Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MOAG-Polyol with Diethanolamine via Amidation Reaction
FTIR and NMR Data of MOAG-Polyol
2.3. Characterization of Alkanolamide Polyol
2.3.1. Wet Chemical Analysis
2.3.2. Fourier Transform Infrared Analysis
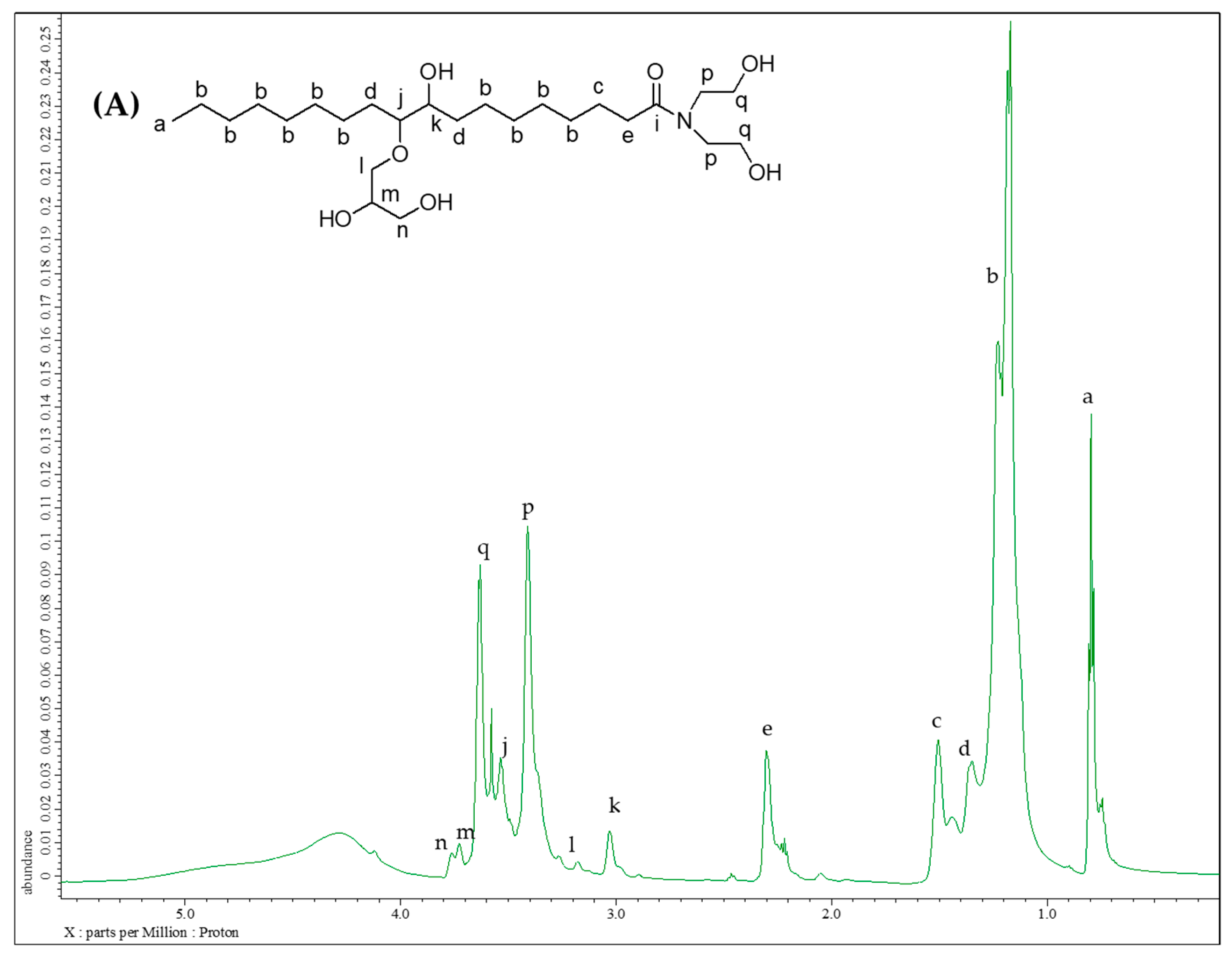
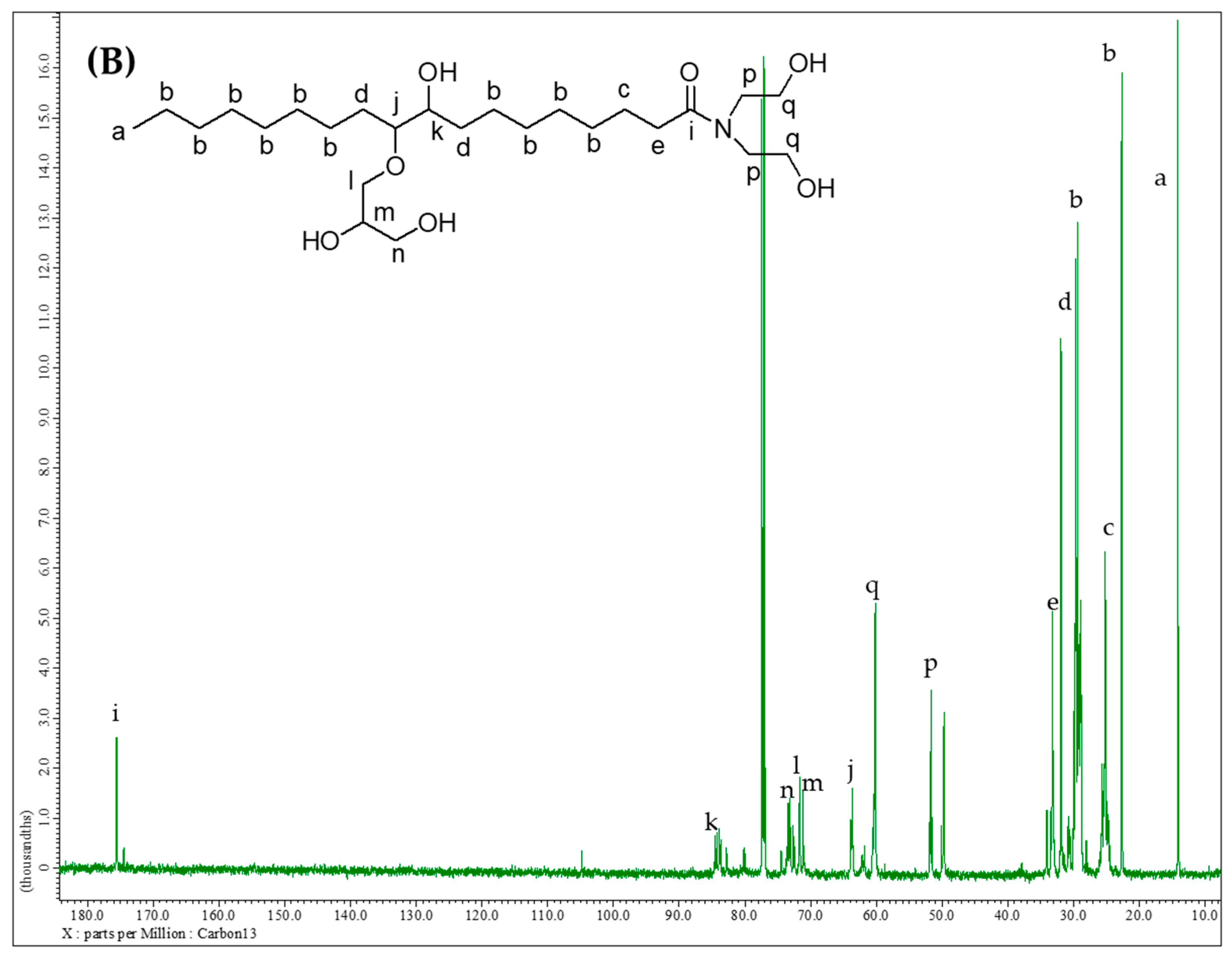
2.3.3. Nuclear Magnetic Resonance Analysis
2.3.4. Molecular Weight Determination
2.3.5. Viscosity Analysis
2.4. Preparation of Rigid PU Foam
2.4.1. Formulation of Rigid PU Foam Made Using Alkanolamide Polyol from MOAG-Polyol
2.4.2. Characterization of Rigid PU Foam
Fourier Transform Infrared Analysis (FTIR)
Foaming Process
Apparent Density
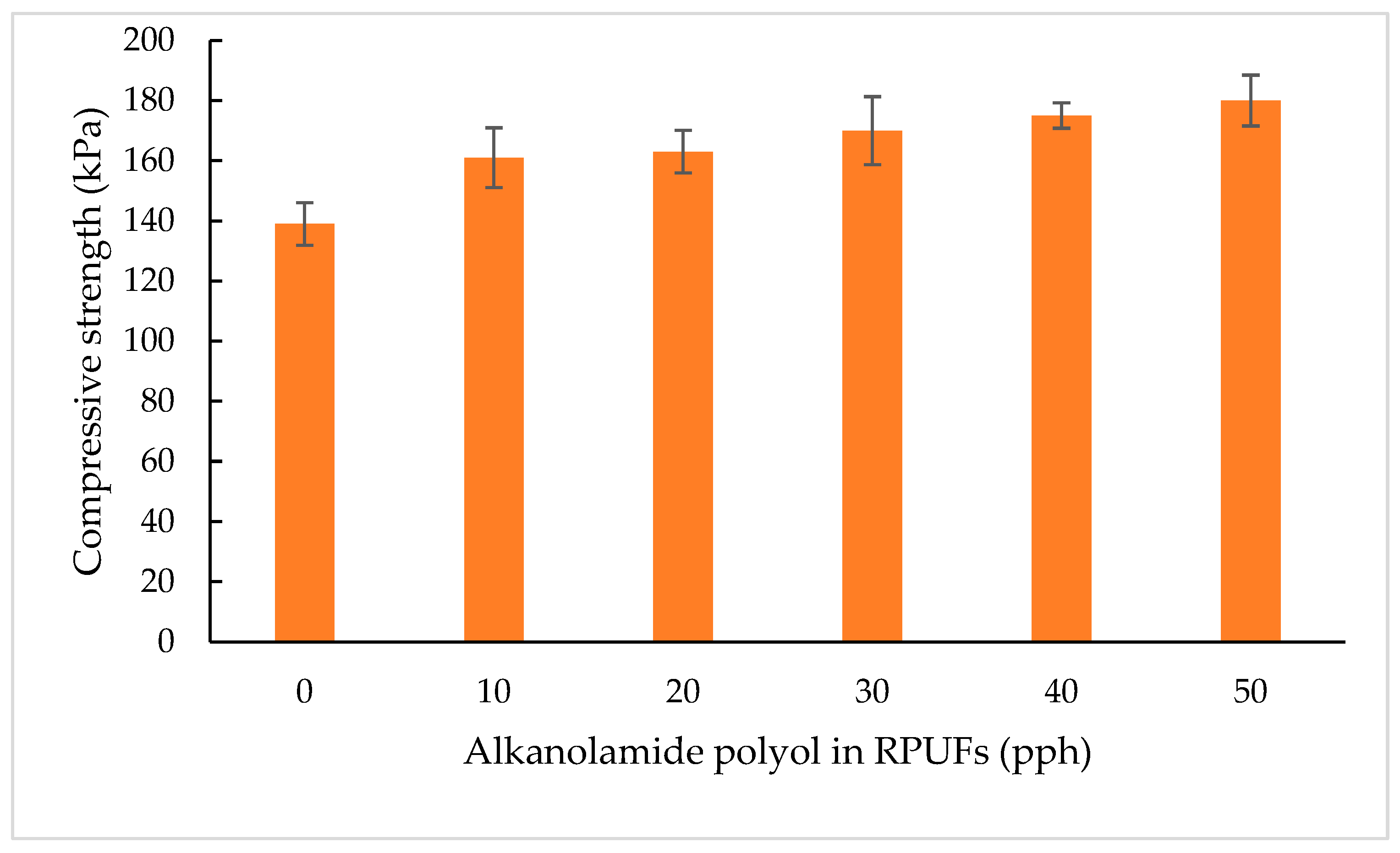
Compressive Strength
Dimensional Stability
Water Absorption
Closed Cell Content
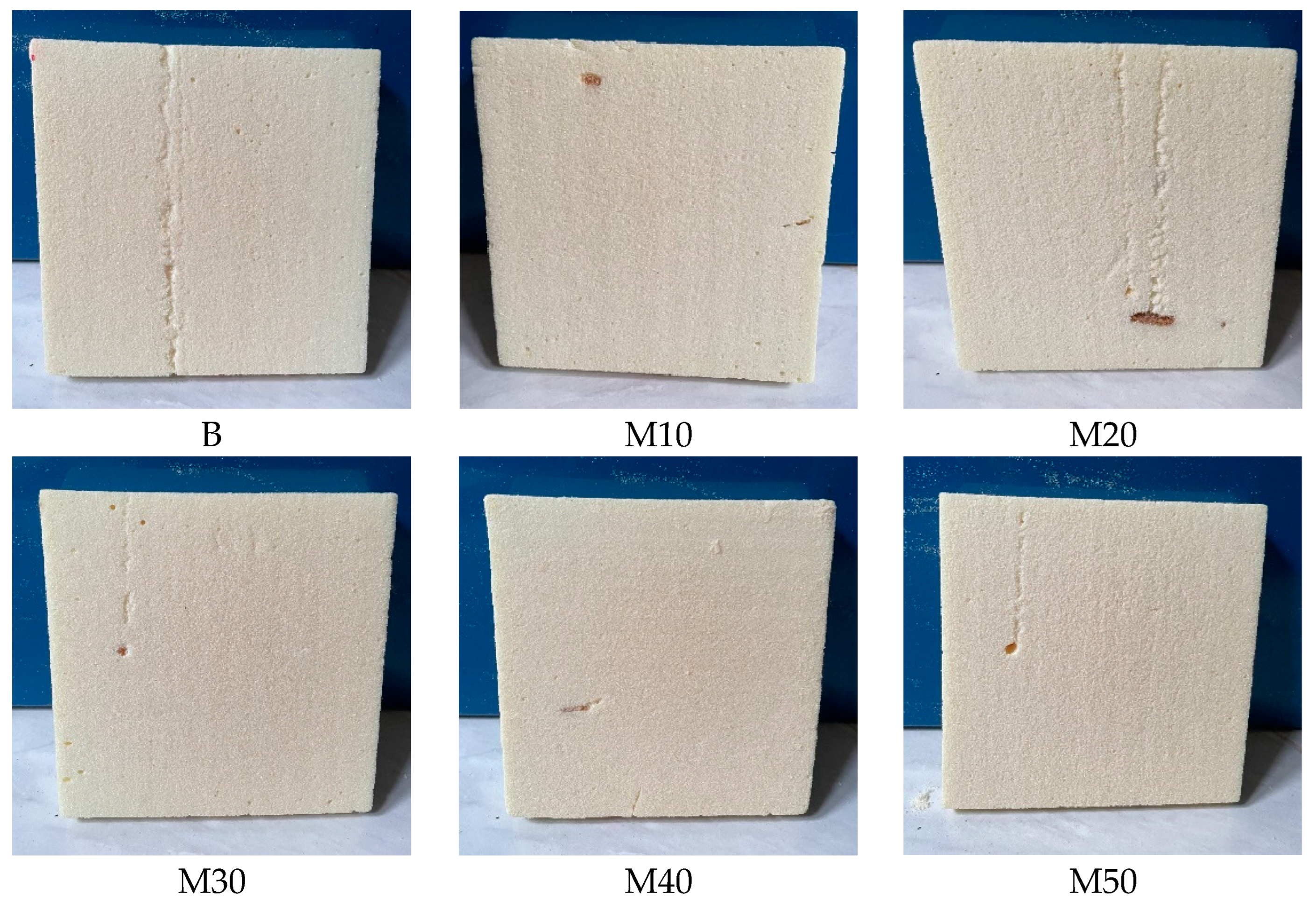
Morphology Analysis
Thermal Conductivity
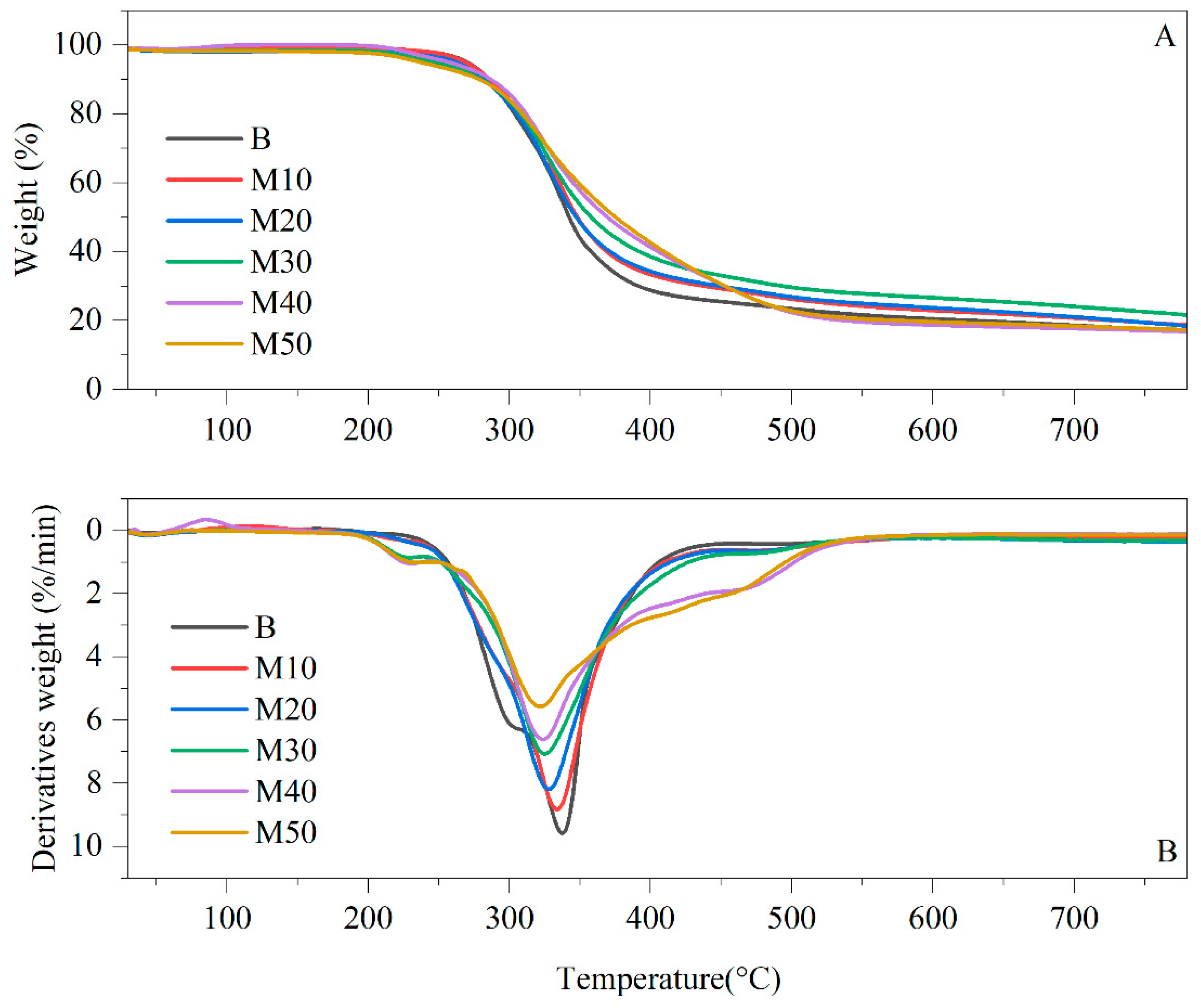
Thermal Gravimetric Analysis
3. Results and Discussion
3.1. Production of Alkanolamide Polyol (MOAG-Polyol) via Amidation Reaction
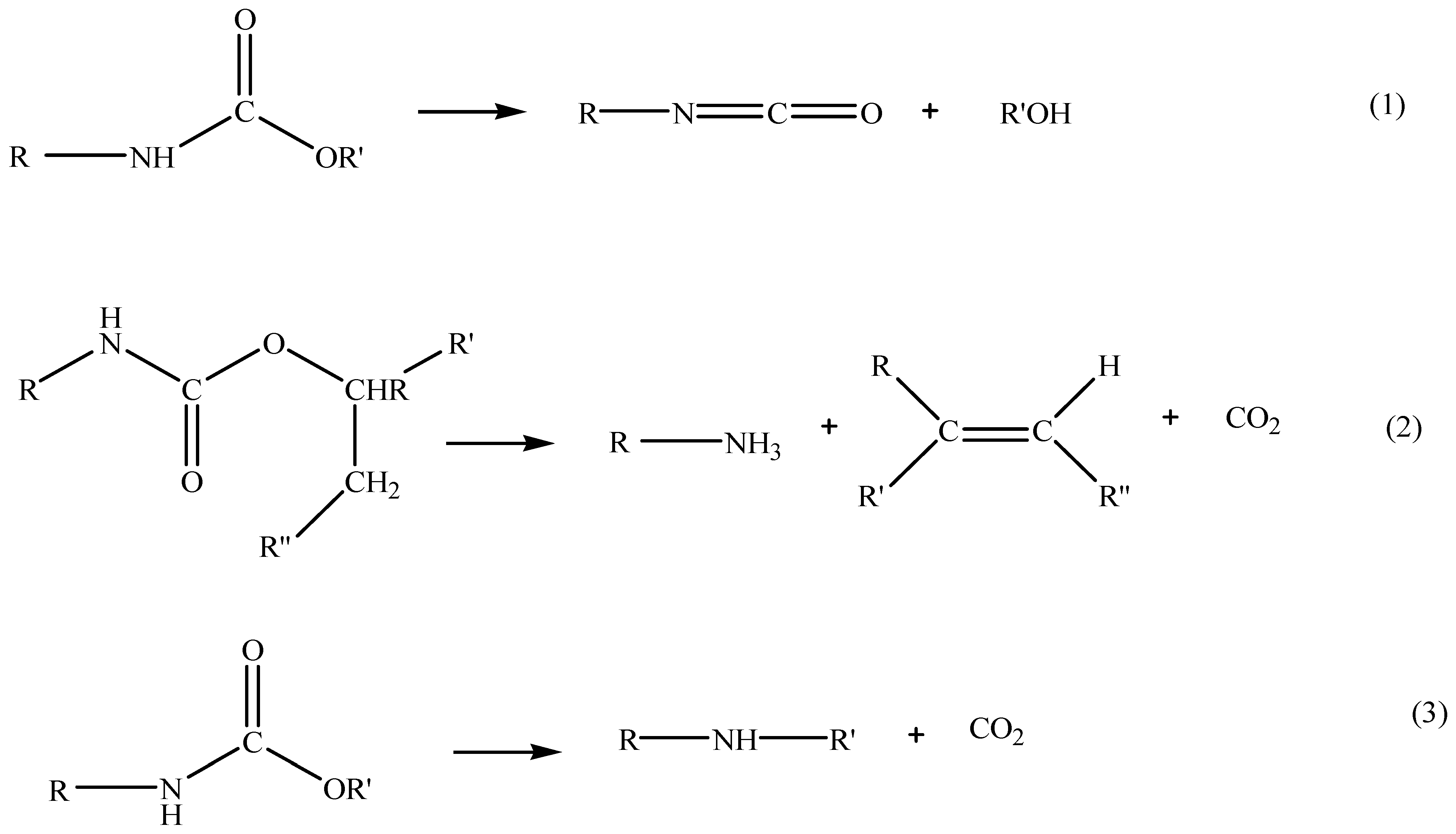
3.1.1. Reaction Mechanism
3.1.2. Optimization of Reaction Parameters of Amidation Reaction (MOAG-Polyol)
Effect of Molar Ratio Reactant: DEA on the Properties of Alkanolamide Polyol (MOAG-Polyol)
Effect of Catalyst Dosage on the Properties of Alkanolamide Polyol (MOAG-Polyol)
Effect of Reaction Time on the Properties of Alkanolamide Polyol (MOAG-Polyol)
3.1.3. Physicochemical Analysis and Molecular Weight Determination
3.1.4. Structure Analysis by FTIR
3.1.5. Structure Analysis by NMR
3.2. Production of Rigid Polyurethane Foams from MOAG-Polyol
3.2.1. Foaming Process
3.2.2. Structure Analysis by FTIR
3.2.3. Apparent Density
3.2.4. Compressive Strength
3.2.5. Dimensional Stability
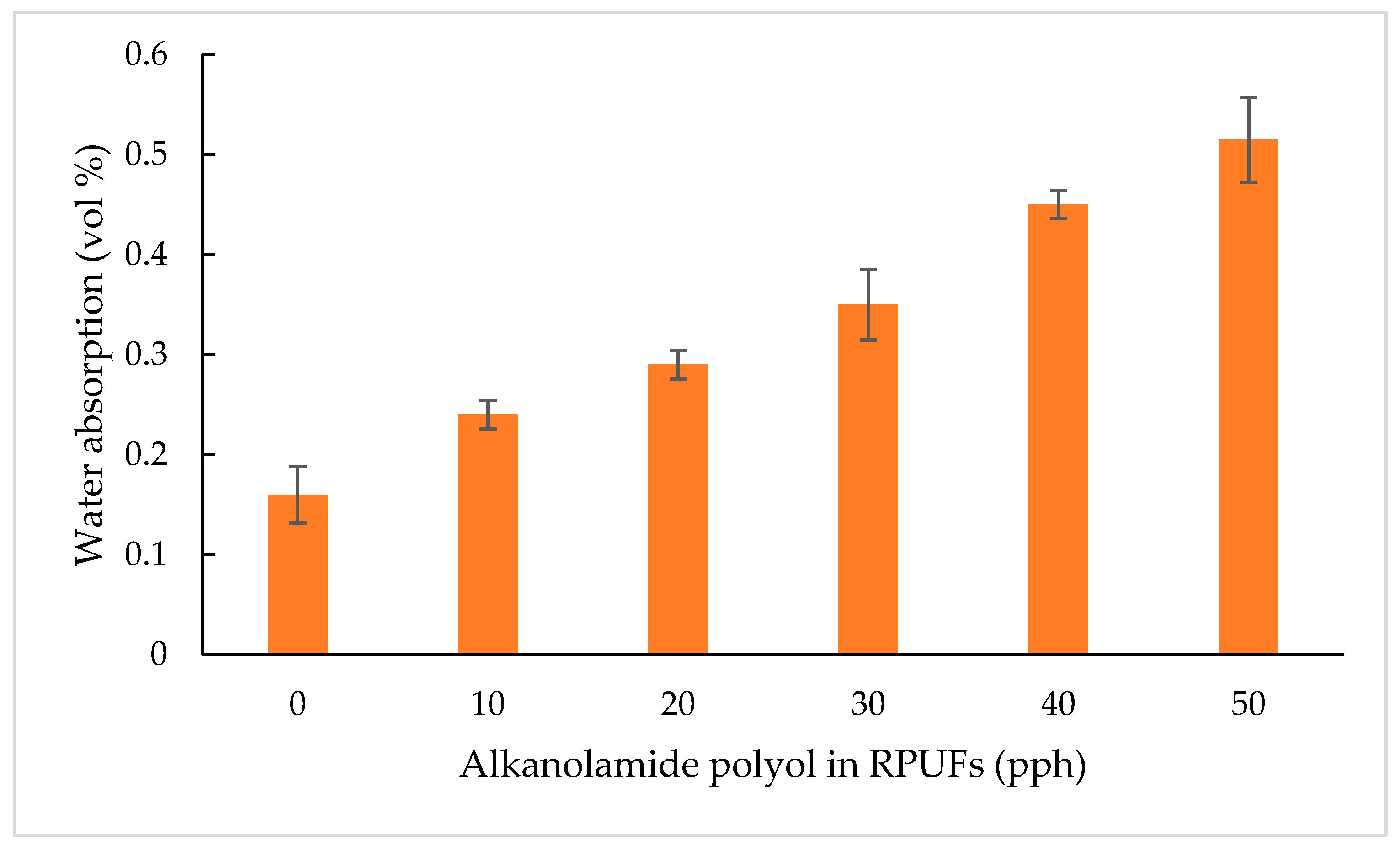
3.2.6. Water Absorption
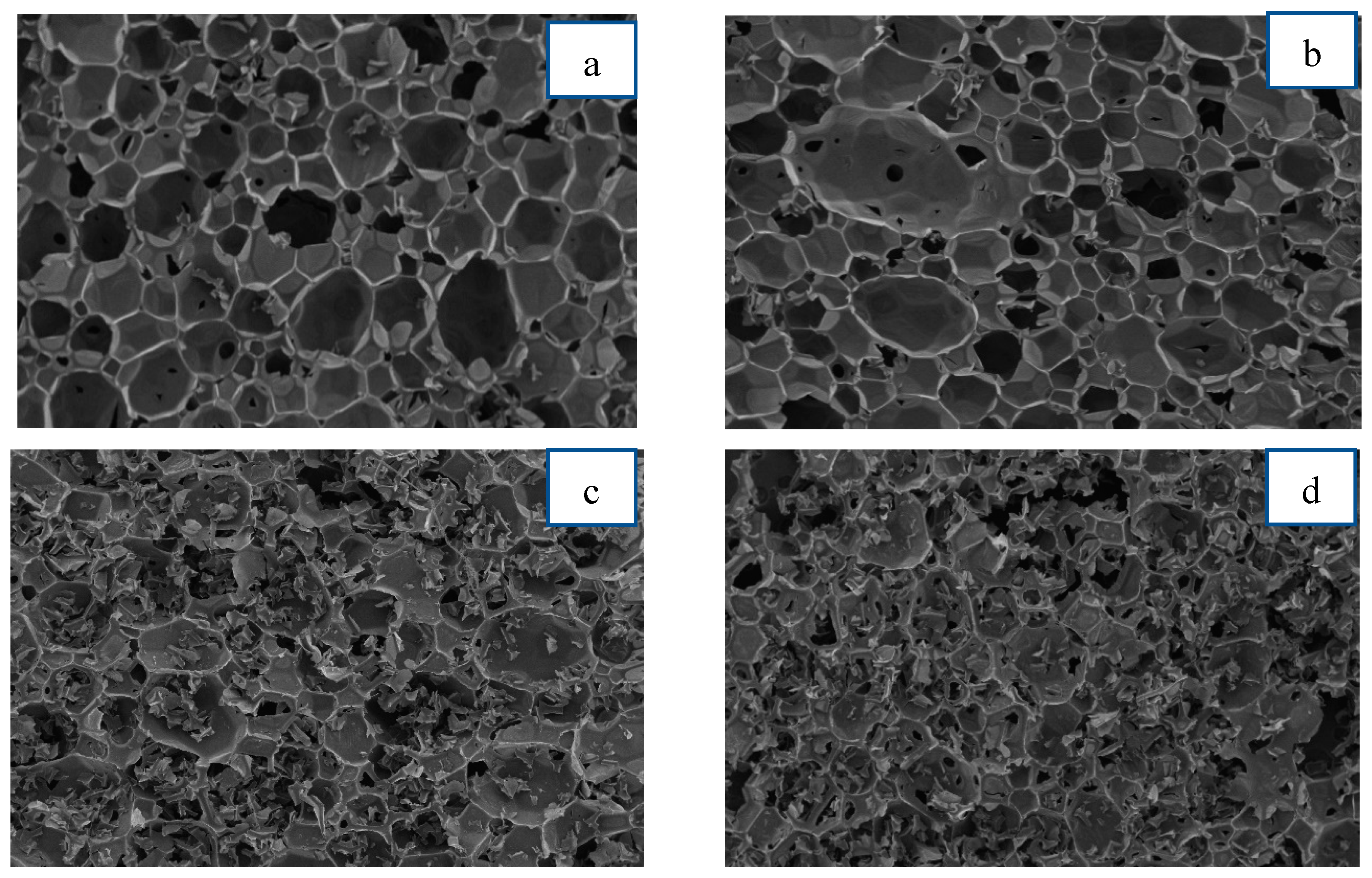
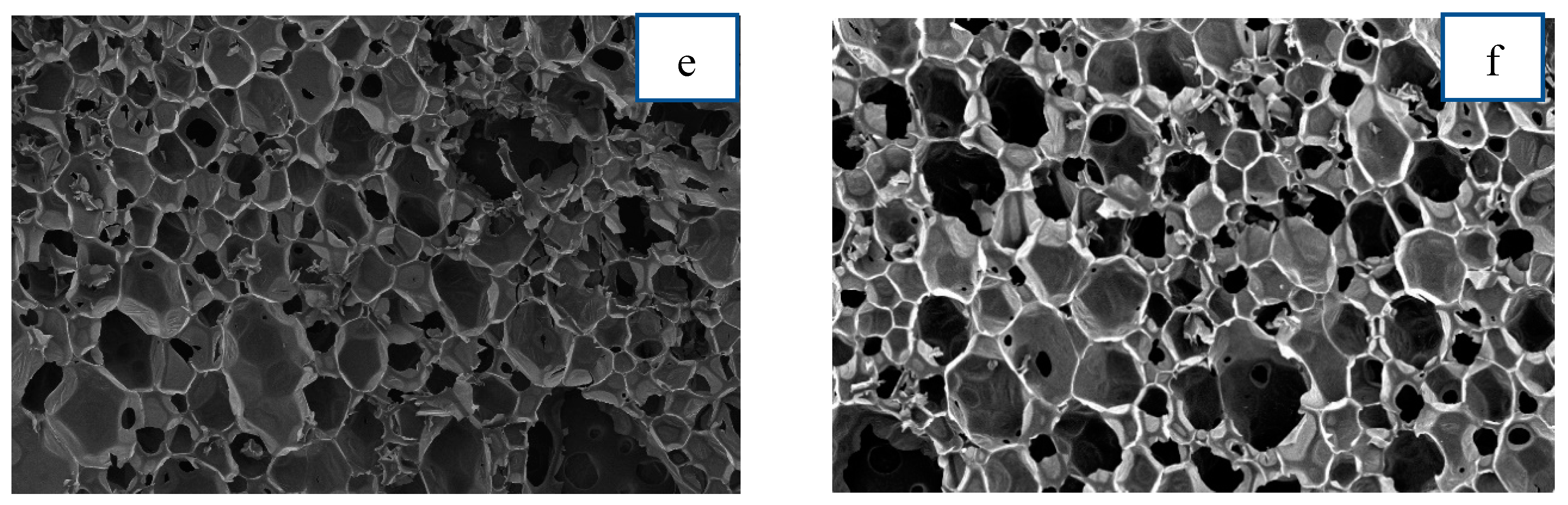
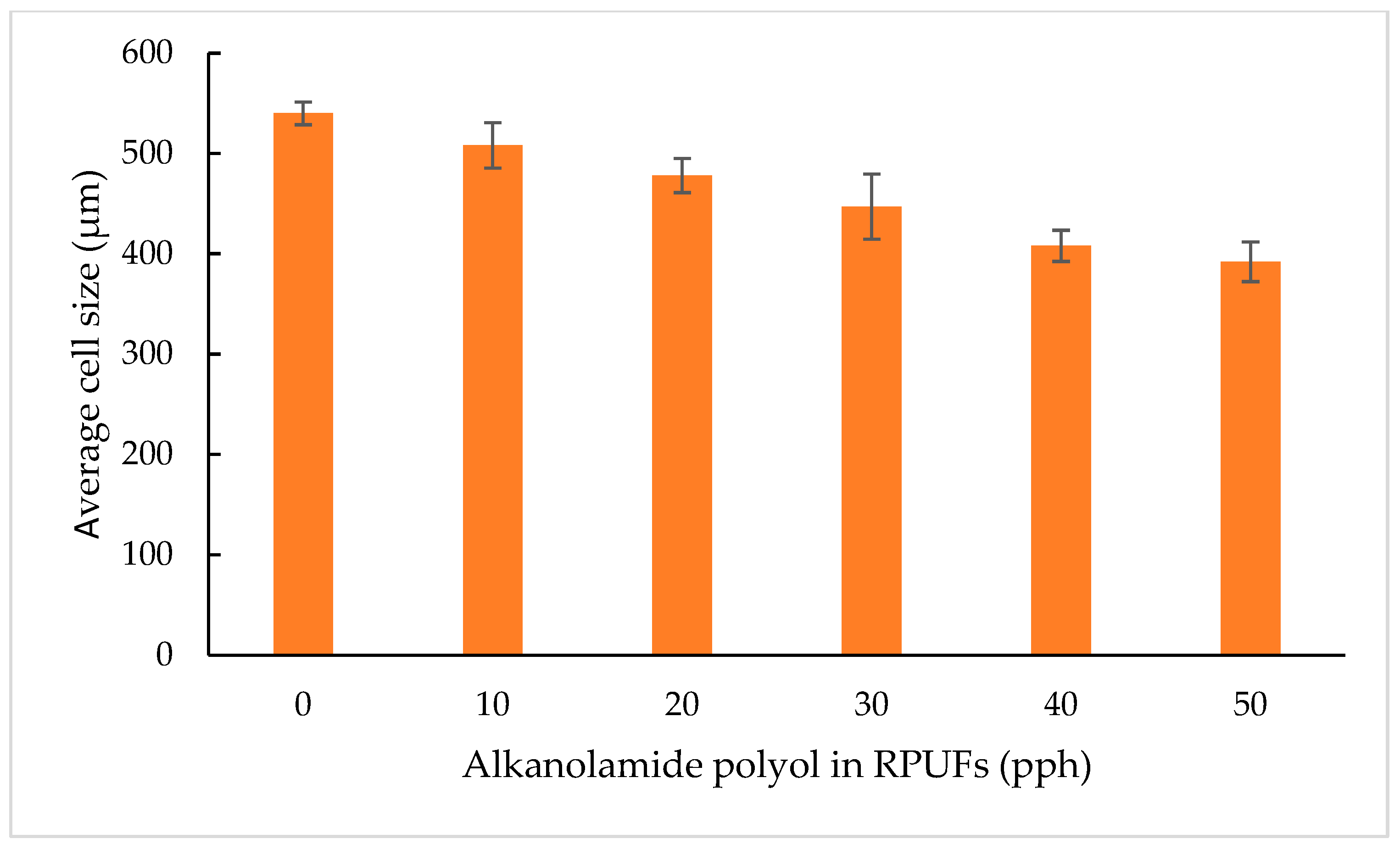
3.2.7. Cell Structure Morphology by SEM
3.2.8. Thermal Conductivity and Closed Cell Content
3.2.9. Thermal Degradation by TGA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Larock, R.C. Soybean-Oil-Based Waterborne Polyurethane Dispersions: Effects of Polyol Functionality and Hard Segment Content on Properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef]
