + HCO3− + 2H2S + H+ 4CH3NH3Cl + 2H2O → 3CH4 + CO2 + 4NH4Cl CH3COO− + H2O → CH4 + HCO3− | [100] |
| Methanobrevibacter millerae | [101] | ||
| Methanobrevibacter smithii | [101] | ||
| Methanobrevibacter thaueri | [102] | ||
| Methanobrevibacter ruminantium | [75] | ||
| Methanobrevibacter olleyae | [103] | ||
| Methanosphaera stadtmanae | [104] | ||
| Thermoplasmata | [101] | ||
| Methanomicrobium mobile | [53] | ||
| Methanobacterium lacus | [53] | ||
| Methanobacterium formicicum | [105] | ||
| Methanomicrobium bryantii | [53] | ||
| Methanosarcina barkeri | [106] | ||
| Methanosarcina mazei | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, K.-L.; Zhang, Y.; Li, Z.; Li, T.; Ou, Y.; Shen, J.; Zhong, S.; Tan, K. Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review. Polymers 2023, 15, 3153. https://doi.org/10.3390/polym15153153
Cheong K-L, Zhang Y, Li Z, Li T, Ou Y, Shen J, Zhong S, Tan K. Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review. Polymers. 2023; 15(15):3153. https://doi.org/10.3390/polym15153153
Chicago/Turabian StyleCheong, Kit-Leong, Yiyu Zhang, Zhuoting Li, Tongtong Li, Yiqing Ou, Jiayi Shen, Saiyi Zhong, and Karsoon Tan. 2023. "Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review" Polymers 15, no. 15: 3153. https://doi.org/10.3390/polym15153153
APA StyleCheong, K.-L., Zhang, Y., Li, Z., Li, T., Ou, Y., Shen, J., Zhong, S., & Tan, K. (2023). Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review. Polymers, 15(15), 3153. https://doi.org/10.3390/polym15153153







