UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization
Abstract
1. Introduction
- A novel synthesis route for transparent coatings preparation based on selection (e.g., different crosslinking agents), and curing conditions (e.g., exposure time) with high conversion rates over extended UV LED exposure time range.
- A selection of fluorinated resin-based transparent coatings deliverables under different curing exposure times.
- Data processing and analysis on the comprehensive database containing viscoelastic and optical properties (i.e., refractive indices, thermo-optical coefficients, and optical losses).
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation and UV LED-Assisted Curing Process
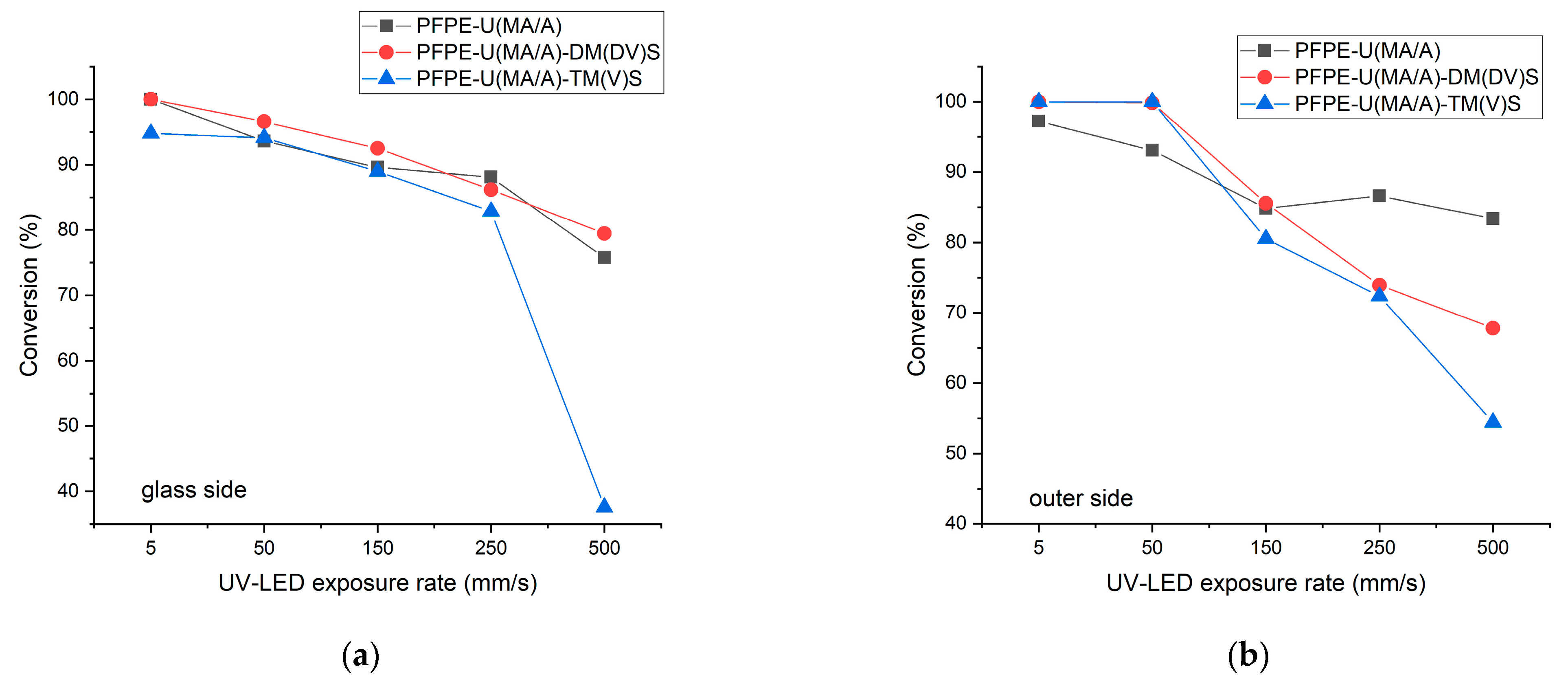
2.3. Curing Behavior and Characterization
2.4. Viscoelastic Properties by Means of Dynamic Mechanical Analysis (DMA)
2.5. Optical Properties Measurements
2.5.1. Refractive Index (n)
2.5.2. Thermo-Optic Coefficients (TOC)
2.5.3. Optical Loss
3. Results and Discussions
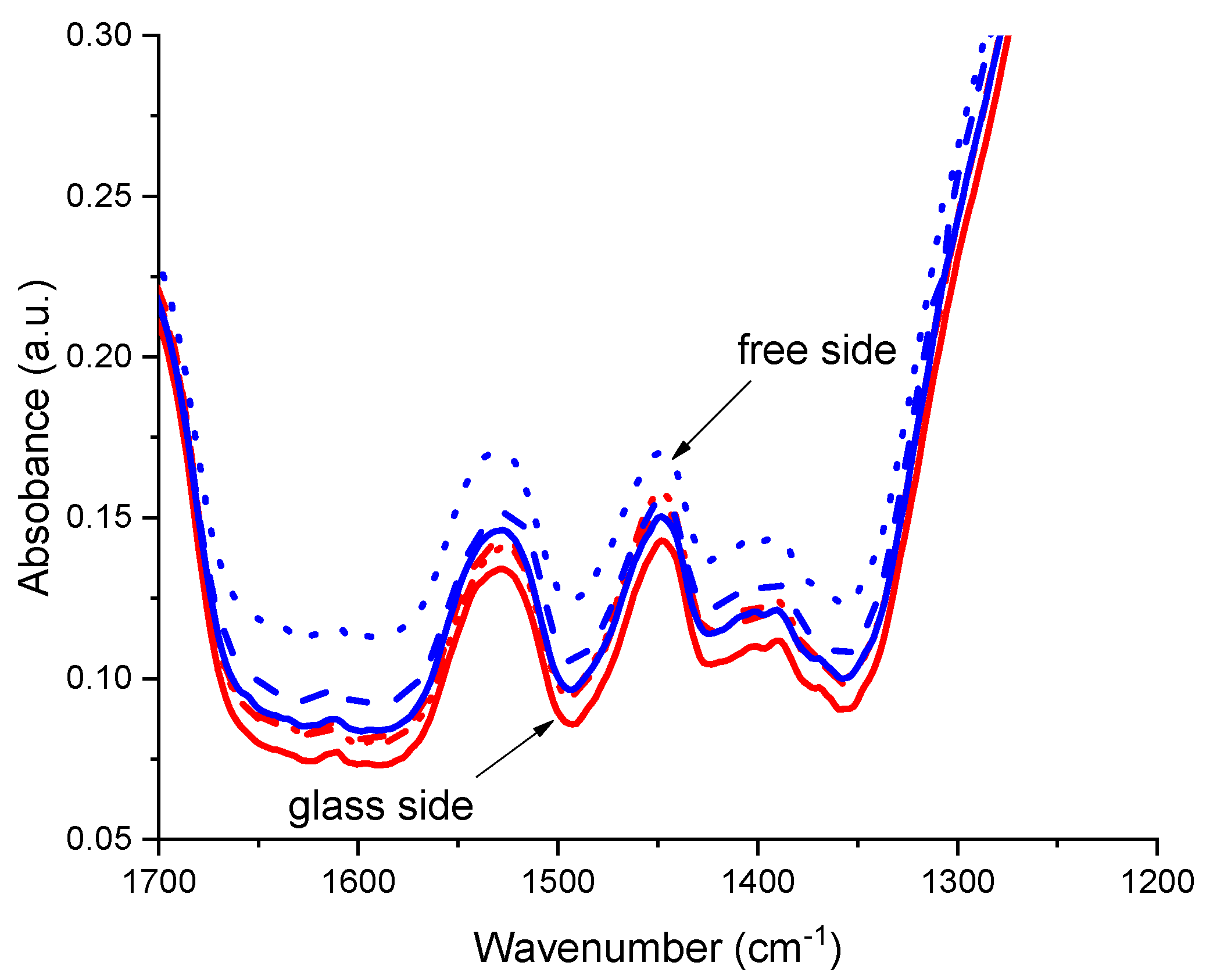
3.1. Structural Characterization of UV LED Cured Coatings
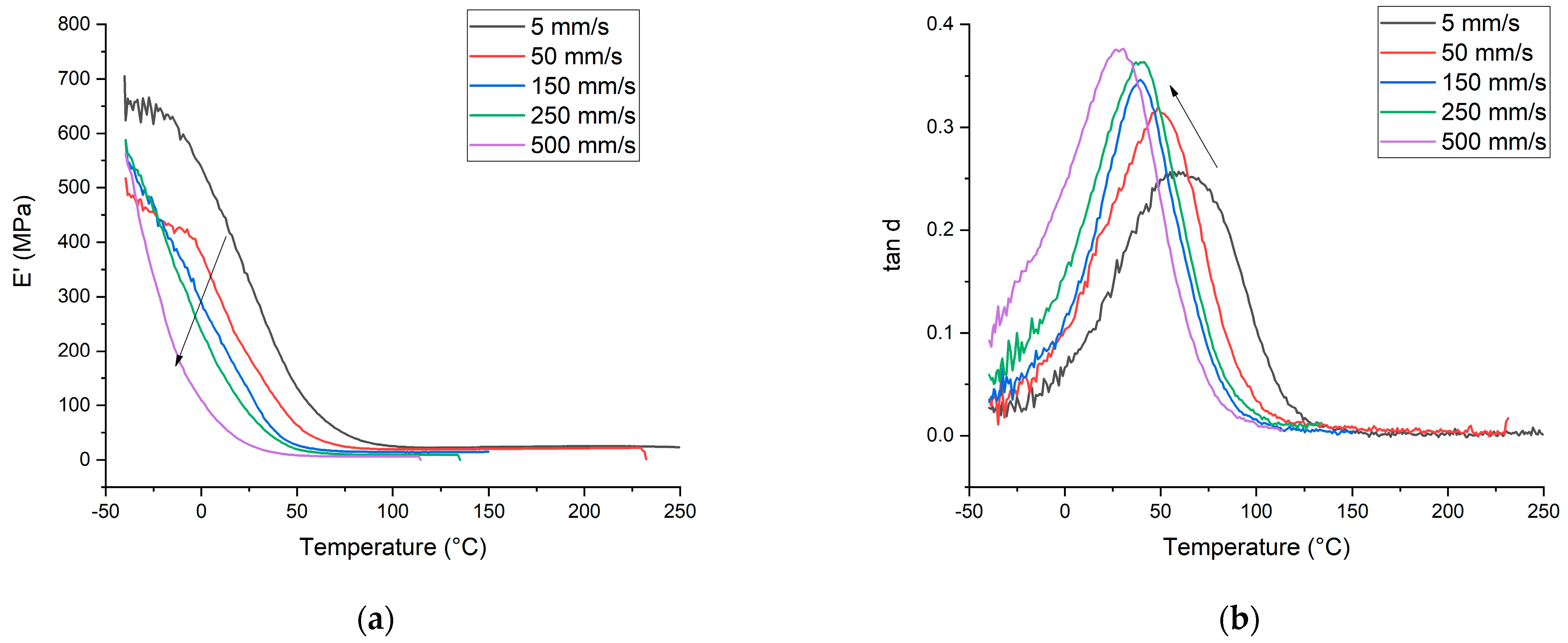
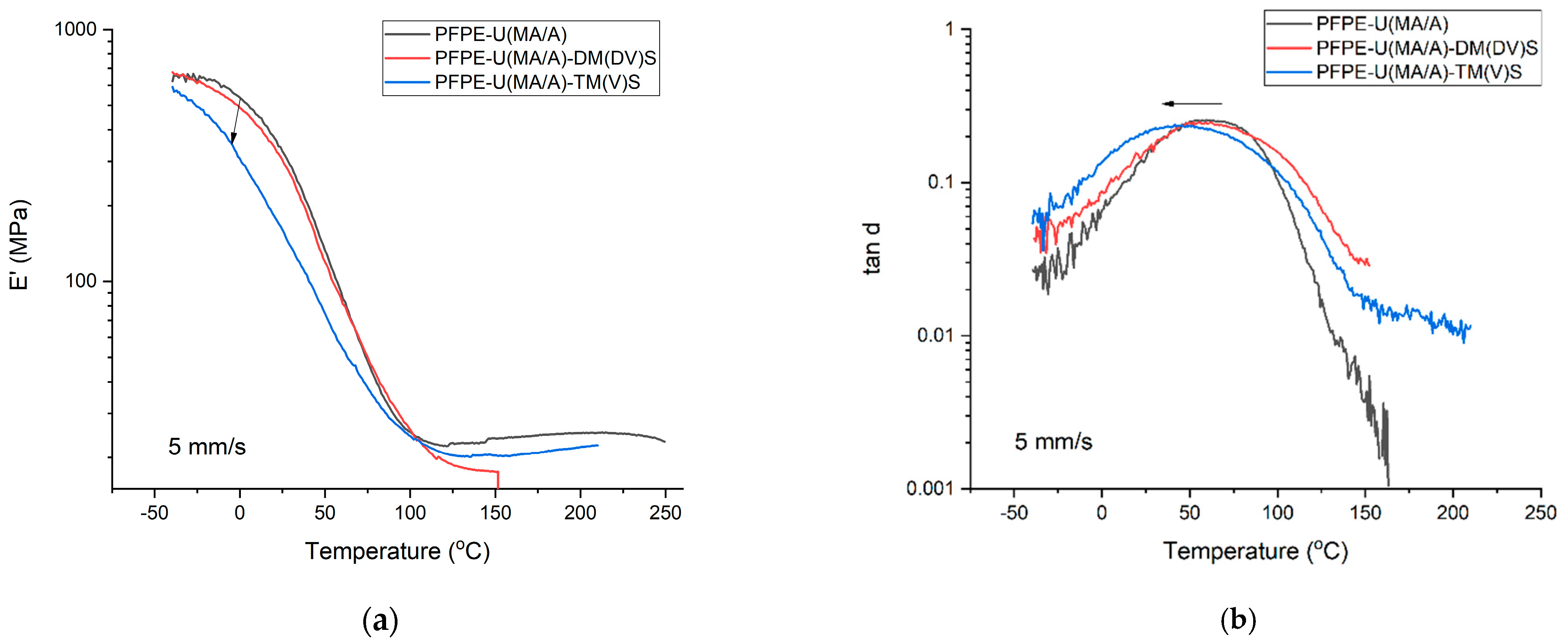
3.2. DMA Properties of UV LED-Cured Coatings
3.3. Optical Properties of UV LED Cured Coatings
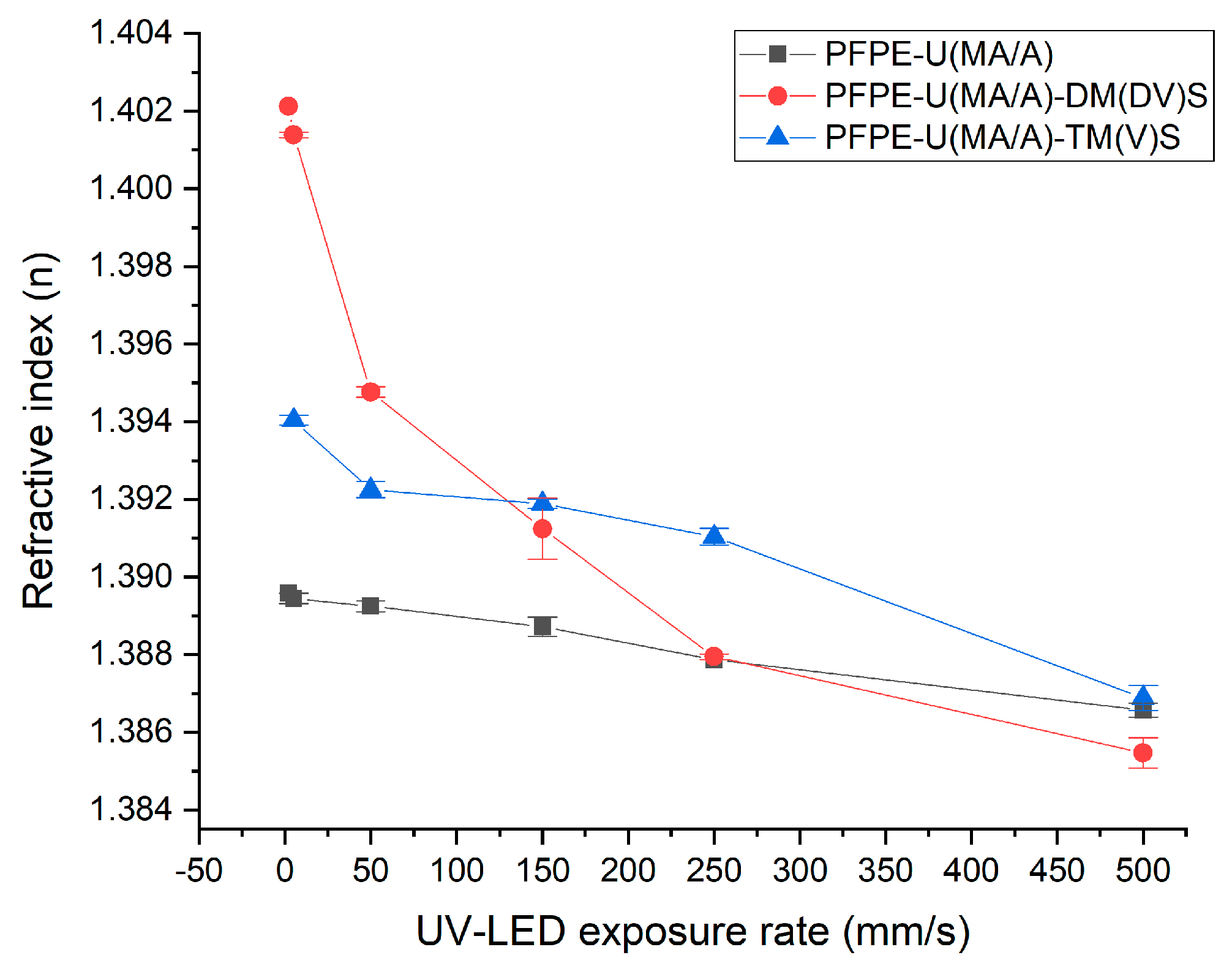
3.3.1. Refractive Index (n)
3.3.2. Thermo-Optic Coefficients (TOC)
3.3.3. Optical Loss
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figoli, A.; Ursino, C.; Galiano, F.; Di Nicolò, E.; Campanelli, P.; Carnevale, M.C.; Criscuoli, A. Innovative hydrophobic coating of perfluoropolyether (PFPE) on commercial hydrophilic membranes for DCMD application. J. Membr. Sci. 2017, 522, 192–201. [Google Scholar] [CrossRef]