- Lee, C.S.; Ooi, T.L.; Chuah, C.H.; Ahmad, S. Rigid Polyurethane Foam Production from Palm Oil-Based Epoxidized Diethanolamides. J. Am. Oil Chem. Soc. 2007, 84, 1161–1167. [Google Scholar] [CrossRef]
- Prociak, A.; Szczepkowski, L.; Ryszkowska, J.; Kurańska, M.; Auguścik, M.; Malewska, E.; Gloc, M.; Michałowski, S. Influence of Chemical Structure of Petrochemical Polyol on Properties of Bio-polyurethane Foams. J. Polym. Environ. 2019, 27, 2360–2368. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Xu, L.; Zhao, C.; Zhang, X.; Li, J. Synthesis and characterization of rigid polyurethane foam with dimer fatty acid-based polyols. Polym. Bull. 2018, 76, 3753–3768. [Google Scholar] [CrossRef]
- Adnan, S.; Tuan Ismail, T.N.M.; Poo, P.K.D.; Soi, S.H.; Hanzah, N.A.; Mohd Satar, N.; Nek Mat Din, N.S.M.; Yeong, S.K. Low density rigid polyurethane foam incorporated with renewable polyol as sustainable thermal insulation material. J. Cell. Plast. 2022, 58, 485–503. [Google Scholar] [CrossRef]
- Luo, X.; Hu, S.; Zhang, X.; Li, Y. Thermochemical conversion of crude glycerol to biopolyols for the production of polyurethane foams. Bioresour. Technol. 2013, 139, 323–329. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of bio-polyols with different chemical structures on foaming of polyurethane systems and foam properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyżowska, M.; Ryszkowska, J. Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crops Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Polyurethane–polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind. Crops Prod. 2015, 74, 849–857. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Lazdiņa, B.; Misāne, M.; Vilsone, D. Biobased Polyurethanes from Rapeseed Oil Polyols: Structure, Mechanical and Thermal Properties. J. Polym. Environ. 2012, 21, 952–962. [Google Scholar] [CrossRef]
- Tuan Ismail, T.N.M.; Ibrahim, N.A.; Mohd Noor, M.A.; Hoong, S.S.; Poo Palam, K.D.; Yeong, S.K.; Idris, Z.; Schiffman, C.M.; Sendijarevic, I.; Abd Malek, E.; et al. Oligomeric Composition of Polyols from Fatty Acid Methyl Ester: The Effect of Ring-Opening Reactants of Epoxide Groups. J. Am. Oil Chem. Soc. 2018, 95, 509–523. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams of very low density modified with various palm oil-based bio-polyols in accordance with cleaner production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of Transesterified Palm Olein-Based Polyol and Rigid Polyurethanes from this Polyol. J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H.; Oh, K.W. Bio-Based Polyurethane Foams with Castor Oil Based Multifunctional Polyols for Improved Compressive Properties. Polymers 2021, 13, 576. