Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon
Abstract
1. Introduction
2. Materials and Methods
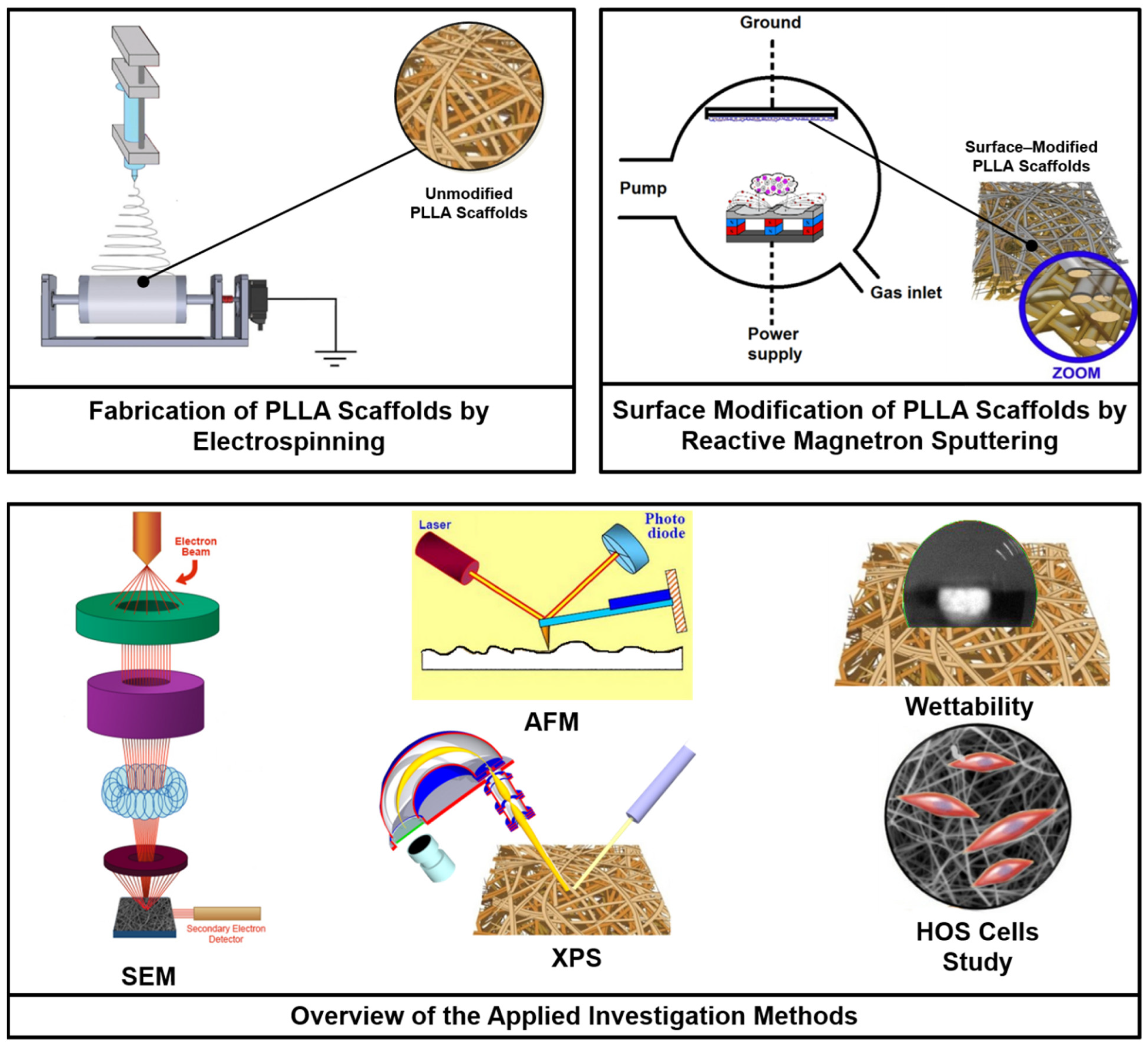
2.1. Electrospun Scaffold Fabrication
2.2. Scaffold Modification
2.3. Scaffold Properties Investigations
2.4. Statistical Evaluation
3. Results and Discussion
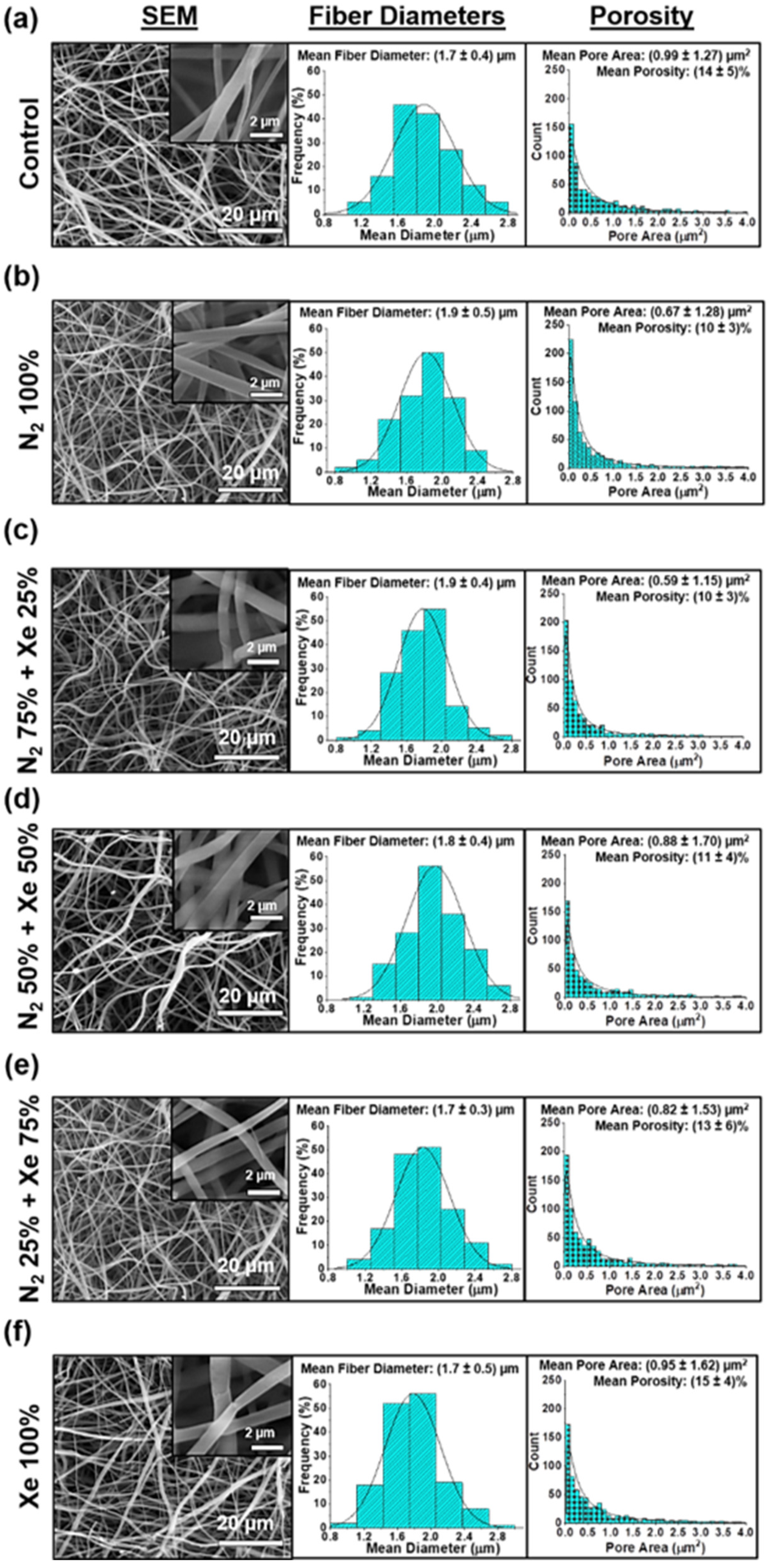
3.1. Surface Morphology
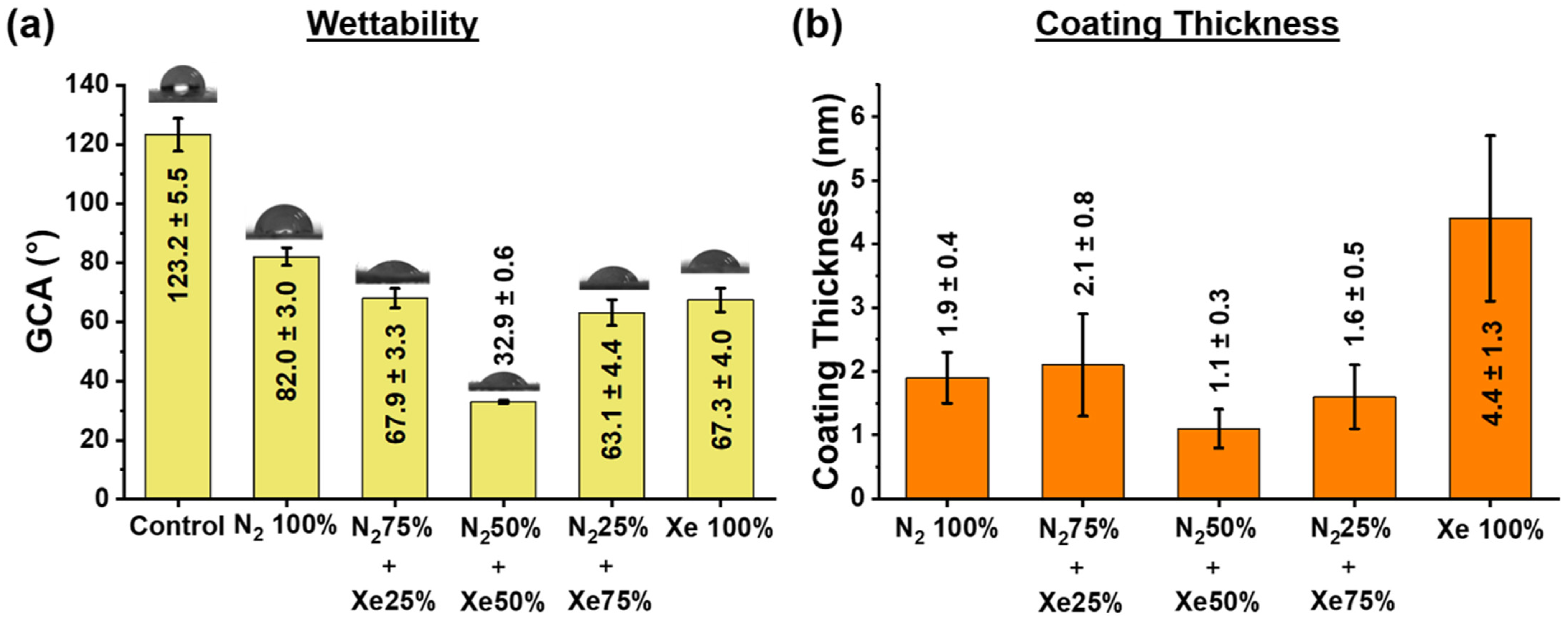
3.2. Results of the Wettability Measurement
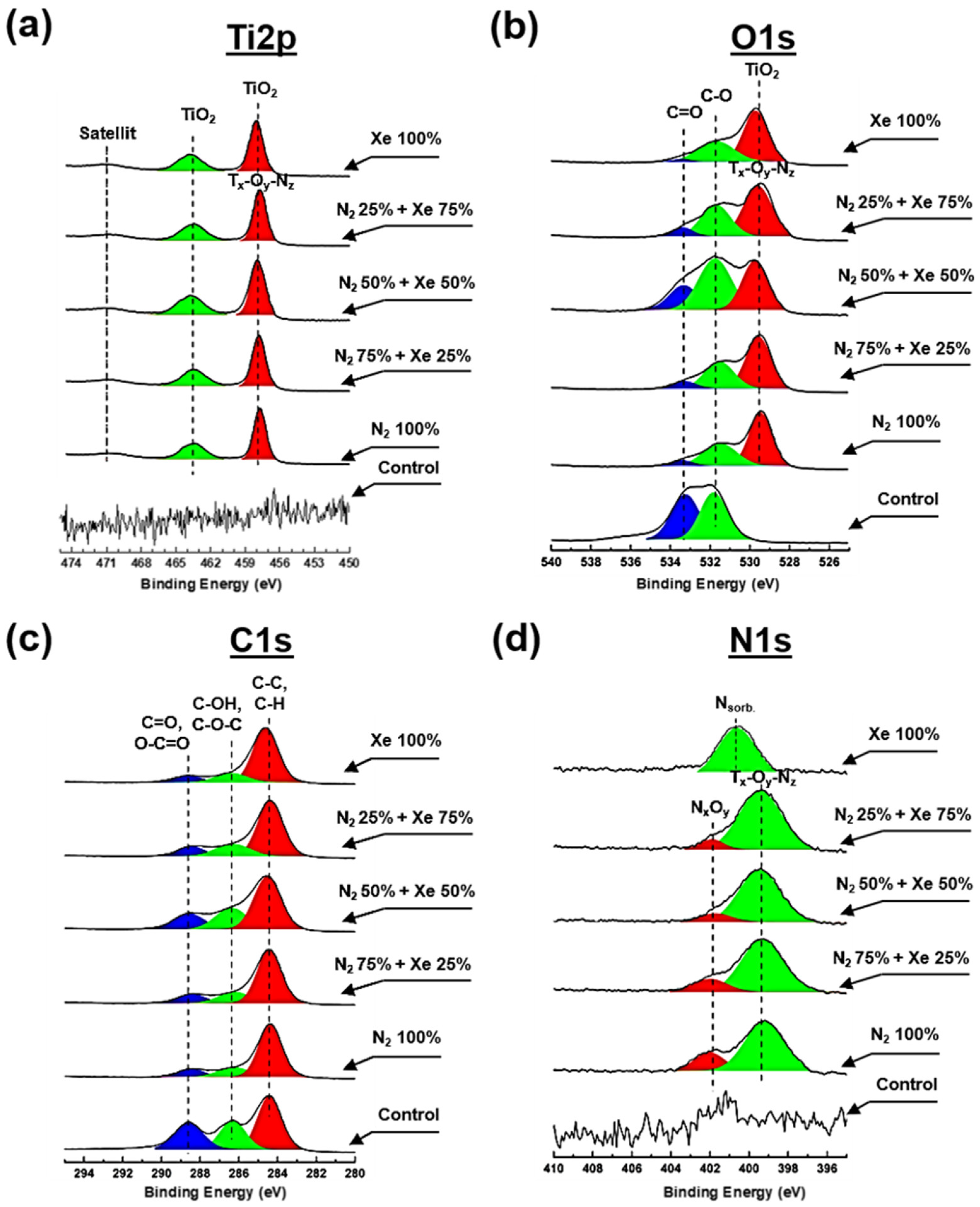
3.3. Chemical Composition of the PLLA Scaffolds
3.4. Roughness of the PLLA Scaffolds
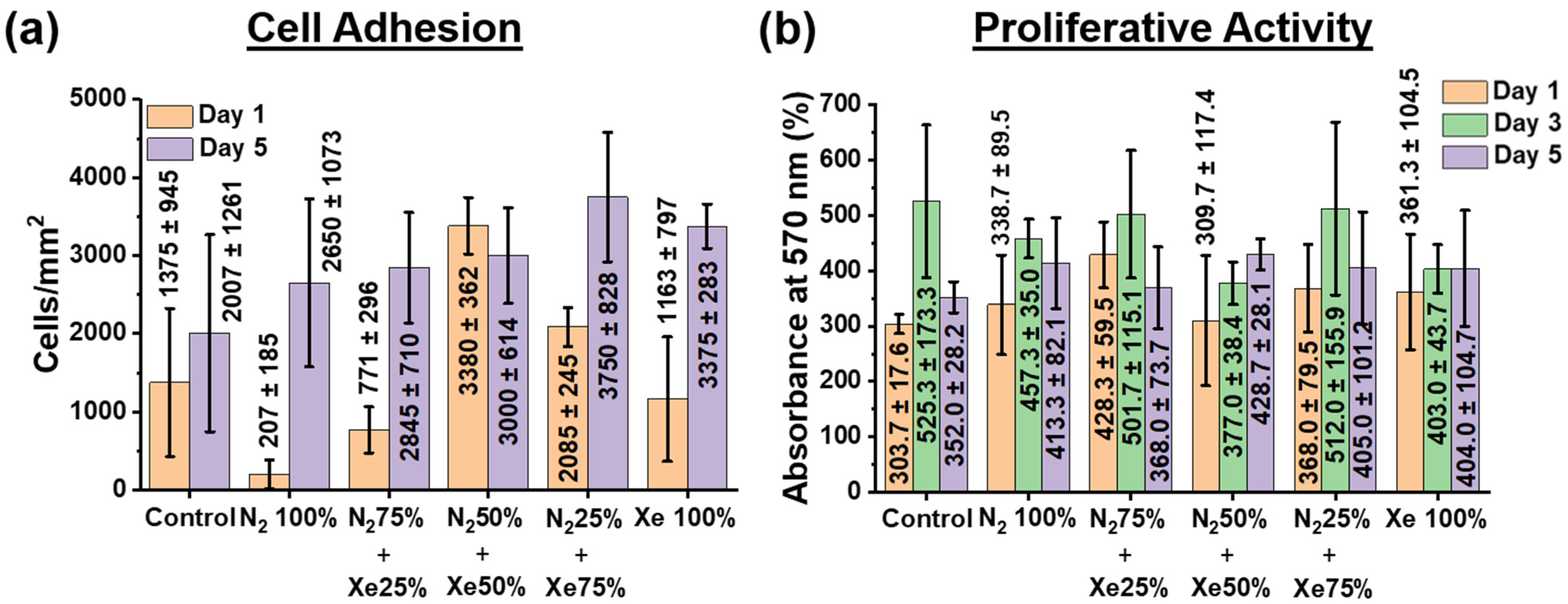
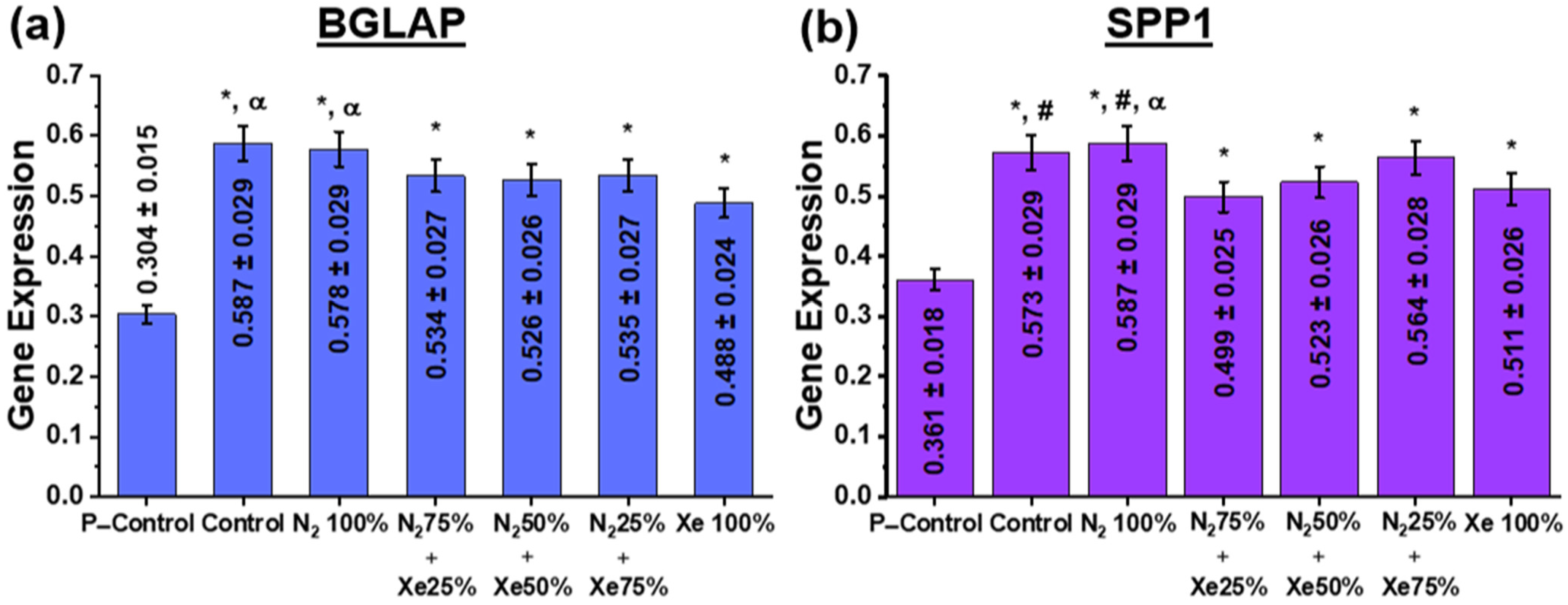
3.5. Cell Adhesion, Proliferative Activity and Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA Scaffold Surface for Medical Applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Tonino, P.A.L.; Pijls, N.H.J.; Collet, C.; Nammas, W.; Van der Heyden, J.; Romppanen, H.; Kervinen, K.; Airaksinen, J.K.E.; Sia, J.; Lalmand, J.; et al. Titanium-Nitride-Oxide–Coated Versus Everolimus-Eluting Stents in Acute Coronary Syndrome. JACC Cardiovasc. Interv. 2020, 13, 1697–1705. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Maryin, P.V.; Stankevich, K.S.; Goreninskii, S.I.; Kudryavtseva, V.L.; Mishanin, A.I.; Golovkin, A.S.; Malashicheva, A.B.; Zhukov, Y.M.; Anissimov, Y.G.; et al. Nitrogen-Doped Titanium Dioxide Thin Films Formation on the Surface of PLLA Electrospun Microfibers Scaffold by Reactive Magnetron Sputtering Method. Plasma Chem. Plasma Process. 2019, 39, 503–517. [Google Scholar] [CrossRef]