- Baik, J.-H.; Kim, D.-G.; Lee, J.H.; Kim, S.; Hong, D.G.; Lee, J.-C. Nonflammable and thermally stable gel polymer electrolytes based on crosslinked perfluoropolyether (PFPE) network for lithium battery applications. J. Ind. Eng. Chem. 2018, 64, 453–460. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Li, P.; Ma, G.; Hou, C.; Zhang, H. Synthesis of fluorinated polyacrylic acrylate oligomer for the UV-curable coatings. J. Coat. Technol. Res. 2019, 16, 681–688. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kim, H.-J. UV-curable low surface energy fluorinated polycarbonate-based polyurethane dispersion. J. Colloid Interface Sci. 2011, 362, 274–284. [Google Scholar] [CrossRef]
- Zhou, M. Low-Loss Polymeric Materials for Passive Waveguide Components in Fiber Optical Telecommunication. In Proceedings of the Asia-Pacific Optical and Wireless Communications Conference and Exhibit, Beijing, China, 12–15 November 2001; SPIE: Chuo City, Tokyo, 2001; Volume 4580. [Google Scholar]
- Jang, J.Y.; Do, J.Y. Synthesis and evaluation of thermoplastic polyurethanes as thermo-optic waveguide materials. Polym. J. 2014, 46, 349–354. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Lin, P.; Sun, F. Thermo-optic coefficients of polymers for optical waveguide applications. Polymer 2006, 47, 4893–4896. [Google Scholar] [CrossRef]
- Jančovičová, V.; Mikula, M.; Havlínová, B.; Jakubíková, Z. Influence of UV-curing conditions on polymerization kinetics and gloss of urethane acrylate coatings. Prog. Org. Coat. 2013, 76, 432–438. [Google Scholar] [CrossRef]