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. Synthesis and application of new bio-polyols based on mustard oil for the production of selected polyurethane materials. Ind. Crops Prod. 2020, 155, 112831. [Google Scholar] [CrossRef]
- Kurańska, M.; Benes, H.; Salasinska, K.; Prociak, A.; Malewska, E.; Polaczek, K. Development and Characterization of “Green Open-Cell Polyurethane Foams” with Reduced Flammability. Materials 2020, 13, 5459. [Google Scholar] [CrossRef]
- Kurańska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Marcovich, N.; Kurańska, M.; Prociak, A.; Malewska, E.; Bujok, S. The effect of different palm oil-based bio-polyols on foaming process and selected properties of porous polyurethanes. Polym. Int. 2017, 66, 1522–1529. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Insulating rigid polyurethane foams from laurel tree pruning based polyol. J. Appl. Polym. Sci. 2020, 138, 49789. [Google Scholar] [CrossRef]
- Kamairudin, N.; Hoong, S.S.; Abdullah, L.C.; Ariffin, H.; Biak, D.R.A. Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology. Molecules 2021, 26, 648. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Kirpluks, M.; Priya, A.; Kaur, G. Conversion of food waste-derived lipid to bio-based polyurethane foam. Case Stud. Chem. Environ. Eng. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Palanisamy, A.; Rao, B.S.; Mehazabeen, S. Diethanolamides of Castor Oil as Polyols for the Development of Water-Blown Polyurethane Foam. J. Polym. Environ. 2011, 19, 698–705. [Google Scholar] [CrossRef]
- Ang, K.P.; Lee, C.S.; Cheng, S.F.; Chuah, C.H. Synthesis of palm oil-based polyester polyol for polyurethane adhesive production. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Luo, Z.; Jiang, X.; Wang, T.; Guo, K. Polyurethane rigid foams formed from different soy-based polyols by the ring opening of epoxidised soybean oil with methanol, phenol, and cyclohexanol. Ind. Crops Prod. 2015, 74, 76–82. [Google Scholar] [CrossRef]
- Petrovic, Z. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation. Ind. Crops Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Žagar, E.; Grdadolnik, J. An infrared spectroscopic study of H-bond network in hyperbranched polyester polyol. J. Mol. Struct. 2003, 658, 143–152. [Google Scholar] [CrossRef]