- Balazic, M.; Kopac, J.; Jackson, M.J.; Ahmed, W. Review: Titanium and titanium alloy applications in medicine. Int. J. Nano Biomater. 2007, 1, 3. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef]
- Khwansungnoen, P.; Chaiyakun, S.; Rattana, T. Room temperature sputtered titanium oxynitride thin films: The influence of oxygen addition. Thin Solid Films 2020, 711, 138269. [Google Scholar] [CrossRef]
- Bowes, M.; Bradley, J.W. Inert gas effects on the deposition rate of TiO2 during reactive HiPIMS. Surf. Coat. Technol. 2014, 250, 2–6. [Google Scholar] [CrossRef]
- Pana, I.; Braic, V.; Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Petrik, L.F.; Braic, M. In Vitro Corrosion of Titanium Nitride and Oxynitride-Based Biocompatible Coatings Deposited on Stainless Steel. Coatings 2020, 10, 710. [Google Scholar] [CrossRef]
- Clément, F.; Held, B.; Soulem, N. Polystyrene thin films treatment under DC pulsed discharges conditions in nitrogen-argon and oxygen-argon mixtures. Eur. Phys. J. Appl. Phys. 2002, 17, 119–130. [Google Scholar] [CrossRef]
- Petrov, I.; Ivanov, I.; Orlinov, V.; Sundgren, J.-E. Comparison of magnetron sputter deposition conditions in neon, argon, krypton, and xenon discharges. J. Vac. Sci. Technol. A 1993, 11, 2733–2741. [Google Scholar] [CrossRef]
- Fedotkin, A.Y.; Maryin, P.V.; Miklashevich, L.A.; Tverdokhlebov, S.I. Fabrication of NO-containing calcium phosphate coatings via direct introduction of argon-nitrogen-mixtures applied in reactive RF-magnetron sputtering. J. Phys. Conf. Ser. 2021, 1954, 012007. [Google Scholar] [CrossRef]