- Jeon, J.H.; Park, Y.G.; Lee, Y.H.; Lee, D.J.; Kim, H.-D. Preparation and Properties of UV-curable fluorinated polyurethane acrylates containing crosslinkable vinyl methacrylate for antifouling coatings. J. Appl. Polym. Sci. 2015, 132, 42168. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.; Chen, Y.; Cheng, Z.; Su, Y.; Hang, J.; Jin, L.; Li, N.; Shang, D.; Shi, L. Preparation and properties of crosslinked network coatings based on perfluoropolyether/poly(dimethyl siloxane)/acrylic polyols for marine fouling–release applications. J. Appl. Polym. Sci. 2015, 132, 41860. [Google Scholar] [CrossRef]
- June, Y.-G.; Jung, K.I.; Lee, D.G.; Jeong, S.; Lee, T.-H.; Park, Y.I.; Noh, S.M.; Jung, H.W. Influence of functional group content in hydroxyl-functionalized urethane methacrylate oligomers on the crosslinking features of clearcoats. J. Coat. Technol. Res. 2021, 18, 229–237. [Google Scholar] [CrossRef]
- Sung, S.; Kim, S.Y.; Lee, T.H.; Favaro, G.; Park, Y.I.; Lee, S.-H.; Ahn, J.B.; Noh, S.M.; Kim, J.C. Thermally reversible polymer networks for scratch resistance and scratch healing in automotive clear coats. Prog. Org. Coat. 2019, 127, 37–44. [Google Scholar] [CrossRef]
- Huang, S.; Guan, R.; Wang, S.; Xiao, M.; Han, D.; Sun, L.; Meng, Y. Polymers for high performance Li-S batteries: Material selection and structure design. Prog. Polym. Sci. 2019, 89, 19–60. [Google Scholar] [CrossRef]
- Li, S.; Lorandi, F.; Wang, H.; Liu, T.; Whitacre, J.F.; Matyjaszewski, K. Functional polymers for lithium metal batteries. Prog. Polym. Sci. 2021, 122, 101453. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liao, K.H.; Chou, N.K.; Wang, S.S.; Chu, S.H.; Hsieh, K.H. UV-curable low-surface-energy fluorinated poly(urethane-acrylate)s for biomedical applications. Eur. Polym. J. 2008, 44, 2927–2937. [Google Scholar] [CrossRef]
