Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Elastomers
2.1.3. Fillers
2.2. Preparation of SP and CB/SP Adducts
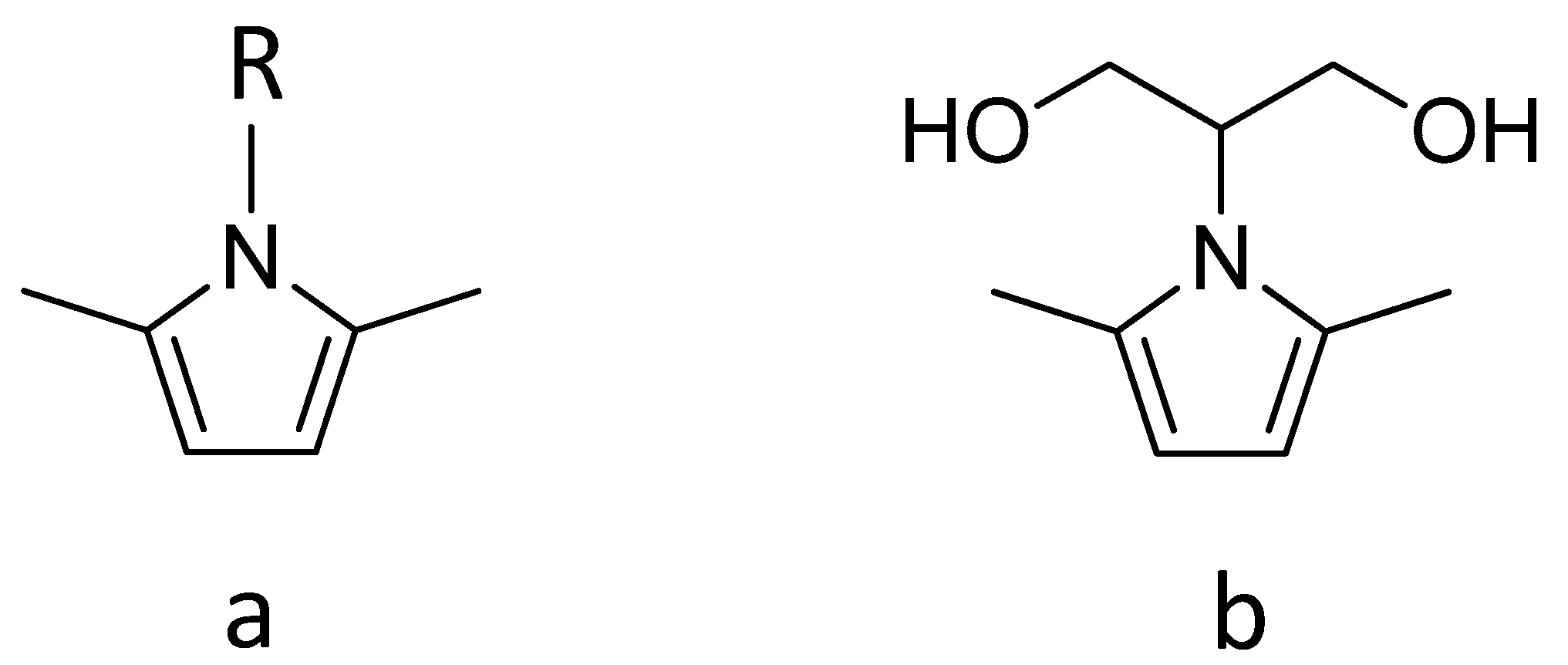
2.2.1. Synthesis of 2-2,5-Dimethyl-1H-pyrrol-1-yl-1,3-propanediol (SP)
2.2.2. Preparation of Adducts of CB N326 with SP
2.3. Preparation of Elastomer Composites
2.3.1. Elastomer Composite with CB/SP-6
2.3.2. Elastomer Composite with CB/SP-4 and CB/SP-5
2.4. Characterization Techniques
2.4.1. Thermogravimetry Analysis (TGA) of CB/SP Adducts
2.4.2. Wide-Angle X-ray Diffraction
2.4.3. Crosslinking
2.4.4. Dynamic-Mechanical Analysis in the Shear Mode Strain Sweep Test
2.4.5. Dynamic-Mechanical Analysis in the Axial Mode
2.4.6. Tensile Test
2.4.7. Electrical Resistance
2.4.8. Headspace Analyses
3. Results and Discussion
3.1. Preparation and Characterization of CB/SP Adducts
3.2. Preparation and Characterization of Rubber Composites
3.3. Silanization of CB/SP
3.4. On the Reactivity of CB/SP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://ourworldindata.org/greenhouse-gas-emissions#annual-greenhouse-gas-emissions-how-much-do-we-emit-each-year (accessed on 15 May 2023).