- Soloi, S.; Abd Majid, R.; Abdul Razak, R. Novel palm oil based polyols with amine functionality synthesis via ring opening reaction of epoxidized palm oil. J. Teknol. 2018, 80, 1–7. [Google Scholar] [CrossRef]
- Nurul ‘Ain, H.; Maznee, T.I.T.N.; Norhayati, M.N.; Noor, M.A.M.; Adnan, S.; Devi, P.P.K.; Norhisham, S.M.; Yeong, S.K.; Hazimah, A.H.; Campara, I.; et al. Natural Palm Olein Polyol as a Replacement for Polyether Polyols in Viscoelastic Polyurethane Foam. J. Am. Oil Chem. Soc. 2016, 93, 983–993. [Google Scholar] [CrossRef]
- Kurańska, M.; Leszczyńska, M.; Kubacka, J.; Prociak, A.; Ryszkowska, J. Effects of Modified Used Cooking Oil on Structure and Properties of Closed-Cell Polyurethane foams. J. Polym. Environ. 2020, 28, 2780–2788. [Google Scholar] [CrossRef]
- Devi, P.P.K.; Maznee, T.I.T.N.; Hoong, S.S.; Zailan, A.B.; Yeong, S.K.; Hazimah, A.H.; Schiffman, C.M.; Sendijarevic, A.; Sendijarevic, V.; Sendijarevic, I. Urethane-forming reaction kinetics of natural oil polyols versus petroleum-based polyether polyol. React. Kinet. Mech. Catal. 2016, 119, 93–106. [Google Scholar] [CrossRef]
- de Souza Felipe, M.; Pawan, K.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. ACS Symp. Ser. 2021, 1380, 1–24. [Google Scholar]
- Ivdre, A.; Fridrihsone-Girone, A.; Abolins, A.; Cabulis, U. Effect of different concentration of rapeseed oil and recycled poly (ethylene terephthalate) in polyols for rigid polyurethane foams. J. Cell. Plast. 2016, 54, 161–177. [Google Scholar] [CrossRef]
- Paruzel, A.; Michałowski, S.; Hodan, J.; Horák, P.; Prociak, A.; Beneš, H. Rigid Polyurethane Foam Fabrication Using Medium Chain Glycerides of Coconut Oil and Plastics from End-of-Life Vehicles. ACS Sustain. Chem. Eng. 2017, 5, 6237–6246. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Ryszkowska, J.; Szczepkowski, L.; Kurańska, M.; Prociak, A.; Leszczyński, M.K.; Gloc, M.; Antos-Bielska, M.; Mizera, K. Cooperative effect of rapeseed oil-based polyol and egg shells on the structure and properties of rigid polyurethane foams. Polym. Test. 2020, 90, 106696. [Google Scholar] [CrossRef]
- Ryszkowska, J.; Auguscik, M.; Zieleniewska, M.; Szczepkowski, L.; Kuranska, M.; Bak, S.; Antos-Bielska, M.; Prociak, A. Semi-rigid polyurethane foams with rapeseed polyol of different viscosity. Polimery 2018, 63, 10–17. [Google Scholar] [CrossRef]