- Vitale, A.; Bongiovanni, R.; Ameduri, B. Fluorinated Oligomers and Polymers in Photopolymerization. Chem. Rev. 2015, 115, 8835–8866. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Risch, P.; Helmer, D.; Rapp, B.E. Highly Fluorinated Methacrylates for Optical 3D Printing of Microfluidic Devices. Micromachines 2018, 9, 115. [Google Scholar]
- McKeen, L.W. 19—Fluorinated Coatings; Technology, History, and Applications. In Introduction to Fluoropolymers, 2nd ed.; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, UK, 2013; pp. 339–367. [Google Scholar] [CrossRef]
- Ursino, C.; Di Nicolò, E.; Gabriele, B.; Criscuoli, A.; Figoli, A. Development of a novel perfluoropolyether (PFPE) hydrophobic/hydrophilic coated membranes for water treatment. J. Membr. Sci. 2019, 581, 58–71. [Google Scholar] [CrossRef]
- Gibbons, J.; Patterson, S.B.H.; Zhakeyev, A.; Vilela, F.; Marques-Hueso, J. Spectroscopic ellipsometric study datasets of the fluorinated polymers: Bifunctional urethane methacrylate perfluoropolyether (PFPE) and polyvinylidene fluoride (PVDF). Data Brief 2021, 39, 107461. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Di Meo, A.; Pollicino, A.; Priola, A.; Tonelli, C. New perfluoropolyether urethane methacrylates as surface modifiers: Effect of molecular weight and end group structure. React. Funct. Polym. 2008, 68, 189–200. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Medici, A.; Zompatori, A.; Garavaglia, S.; Tonelli, C. Perfluoropolyether polymers by UV curing: Design, synthesis and characterization. Polym. Int. 2012, 61, 65–73. [Google Scholar] [CrossRef]