- Available online: https://www.un.org/en/conferences/environment/rio1992/ (accessed on 2 June 2023).
- Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 2 June 2023).
- Available online: https://ourworldindata.org/emissions-by-sector (accessed on 16 May 2023).
- Global Mobility Report 2017, Tracking Sector Performance; Sustainable Mobility for All: Washington, DC, USA, 2017.
- Available online: https://www.reportlinker.com/p05379599/?utm_source=GNW (accessed on 2 June 2023).
- Hall, D.E.; Moreland, J.C. Fundamentals of Rolling Resistance. Rubber Chem. Technol. 2001, 74, 525–539. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Robertson, C.G. Interpretation of the tanδ peak height for particle-filled rubber and polymer nanocomposites with relevance to tire tread performance balance. Rubber Chem. Technol. 2018, 91, 577–594. [Google Scholar] [CrossRef]
- Tao, Y.C.; Dong, B.; Zhang, L.Q.; Wu, Y.P. Reactions of silica–silane rubber and properties of silane–silica/solution-polymerized styrene–butadiene rubber composite. Rubber Chem. Technol. 2016, 89, 526–539. [Google Scholar] [CrossRef]
- Tunnicliffe, L.B.; Busfield, J.J. Reinforcement of rubber and filler network dynamics at small strains. In Designing of Elastomer Nanocomposites: From Theory to Applications; Advances in Polymer Science; Stöckelhuber, K., Das, A., Klüppel, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 275, pp. 71–102. [Google Scholar]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Progr. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Donnet, J.B.; Custodero, E. Reinforcement of Elastomers by Particulate Fillers. In The Science and Technology of Rubber, 3rd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 367–400. [Google Scholar]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler-filler and filler-elastomer interaction on rubber reinforcement. Compos. Part A 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Kraus, G. Reinforcement of Elastomers by Carbon Black. In Fortschritte der Hochpolym; Springer: Berlin, Heidelberg, 1971; pp. 155–237. [Google Scholar]
- Medalia, A.I. Effect of carbon black on dynamic properties of rubber vulcanizates. Rubber Chem. Technol. 1978, 51, 437–523. [Google Scholar] [CrossRef]
- Voll, M.; Kleinschmit, P. Carbon, 6. Carbon Black. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Donnet, J.B.; Bansal, R.C.; Wang, M.J. Carbon Black: Science and Technology, 2nd ed.; Dekker: New York, NY, USA, 1993. [Google Scholar]
- Fan, Y.; Fowler, D.G.; Zhao, M. The Past, present and future of carbon black as a rubber reinforcing filler—A review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef]
- Chevalier, Y.; Morawski, J.C. Precipitated Silica with Morphological Properties, Process for Producing It and Its Application, Especially as a Filler. European Patent EP 0157703 B1, 31 May 1989. [Google Scholar]
- Legrand, A.P. On the silica edge. In The Surface Properties of Silicas; Legrand, A.P., Ed.; Wiley and Sons: New York, NY, USA, 1998; pp. 1–20. [Google Scholar]
- Ten Brinke, J.W.; Debnath, S.C.; Reuvekamp, L.A.; Noordermeer, J.W. Mechanistic aspects of the role of coupling agents in silica–rubber composites. Compos. Sci. Technol. 2003, 63, 1165–1174. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.S.; Lim, S.H.; Jang, S.H.; Kim, D.H.; Park, N.H.; Jung, J.W.; Choi, J. The investigation of the silica-reinforced rubber polymers with the methoxy type silane coupling agents. Polymers 2020, 12, 3058. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Sebastiano, R.; Citterio, A.; Leonardi, G.; Valerio, A.M. Adducts between Carbon Allotropes and Serinol Derivatives. U.S. Patent 10,160,652 B2, 25 December 2018. [Google Scholar]