- Kuranska, M.; Leszczynska, M.; Malewska, E.; Prociak, A.; Ryszkowska, J. Implementation of Circular Economy Principles in the Synthesis of Polyurethane Foams. Polymers 2020, 12, 2068. [Google Scholar] [CrossRef]
- Coman, A.E.; Peyrton, J.; Hubca, G.; Sarbu, A.; Gabor, A.R.; Nicolae, C.A.; Iordache, T.V.; Averous, L. Synthesis and characterization of renewable polyurethane foams using different biobased polyols from olive oil. Eur. Polym. J. 2021, 149. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part. B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Prociak, A. Open-cell bio-polyurethane foams based on bio-polyols from used cooking oil. J. Clean. Prod. 2022, 359. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: II. Foams. J. Am. Oil Chem. Soc. 2006, 84, 65–72. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid Polyurethane Foams with Various Isocyanate Indices Based on Polyols from Rapeseed Oil and Waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef]
- Song, S.A.; Chung, Y.S.; Kim, S.S. The mechanical and thermal characteristics of phenolic foams reinforced with carbon nanoparticles. Compos. Sci. Technol. 2014, 103, 85–93. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Lin, Y.; Hsieh, F.; Huff, H.E. Physical, mechanical and thermal properties of water-blown rigid polyurethane foam containing soy protein isolate. Cereal Chem. 1996, 73, 189–196. [Google Scholar]
- Cecchini, C. Dimensional stability of polyurethane rigid foams blown with CO2. J. Cell. Plast. 1999, 15, 514–530. [Google Scholar] [CrossRef]
- Cunningham, R.L.; Carr, M.E.; Bagley, E.B. Polyurethane foams extended with corn flour. Cereal Chem. 1991, 68, 258–261. [Google Scholar]
- Czlonka, S.; Strakowska, A.; Strzelec, K.; Kairyte, A.; Kremensas, A. Bio-Based Polyurethane Composite Foams with Improved Mechanical, Thermal, and Antibacterial Properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef]
- Kairytė, A.; Vėjelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crops Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Czlonka, S.; Strakowska, A. Rigid Polyurethane Foams Based on Bio-Polyol and Additionally Reinforced with Silanized and Acetylated Walnut Shells for the Synthesis of Environmentally Friendly Insulating Materials. Materials 2020, 13, 3245. [Google Scholar] [CrossRef]