- Dalle Vacche, S.; Forzano, S.; Vitale, A.; Corrado, M.; Bongiovanni, R. Glass lap joints with UV-cured adhesives: Use of a perfluoropolyether methacrylic resin in the presence of an acrylic silane coupling agent. Int. J. Adhes. Adhes. 2019, 92, 16–22. [Google Scholar] [CrossRef]
- Goralczyk, A.; Mayoussi, F.; Sanjaya, M.; Corredor, S.F.; Bhagwat, S.; Song, Q.; Schwenteck, S.; Warmbold, A.; Pezeshkpour, P.; Rapp, B.E. On-Chip Chemical Synthesis Using One-Step 3D Printed Polyperfluoropolyether. Chem. Ing. Tech. 2022, 94, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Masson, F.; Decker, C.; Andre, S.; Andrieu, X. UV-curable formulations for UV-transparent optical fiber coatings: I. Acrylic resins. Prog. Org. Coat. 2004, 49, 1–12. [Google Scholar] [CrossRef]
- Gleißner, U.; Hanemann, T.; Megnin, C.; Wieland, F. The influence of photo initiators on refractive index and glass transition temperature of optically and rheologically adjusted acrylate based polymers. Polym. Adv. Technol. 2016, 27, 1294–1300. [Google Scholar] [CrossRef]
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Kredel, J.; Schmitt, D.; Schäfer, J.-L.; Biesalski, M.; Gallei, M. Cross-Linking Strategies for Fluorine-Containing Polymer Coatings for Durable Resistant Water- and Oil-Repellency. Polymers 2021, 13, 723. [Google Scholar] [PubMed]
- Ghazali, S.K.; Adrus, N.; Majid, R.A.; Ali, F.; Jamaluddin, J. UV-LED as a New Emerging Tool for Curable Polyurethane Acrylate Hydrophobic Coating. Polymers 2021, 13, 487. [Google Scholar] [CrossRef]
- Dall Agnol, L.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review. Prog. Org. Coat. 2021, 154, 106156. [Google Scholar] [CrossRef]