- Galimberti, M.; Barbera, V. Adducts of Pyrrole Derivatives to Carbon Allotropes. U.S. Patent 11,098,012 B2, 24 August 2018. [Google Scholar]
- Galimberti, M.; Barbera, V.; Guerra, S.; Conzatti, L.; Castiglioni, C.; Brambilla, L.; Serafini, A. Biobased Janus Molecule for the Facile Preparation of Water Solutions of Few Layer Graphene Sheets. RSC Adv. 2015, 5, 81142–81152. [Google Scholar] [CrossRef]
- Barbera, V.; Bernardi, A.; Palazzolo, A.; Rosengart, A.; Brambilla, L.; Galimberti, M. Facile and Sustainable Functionalization of Graphene Layers with Pyrrole Compounds. Pure Appl. Chem. 2018, 90, 253–270. [Google Scholar] [CrossRef]
- Locatelli, D.; Barbera, V.; Brambilla, L.; Castiglioni, C.; Sironi, A.; Galimberti, M. Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds. Nanomaterials 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Bongiovanni, R.; Galimberti, M. Domino reaction for the sustainable functionalization of few-layer graphene. Nanomaterials 2018, 9, 44. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.L.; Zong, S.; Du, H.X.; Shi, X.X. Clean synthesis process of 2,5-hexanedione. Adv. Mat. Res. 2012, 518, 3947–3950. [Google Scholar] [CrossRef]
- Waidmann, C.R.; Pierpont, A.W.; Batista, E.R.; Gordon, J.C.; Martin, R.L.; West, R.M.; Wu, R. Functional group dependence of the acid catalyzed ring opening of biomass derived furan rings: An experimental and theoretical study. Catal. Sci. Technol. 2013, 3, 106–115. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Vlachos, D.G.; Xu, B. Poisoning of Ru/C by homogeneous Brønsted acids in hydrodeoxygenation of 2,5-dimethylfuran via catalytic transfer hydrogenation. Appl. Catal. A Gen. 2017, 542, 327–335. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Giannini, L.; Naddeo, S. Processo per la Preparazione di Dichetoni e Derivati Pirrolici. Italian Patent Application n. 102021000032138, 1 January 2021. [Google Scholar]
- de Gennes, P.G. Soft matter (nobel lecture). Angew. Chem. Int. Ed. Engl. 1992, 31, 842–845. [Google Scholar] [CrossRef]
- Available online: https://corporate.pirelli.com/var/files2020/EN/PDF/PIRELLI_ANNUAL_REPORT_2020_ENG.pdf (accessed on 2 June 2023).
- Standard ISO37/UNI 6065; Rubber—Tests on Vulcanized or Thermoplastic Rubber—Tensile Test. Ente Nazionale Italiano di Unificazione (UNI): Milano, Italy, 2001.
- Vogel, A.I.; Tatchell, A.R.; Furnis, B.S.; Hannaford, A.J.; Smith, P.W.G. (Eds.) Vogel’s Textbook of Practical Organic Chemistry; Prentice Hall: Hoboken, NJ, USA, 1996; ISBN 978-0-582-46236-6. [Google Scholar]
- McCabe, W.L.; Julian, C.S.; Harriot, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 1993; Volume 5. [Google Scholar]
- Alfe, M.; Gargiulo, V.; Di Capua, R.; Chiarella, F.; Rouzaud, J.N.; Vergara, A.; Ciajolo, A. Wet chemical method for making graphene-like films from carbon black. ACS Appl. Mater. Interfaces 2012, 4, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.H.; Prettyman, I.B.; Hall, G.L. Hysteretic and elastic properties of rubberlike materials under dynamic shear stresses. J. Appl. Phys. 1944, 15, 309–323. [Google Scholar] [CrossRef]
- Fletcher, W.P.; Gent, A.N. Nonlinearity in the dynamic properties of vulcanized rubber compounds. Trans. Inst. Rubber Ind. 1953, 29, 266–280. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Genix, A.C.; Sztucki, M.; Oberdisse, J.; Robertson, C.G. The Payne effect: Primarily polymer-related or filler-related phenomenon? Rubber Chem. Technol. 2019, 92, 599–611. [Google Scholar] [CrossRef]