- Maryin, P.V.; Fedotkin, A.Y.; Bolbasov, E.N.; Kozelskaya, A.I.; Buldakov, M.A.; Evtina, A.A.; Cherdyntseva, N.V.; Rutkowski, S.; Tverdokhlebov, S.I. Surface modification of PLLA scaffolds via reactive magnetron sputtering in mixtures of nitrogen with noble gases for higher cell adhesion and proliferation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129464. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Antonova, L.V.; Stankevich, K.S.; Ashrafov, A.; Matveeva, V.G.; Velikanova, E.A.; Khodyrevskaya, Y.I.; Kudryavtseva, Y.A.; Anissimov, Y.G.; Tverdokhlebov, S.I.; et al. The use of magnetron sputtering for the deposition of thin titanium coatings on the surface of bioresorbable electrospun fibrous scaffolds for vascular tissue engineering: A pilot study. Appl. Surf. Sci. 2017, 398, 63–72. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Kazarian, S.G. Tip-enhanced Raman mapping with top-illumination AFM. Nanotechnology 2011, 22, 175701. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Bolbasov, E.N.; Maryin, P.V.; Stankevich, K.S.; Kozelskaya, A.I.; Shesterikov, E.V.; Khodyrevskaya, Y.I.; Nasonova, M.V.; Shishkova, D.K.; Kudryavtseva, Y.A.; Anissimov, Y.G.; et al. Surface modification of electrospun poly-(l-lactic) acid scaffolds by reactive magnetron sputtering. Colloids Surf. B Biointerfaces 2018, 162, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, K.; Rytlewski, P.; Malinowski, R.; Żenkiewicz, M. Comparison of some effects of modification of a polylactide surface layer by chemical, plasma, and laser methods. Appl. Surf. Sci. 2015, 346, 11–17. [Google Scholar] [CrossRef]
- Hirotsu, T.; Nakayama, K.; Tsujisaka, T.; Mas, A.; Schue, F. Plasma surface treatments of melt-extruded sheets of poly(L-lactic acid). Polym. Eng. Sci. 2002, 42, 299–306. [Google Scholar] [CrossRef]