- Tu, Y.-C.; Suppes, G.J.; Hsieh, F.-H. Water-blown rigid and flexible polyurethane foams containing epoxidized soybean oil triglycerides. J. Appl. Polym. Sci. 2008, 109, 537–544. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Suchorzewski, J.; Piszczyk, L.; Haponiuk, J.T. Study on the Structure-Property Dependences of Rigid PUR-PIR Foams Obtained from Marine Biomass-Based Biopolyol. Materials 2020, 13, 1257. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Q.; Liu, H.; Guo, W.; Jiang, Y.; Chen, L.; He, L.; Qi, J.; Xiao, H.; Chen, Y.; et al. Renewable natural resources reinforced polyurethane foam for use of lightweight thermal insulation. Mater. Res. Express 2020, 7, 055302. [Google Scholar] [CrossRef]
- Maria, K.; Malewska, E.; Polaczek, K.; Prociak, A.; Kubacka, J. A Pathway toward a New Era of Open-Cell Polyurethane Foams-Influence of Bio-Polyols Derived from Used Cooking Oil on Foams Properties. Materials 2020, 13, 5161. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell rigid polyurethane bio-foams based on modified used cooking oil. Polymer 2020, 190, 122164. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, H.; Zhang, L.; Hu, L.; Zhou, Y. Study of the mechanical, thermal properties and flame retardancy of rigid polyurethane foams prepared from modified castor-oil-based polyols. Ind. Crops Prod. 2014, 59, 135–143. [Google Scholar] [CrossRef]




| Formulation Designation | B | M10 | M20 | M30 | M40 | M50 |
|---|---|---|---|---|---|---|
| Ratio (pph) a | ||||||
| YD6205 | 100 | 90 | 80 | 70 | 60 | 50 |
| MOAG | - | 10 | 20 | 30 | 40 | 50 |
| Water | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Dabco DC193 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Dabco 33LV | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Niax A1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Isocyanate system | ||||||
| Desmodur 44V20L | 175.98 | 175.56 | 175.15 | 174.73 | 174.32 | 173.91 |
| Isocyanate index b | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Entry | Molar Ratio | Catalyst | Time | OHV | GPC | ||
|---|---|---|---|---|---|---|---|
| Reactant: DEA | Mw | Mn | PDI | ||||
| Group 1 | |||||||
| 1 | 1:1 | 0.25 | 3 | 279 | 570 | 509 | 1.12 |
| 2 | 1:2 | 0.25 | 3 | 313 | 664 | 600 | 1.11 |
| 3 | 1:3 | 0.25 | 3 | 300 | 575 | 517 | 1.11 |
| Group 2 | |||||||
| 4 | 1:2 | 0.15 | 3 | 285 | 600 | 547 | 1.10 |