- Locatelli, D.; Bernardi, A.; Rubino, L.R.; Gallo, S.; Vitale, A.; Bongiovanni, R.; Barbera, V.; Galimberti, M. Biosourced Janus Molecules as Silica Coupling Agents in Elastomer Composites for Tires with Lower Environmental Impact. ACS Sustain. Chem. Eng. 2023, 11, 2713–2726. [Google Scholar] [CrossRef]










| Ingredient | Silica | CB/SP-6 |
|---|---|---|
| NR (SIR-20) | 70 | 70 |
| BR | 30 | 30 |
| Silane/CB | 5.6 | 5.6 |
| N326 | 30 | 30.0 |
| Silica | 35 | 12 |
| CB/SP-6 | 0 | 19.72 |
| Ingredients | Silica | CB/SP b |
|---|---|---|
| IR | 100.00 | 100.00 |
| Silica | 35.00 | 12.00 |
| CB/TESPT | 5.60 | 5.60 |
| CB N326 | 30.00 | 30.00 |
| CB—SP | 0.00 | 19.72 |
| Sample | SP a (phc) | Reaction T (°C) | Mass Loss (%) | SP in CB/SP (phc) | F.Y. b (%) | ||
|---|---|---|---|---|---|---|---|
| T < 150 °C | 150 °C < T < 900 °C | T > 900 °C | |||||
| CB/SP-6 | 10 | 150 | 0.2 | 5.6 | 94.2 | 5.9 | 59 |
| CB/SP-4 | 5 | 120 | 0.7 | 3.8 | 95.0 c | 4.0 | 80 |
| CB/SP-5 | 10 | 120 | 0.4 | 4.8 | 94.1 d | 5.1 | 51 |
| Silica | CB/SP-6 | |
|---|---|---|
| G′0.2% [MPa] | 2.52 | 2.30 |
| G′25% [MPa] | 0.94 | 0.94 |
| ΔG′ [MPa] | 1.58 | 1.36 |
| ΔG′/G′0.2% | 0.67 | 0.59 |
| G″max [MPa] | 0.15 | 0.15 |
| Tan(δ)max | 0.11 | 0.11 |
| T (°C) | Silica | CB/SP-6 | |
|---|---|---|---|
| E’ (Mpa) | 10 | 5.33 | 7.20 |
| 23 | 5.03 | 6.72 | |
| 70 | 4.27 | 5.72 | |
| E’’ (MPa) | 10 | 1.13 | 1.41 |
| 23 | 0.86 | 1.04 | |
| 70 | 0.52 | 0.56 | |
| Tan δ | 10 | 0.21 | 0.20 |
| 23 | 0.17 | 0.16 | |
| 70 | 0.12 | 0.10 |
| Silica | CB/SP-6 | |
|---|---|---|
| σ100 (Mpa) | 2.21 ± 0.03 | 3.39 ± 0.03 |
| σ200 (MPa) | 6.28 ± 0.12 | 9.05 ± 0.11 |
| σ300 (Mpa) | 13.61 ± 0.25 | 17.58 ± 0.22 |
| σ300/σ100 | 6.15 ± 0.22 | 5.18 ± 0.13 |
| σB (Mpa) | 30.34 ± 1.15 | 25.91 ± 1.19 |
| εB (%) | 504.22 ± 10.14 | 392.94 ± 7.15 |
| Energy (J/cm3) | 60.18 ± 2.54 | 41.16 ± 4.52 |
| T (°C) | Silica | CB/SP-4 | CB/SP-5 | |
|---|---|---|---|---|
| E′ [Mpa] | 10 | 7.09 | 7.67 | 8.00 |
| 23 | 6.51 | 6.91 | 7.22 | |
| 70 | 5.69 | 5.83 | 6.30 | |
| E″ [Mpa] | 10 | 1.89 | 2.09 | 2.05 |
| 23 | 1.49 | 1.64 | 1.60 | |
| 70 | 0.84 | 0.83 | 0.85 | |
| Tan (δ) | 10 | 0.27 | 0.27 | 0.26 |
| 23 | 0.23 | 0.24 | 0.22 | |
| 70 | 0.15 | 0.14 | 0.13 |
| Sample | Resistance [MΩ] |
|---|---|
| Silica | 1.9 ± 0.3 |
| CB—SP 4 | (5.4 ± 1.0) × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaletti, F.; Margani, F.; Monti, A.; Dezyani, R.; Prioglio, G.; Giese, U.; Barbera, V.; Galimberti, M.S. Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy. Polymers 2023, 15, 3120. https://doi.org/10.3390/polym15143120
Magaletti F, Margani F, Monti A, Dezyani R, Prioglio G, Giese U, Barbera V, Galimberti MS. Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy. Polymers. 2023; 15(14):3120. https://doi.org/10.3390/polym15143120
Chicago/Turabian StyleMagaletti, Federica, Fatima Margani, Alessandro Monti, Roshanak Dezyani, Gea Prioglio, Ulrich Giese, Vincenzina Barbera, and Maurizio Stefano Galimberti. 2023. "Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy" Polymers 15, no. 14: 3120. https://doi.org/10.3390/polym15143120
APA StyleMagaletti, F., Margani, F., Monti, A., Dezyani, R., Prioglio, G., Giese, U., Barbera, V., & Galimberti, M. S. (2023). Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy. Polymers, 15(14), 3120. https://doi.org/10.3390/polym15143120