- Semal, S.; Blake, T.D.; Geskin, V.; de Ruijter, M.J.; Castelein, G.; De Coninck, J. Influence of Surface Roughness on Wetting Dynamics. Langmuir 1999, 15, 8765–8770. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Tozer, B.A.; Craggs, J.D. Cross Sections for Ionization of the Inert Gases by Electron Impact†. J. Electron. Control 1960, 8, 103–109. [Google Scholar] [CrossRef]
- Charles, M.W. Gas gain measurements in proportional counters. J. Phys. E 1972, 5, 95–100. [Google Scholar] [CrossRef]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Low voltage environmentally friendly plasma electrolytic oxidation process for titanium alloys. Sci. Rep. 2022, 12, 6037. [Google Scholar] [CrossRef]
- Okada, K.; Uozumi, T.; Kotani, A. Charge-Transfer Satellites in Ti 2p XAS and XPS of Ti Compounds. Jpn. J. Appl. Phys. 1993, 32, 113. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Auger, M.A.; Sánchez, O.; Albella, J.M. Chemical stability of TiN, TiAlN and AlN layers in aggressive SO2 environments. Surf. Interface Anal. 2005, 37, 1082–1091. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Pellegrin, E.; Šics, I.; Reyes-Herrera, J.; Perez Sempere, C.; Lopez Alcolea, J.J.; Langlois, M.; Fernandez Rodriguez, J.; Carlino, V. Characterization, optimization and surface physics aspects of in situ plasma mirror cleaning. J. Synchrotron Radiat. 2014, 21, 300–314. [Google Scholar] [CrossRef]