- Startek, K.; Arabasz, S.; Bachmatiuk, A.; Lukowiak, A. Influence of fluoroalkyl chains on structural, morphological, and optical properties of silica-based coatings on flexible substrate. Opt. Mater. 2021, 121, 111524. [Google Scholar] [CrossRef]
- Gao, H.; Yorifuji, D.; Jiang, Z.; Ando, S. Thermal and optical properties of hyperbranched fluorinated polyimide/mesoporous SiO2 nanocomposites exhibiting high transparency and reduced thermo-optical coefficients. Polymer 2014, 55, 2848–2855. [Google Scholar] [CrossRef]
- Mumtaz, M.; Mahmood, M.A.; Khan, S.D.; Zia, M.A.; Ahmed, M.; Ahmad, I. Experimental measurement of temperature-dependent sellmeier coefficients and thermo-optic coefficients of polymers in terahertz spectral range. Opt. Mater. 2019, 91, 126–129. [Google Scholar] [CrossRef]
- Dreyer, C.; Köhler, M.; Schrader, S.; Yao, H. Chapter 9—Fluorinated thermosetting resins for photonic applications. In Fascinating Fluoropolymers and Their Applications; Ameduri, B., Fomin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–335. [Google Scholar] [CrossRef]
- Bertolotti, B.; Messaoudi, H.; Chikh, L.; Vancaeyzeele, C.; Alfonsi, S.; Fichet, O. Stability in alkaline aqueous electrolyte of air electrode protected with fluorinated interpenetrating polymer network membrane. J. Power Sources 2015, 274, 488–495. [Google Scholar]
- González Lazo, M.A.; Katrantzis, I.; Dalle Vacche, S.; Karasu, F.; Leterrier, Y. A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces. Materials 2016, 9, 738. [Google Scholar] [PubMed]