| 5 | 1:2 | 0.25 | 3 | 313 | 664 | 600 | 1.11 |
| 6 | 1:2 | 0.50 | 3 | 292 | 623 | 525 | 1.20 |
| Group 3 | |||||||
| 7 | 1:2 | 0.25 | 2 | 270 | 571 | 527 | 1.10 |
| 8 | 1:2 | 0.25 | 3 | 313 | 664 | 600 | 1.11 |
| 9 | 1:2 | 0.25 | 6 | 274 | 701 | 619 | 1.13 |
| Parameters | MOG-Polyol | MOAG-Polyol |
|---|---|---|
| IV (g I2 100/g) | 5.38 | 6.76 |
| AV (mg KOH/g) | 1.18 | 2.33 |
| OHV (mg KOH/g) | 306 | 313 |
| SV (mg KOH/g) | 140 | 106 |
| Viscosity at 25 °C (mPa∙s) | 513 | 22,800 |
| Moisture content (wt%) | 0.10 | 0.84 |
| MW (Da) | 488 | 664 |
| Average number, Mn (Da) | 428 | 600 |
| % Oligomer | 20 | 17 |
| PDI = MW/Mn | 1.14 | 1.11 |
| Physical appearance | Liquid | Liquid |
| Designations | Cream Time (s) | Gel Time (s) | Free Rise Time (s) | Tack-Free Time (s) |
|---|---|---|---|---|
| B | 20 | 58 | 115 | 153 |
| M10 | 17 | 48 | 99 | 128 |
| M20 | 17 | 45 | 90 | 114 |
| M30 | 16 | 42 | 84 | 93 |
| M40 | 16 | 42 | 76 | 76 |
| M50 | 15 | 40 | 68 | 68 |
| 100 PP/ 0 BP | 90 PP/ 10 BP | 80 PP/ 20 BP | 70 PP/ 30 BP | 60 PP/ 40 BP | 50 PP/ 50 BP | |
|---|---|---|---|---|---|---|
| Wavenumbers (cm−1) | bond vibration | |||||
| 3314 | 3319 | 3314 | 3317 | 3318 | 3308 | N-H stretching |
| 2923 | 2927 | 2925 | 2926 | 2924 | 2925 | C-H asymmetric |
| 2853 | 2852 | 2854 | 2855 | 2854 | 2854 | C-H symmetric |
| 1708 | 1706 | 1704 | 1704 | 1703 | 1706 | C=O stretching |
| 1595 | 1595 | 1595 | 1595 | 1595 | 1596 | C=C (aromatic, stretching) |
| 1510 | 1510 | 1509 | 1509 | 1509 | 1509 | N-H bending |
| 1411 | 1411 | 1411 | 1411 | 1411 | 1411 | PIR (deformation) |
| 1222 | 1221 | 1221 | 1222 | 1222 | 1222 | C-N (stretching) |
| 1074 | 1073 | 1071 | 1073 | 1072 | 1068 | C-O-C (stretching) |
| 764 | 764 | 764 | 763 | 763 | 763 | C-H (deformation) |
| Designations | Thermal Conductivity Coefficient (W/m.K) | Closed Cell Content (%) | ||
|---|---|---|---|---|
| 20 °C | 50 °C | 70 °C | ||
| B | 0.034304 | 0.039998 | 0.043448 | 45 |
| M10 | 0.035745 | 0.040474 | 0.044021 | 28 |
| M20 | 0.036045 | 0.0405060 | 0.044162 | 20 |
| M30 | 0.036147 | 0.040595 | 0.045168 | 18 |
| M40 | 0.036180 | 0.040962 | 0.045192 | 15 |
| M50 | 0.037045 | 0.0410560 | 0.046011 | 13 |
| Designations | Duration (Days) | Weight Changes (%) | Volume Changes (%) | ||
|---|---|---|---|---|---|
| at 70 °C | at −20 °C | at 70 °C | at −20 °C | ||
| B | 1 | −1.06 ± 0.03 | 0.49 ± 0.03 | −1.19 ± 0.03 | 0.06 ± 0.03 |