- Renò, F.; D’Angelo, D.; Gottardi, G.; Rizzi, M.; Aragno, D.; Piacenza, G.; Cartasegna, F.; Biasizzo, M.; Trotta, F.; Cannas, M. Atmospheric Pressure Plasma Surface Modification of Poly(D,L-lactic acid) Increases Fibroblast, Osteoblast and Keratinocyte Adhesion and Proliferation. Plasma Process. Polym. 2012, 9, 491–502. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Goralski, E.G.; Walck, S.D.; Yates, J.T. Photochemical Activity of Nitrogen-Doped Rutile TiO2(110) in Visible Light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, L.; Zhang, M.; Huang, Q.; Cui, C.; Li, X.; Wang, L.; Li, L.; Yang, C.; Li, Y. Embedding α-MnSe nanodots in nitrogen-doped electrospinning carbon nanofibers to enhanced storage properties of lithium-ion batteries. J. Alloys Compd. 2019, 797, 826–833. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Pinheiro, L.M.P.; Nascente, P.A.P.; Ferreira, M.J.; Duek, E.A.R. Plasma surface treatments of poly(l-lactic acid) (PLLA) and poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV). Mater. Sci. Eng. C 2009, 29, 806–813. [Google Scholar] [CrossRef]
- Fraxedas, J.; Schütte, M.; Sauthier, G.; Tallarida, M.; Ferrer, S.; Carlino, V.; Pellegrin, E. In situ XPS analysis of the electronic structure of silicon and titanium thin films exposed to low-pressure inductively-coupled RF plasma. Appl. Surf. Sci. 2021, 542, 148684. [Google Scholar] [CrossRef]
- Braz, D.C.; Alves Junior, C.; de Vitoriano, O.J.; Rocha, H.A.; Biscaia, S.M.P.; Franco, C.R.C.; de Moura, C.E.B. Effect of the ratio of oxygen to nitrogen on the physicochemical and biocompatibility properties of titanium oxynitride. Mater. Chem. Phys. 2022, 278, 125508. [Google Scholar] [CrossRef]