- Nourshargh, N.; Starr, E.M.; Fox, N.I.; Jones, S.G. Simple technique for measuring attenuation of integrated optical waveguides. Electron. Lett. 1985, 21, 818–820. [Google Scholar]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Pitois, C.; Vestberg, R.; Rodlert, M.; Malmström, E.; Hult, A.; Lindgren, M. Fluorinated dendritic polymers and dendrimers for waveguide applications. Opt. Mater. 2003, 21, 499–506. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, E. Optical Loss of Photochromic Polymer Films. In Proceedings of the Integrated Optoelectronics Devices, San Jose, CA, USA, 25–31 January 2003; SPIE: Chuo City, Tokyo, 2003; Volume 4991. [Google Scholar]
- Oh, M.-C.; Kim, K.-J.; Chu, W.-S.; Kim, J.-W.; Seo, J.-K.; Noh, Y.-O.; Lee, H.-J. Integrated Photonic Devices Incorporating Low-Loss Fluorinated Polymer Materials. Polymers 2011, 3, 975–997. [Google Scholar]


| Sample | UV LED Exposure Rate | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mm/s | 50 mm/s | 150 mm/s | 250 mm/s | 500 mm/s | ||||||||||||||||
| Tg (°C) | tan δmax | E’ (MPa) | ν * (mol/m3) | Tg (°C) | tan δmax | E’ (MPa) | ν (mol/m3) | Tg (°C) | tan δmax | E’ (MPa) | ν (mol/m3) | Tg (°C) | tan δmax | E’ (MPa) | ν (mol/m3) | Tg (°C) | tan δmax | E’ (MPa) | ν (mol/m3) | |
| PFPE-U(MA/A) | 59.50 | 0.2568 | 328.92 | 2714 | 48.80 | 0.3207 | 190.1 | 2110 | 41.50 | 0.3462 | 126.91 | 1573 | 37.18 | 0.36304 | 85.242 | 1020 | 30.58 | 0.37642 | 28.03 | 691 |
| PFPE-U(MA/A)-DM(DV)S | 57.20 | 0.2447 | 327.72 | 2638 | 54.10 | 0.2660 | 204.7 | 1690 | 39.60 | 0.2964 | 82.714 | 1123 | - | - | - | - | - | - | - | - |
| PFPE-U(MA/A)-TM(V)S | 41.50 | 0.2386 | 170.58 | 2539 | 44.20 | 0.2566 | 122.87 | 1670 | 34.50 | 0.3098 | 63.62 | 919 | - | - | - | - | - | - | - | - |