| 7 | −1.13 ± 0.02 | 0.56 ± 0.03 | −1.29 ± 0.04 | 0.57 ± 0.02 | |
| 14 | −1.36 ± 0.02 | 0.71 ± 0.03 | −1.43 ± 0.05 | 0.79 ± 0.03 | |
| M10 | 1 | −0.97 ± 0.03 | 0.46 ± 0.03 | −1.15 ± 0.05 | 0.11 ± 0.04 |
| 7 | −1.07 ± 0.03 | 0.74 ± 0.4 | −1.39 ± 0.08 | 0.16 ± 0.03 | |
| 14 | −1.18 ± 0.03 | 0.95 ± 0.04 | −1.68 ± 0.06 | 0.21 ± 0.03 | |
| M20 | 1 | −0.08 ± 0.03 | 0.44 ± 0.02 | −0.86 ± 0.02 | 0.41 ± 0.04 |
| 7 | −0.95 ± 0.02 | 0.56 ± 0.05 | −1.14 ± 0.04 | 0.49 ± 0.03 | |
| 14 | −1.06 ± 0.01 | 0.67 ± 0.03 | −1.17 ± 0.04 | 0.74 ± 0.04 | |
| M30 | 1 | −0.96 ± 0.03 | 0.10 ± 0.02 | −1.19 ± 0.08 | 0.64 ± 0.03 |
| 7 | −1.17 ± 0.04 | 0.16 ± 0.04 | −1.25 ± 0.03 | 0.75 ± 0.04 | |
| 14 | −1.28 ± 0.03 | 0.25 ± 0.03 | −1.55 ± 0.03 | 0.95 ± 0.04 | |
| M40 | 1 | −1.12 ± 0.03 | 0.75 ± 0.04 | −0.06 ± 0.06 | 0.43 ± 0.02 |
| 7 | −1.25 ± 0.03 | 0.86 ± 0.02 | −1.74 ± 0.04 | 0.56 ± 0.05 | |
| 14 | −1.42 ± 0.03 | 0.96 ± 0.03 | −1.92 ± 0.05 | 0.73 ± 0.03 | |
| M50 | 1 | −0.92 ± 0.06 | 0.22 ± 0.02 | −1.17 ± 0.02 | 0.07 ± 0.02 |
| 7 | −1.23 ± 0.05 | 0.44 ± 0.04 | −1.45 ± 0.04 | 0.65 ± 0.03 | |
| 14 | −1.31 ± 0.04 | 0.55 ± 0.03 | −1.56 ± 0.04 | 0.84 ± 0.03 | |
| Designations | T5% (°C) | T10% (°C) | T50% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|---|
| B | 273 | 286 | 342 | 338 | 16.84 |
| M10 | 270 | 285 | 348 | 334 | 18.68 |
| M20 | 262 | 282 | 350 | 328 | 18.41 |
| M30 | 257 | 280 | 358 | 325 | 21.63 |
| M40 | 248 | 279 | 370 | 324 | 16.84 |
| M50 | 237 | 278 | 375 | 322 | 17.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamairudin, N.; Abdullah, L.C.; Hoong, S.S.; Biak, D.R.A.; Ariffin, H. Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material. Polymers 2023, 15, 3028. https://doi.org/10.3390/polym15143028
Kamairudin N, Abdullah LC, Hoong SS, Biak DRA, Ariffin H. Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material. Polymers. 2023; 15(14):3028. https://doi.org/10.3390/polym15143028
Chicago/Turabian StyleKamairudin, Norsuhaili, Luqman Chuah Abdullah, Seng Soi Hoong, Dayang Radiah Awang Biak, and Hidayah Ariffin. 2023. "Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material" Polymers 15, no. 14: 3028. https://doi.org/10.3390/polym15143028
APA StyleKamairudin, N., Abdullah, L. C., Hoong, S. S., Biak, D. R. A., & Ariffin, H. (2023). Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material. Polymers, 15(14), 3028. https://doi.org/10.3390/polym15143028