- Fricke, K.; Koban, I.; Tresp, H.; Jablonowski, L.; Schröder, K.; Kramer, A.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T. Atmospheric Pressure Plasma: A High-Performance Tool for the Efficient Removal of Biofilms. PLoS ONE 2012, 7, e42539. [Google Scholar] [CrossRef] [PubMed]
- Rochford, E.T.J.; Subbiahdoss, G.; Moriarty, T.F.; Poulsson, A.H.C.; van der Mei, H.C.; Busscher, H.J.; Richards, R.G. An in vitro investigation of bacteria-osteoblast competition on oxygen plasma-modified PEEK. J. Biomed. Mater. Res. Part A 2014, 102, 4427–4434. [Google Scholar] [CrossRef]
- Lou, H.Q.; Axén, N.; Somekh, R.E.; Hutchings, I.M. Effect of deposition conditions on the characteristics of reactively sputtered titanium nitride films. Surf. Coat. Technol. 1997, 90, 123–127. [Google Scholar] [CrossRef]
- Lampin, M.; Warocquier-Clérout, R.; Legris, C.; Degrange, M.; Sigot-Luizard, M.F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Yokota, T.; Terai, T.; Kobayashi, T.; Meguro, T.; Iwaki, M. Cell adhesion to nitrogen-doped DLCs fabricated by plasma-based ion implantation and deposition method using toluene gas. Surf. Coat. Technol. 2007, 201, 8048–8051. [Google Scholar] [CrossRef]
- Finke, B.; Hempel, F.; Testrich, H.; Artemenko, A.; Rebl, H.; Kylián, O.; Meichsner, J.; Biederman, H.; Nebe, B.; Weltmann, K.-D.; et al. Plasma processes for cell-adhesive titanium surfaces based on nitrogen-containing coatings. Surf. Coat. Technol. 2011, 205, S520–S524. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Hikiji, H.; Shin, W.S.; Oida, S.; Takato, T.; Koizumi, T.; Toyo-oka, T. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. 1997, 410, 238–242. [Google Scholar] [CrossRef]