| Exposure Rate (mm/s) | nTE | dn/dT (∙10−4) | Β * (K1) (∙10−4) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PFPE-U(MA/A) | PFPE-U(MA/A)-DM(DV)S | PFPE-U(MA/A)-TM(V)S | PFPE-U(MA/A) | PFPE-U(MA/A)-DM(DV)S | PFPE-U(MA/A)-TM(V)S | PFPE-U(MA/A) | PFPE-U(MA/A)-DM(DV)S | PFPE-U(MA/A)-TM(V)S | |
| 5 | 1.38945 | 1.40139 | 1.39404 | −2.57 | −2.57 | −2.55 | 5.858 | 5.656 | 5.734 |
| 50 | 1.38925 | 1.39477 | 1.39225 | 5.861 | 5.766 | 5.764 | |||
| 150 | 1.38872 | 1.39124 | 1.39189 | 5.870 | 5.827 | 5.770 | |||
| 200 | 1.38787 | 1.38795 | 1.39104 | 5.885 | 5.884 | 5.785 | |||
| 250 | 1.38657 | 1.38547 | 1.38689 | 5.908 | 5.928 | 5.857 | |||
| Specimen | Optical Loss (dB/cm) | |
|---|---|---|
| @633 nm | @1547 nm | |
| PFPE-U(MA/A) | 1.4 | 1.9 |
| PFPE-U(MA/A)-DM(DV)S | 1.8 | 1.5 |
| PFPE-U(MA/A)-TM(V)S | 1.3 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreyer, C.; Motoc, D.L.; Koehler, M.; Goldenberg, L. UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization. Polymers 2023, 15, 2983. https://doi.org/10.3390/polym15142983
Dreyer C, Motoc DL, Koehler M, Goldenberg L. UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization. Polymers. 2023; 15(14):2983. https://doi.org/10.3390/polym15142983
Chicago/Turabian StyleDreyer, Christian, Dana Luca Motoc, Mathias Koehler, and Leonid Goldenberg. 2023. "UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization" Polymers 15, no. 14: 2983. https://doi.org/10.3390/polym15142983
APA StyleDreyer, C., Motoc, D. L., Koehler, M., & Goldenberg, L. (2023). UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization. Polymers, 15(14), 2983. https://doi.org/10.3390/polym15142983