- Koyama, A.; Otsuka, E.; Inoue, A.; Hirose, S.; Hagiwara, H. Nitric oxide accelerates the ascorbic acid-induced osteoblastic differentiation of mouse stromal ST2 cells by stimulating the production of prostaglandin E2. Eur. J. Pharmacol. 2000, 391, 225–231. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Recek, N.; Mozetič, M.; Jaganjac, M.; Milkovič, L.; Žarkovic, N.; Vesel, A. Improved proliferation of human osteosarcoma cells on oxygen plasma treated polystyrene. Vacuum 2013, 98, 116–121. [Google Scholar] [CrossRef]
- Takitoh, T.; Kato, Y.; Nakasu, A.; Tadokoro, M.; Bessho, M.; Hirose, M.; Ohgushi, H.; Mori, H.; Hara, M. In vitro osteogenic differentiation of HOS cells on two types of collagen gels. J. Biosci. Bioeng. 2010, 110, 471–478. [Google Scholar] [CrossRef]
- Declercq, H.A.; Desmet, T.; Berneel, E.E.M.; Dubruel, P.; Cornelissen, M.J. Synergistic effect of surface modification and scaffold design of bioplotted 3-D poly-ε-caprolactone scaffolds in osteogenic tissue engineering. Acta Biomater. 2013, 9, 7699–7708. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Riancho, J.A.; Salas, E.; Zarrabeitia, M.T.; Olmos, J.M.; Amado, J.A.; Fernández-Luna, J.L.; González-Macías, J. Expression and functional role of nitric oxide synthase in osteoblast-like cells. J. Bone Miner. Res. 1995, 10, 439–446. [Google Scholar] [CrossRef]
- Löwik, C.W.; Nibbering, P.H.; van de Ruit, M.; Papapoulos, S.E. Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J. Clin. Investig. 1994, 93, 1465–1472. [Google Scholar] [CrossRef]
- Ralston, S.H.; Todd, D.; Helfrich, M.; Benjamin, N.; Grabowski, P.S. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology 1994, 135, 330–336. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Elkhooly, T.A.; Liu, Y.; Wu, H.; Feng, Q.; Liu, L.; Fang, Y.; Zhu, W.; Hu, T. Effects of titanium surface roughness on the mediation of osteogenesis via modulating the immune response of macrophages. Biomed. Mater. 2018, 13, 045013. [Google Scholar] [CrossRef]
- Takebe, J.; Champagne, C.M.; Offenbacher, S.; Ishibashi, K.; Cooper, L.F. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A.1 macrophage cell line. J. Biomed. Mater. Res. 2003, 64A, 207–216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maryin, P.V.; Tran, T.-H.; Frolova, A.A.; Buldakov, M.A.; Choinzonov, E.L.; Kozelskaya, A.I.; Rutkowski, S.; Tverdokhlebov, S.I. Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon. Polymers 2023, 15, 2969. https://doi.org/10.3390/polym15132969
Maryin PV, Tran T-H, Frolova AA, Buldakov MA, Choinzonov EL, Kozelskaya AI, Rutkowski S, Tverdokhlebov SI. Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon. Polymers. 2023; 15(13):2969. https://doi.org/10.3390/polym15132969
Chicago/Turabian StyleMaryin, Pavel V., Tuan-Hoang Tran, Anastasia A. Frolova, Mikhail A. Buldakov, Evgeny L. Choinzonov, Anna I. Kozelskaya, Sven Rutkowski, and Sergei I. Tverdokhlebov. 2023. "Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon" Polymers 15, no. 13: 2969. https://doi.org/10.3390/polym15132969
APA StyleMaryin, P. V., Tran, T.-H., Frolova, A. A., Buldakov, M. A., Choinzonov, E. L., Kozelskaya, A. I., Rutkowski, S., & Tverdokhlebov, S. I. (2023). Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon. Polymers, 15(13), 2969. https://doi.org/10.3390/polym15132969