Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
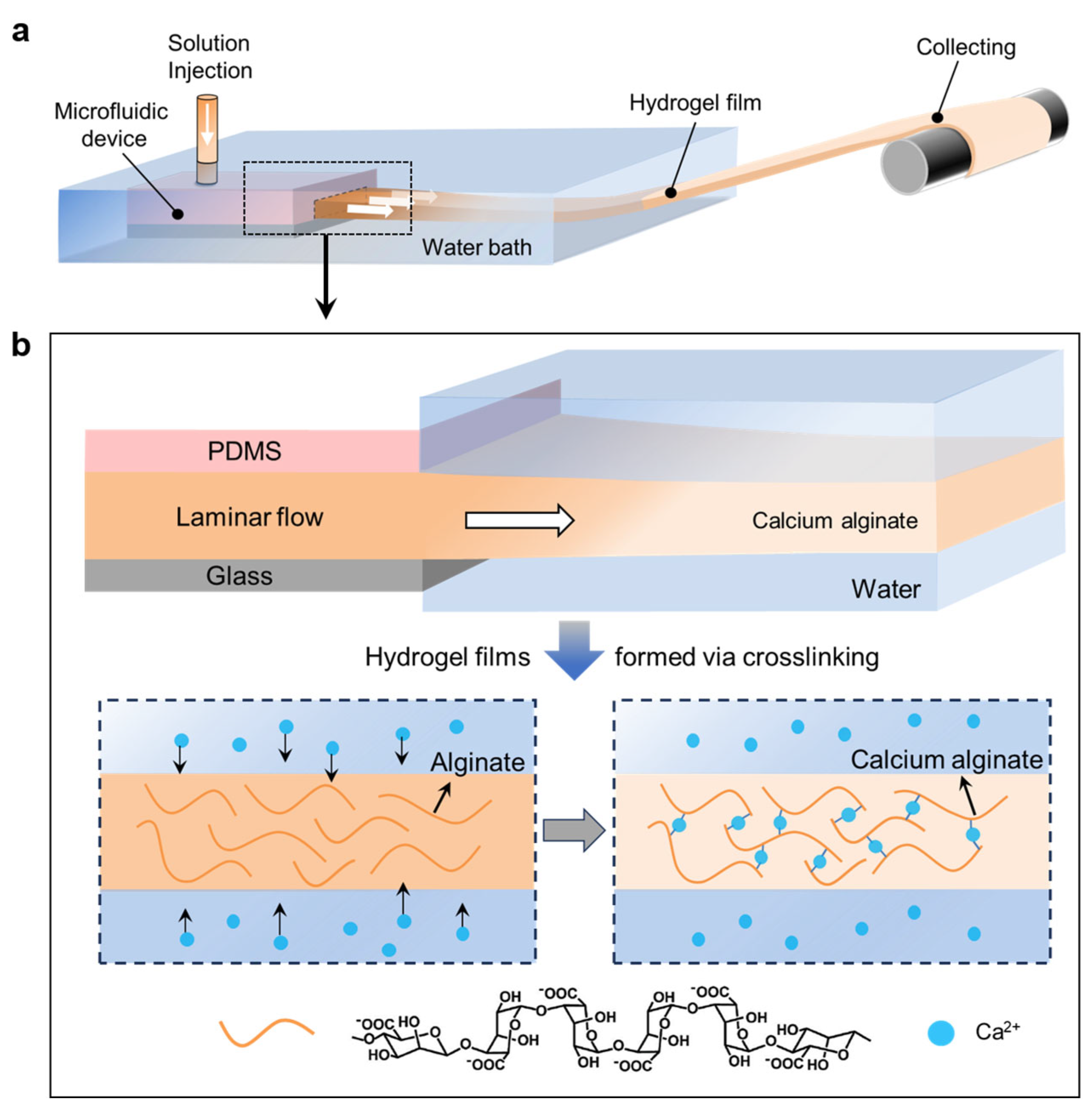
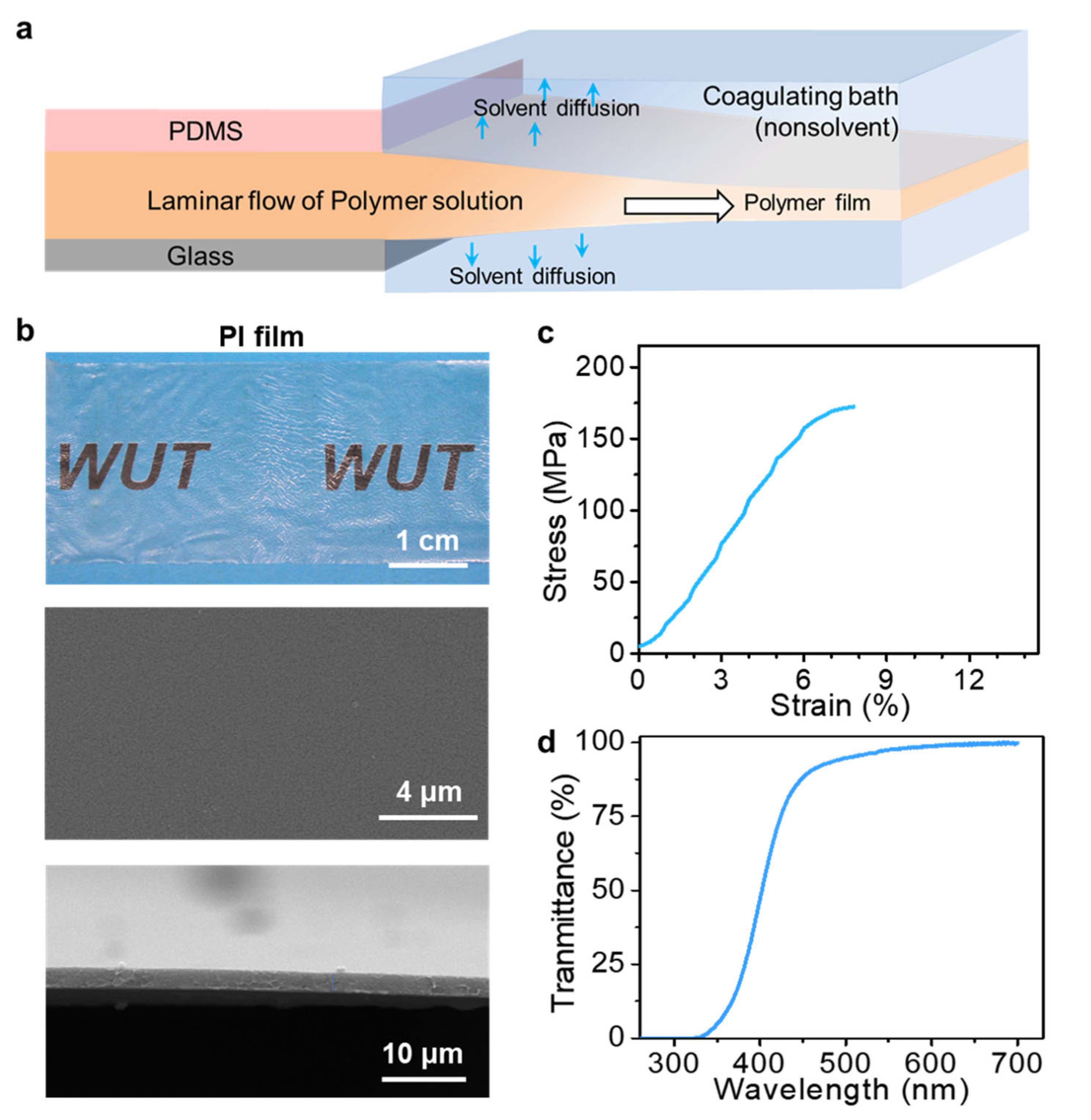
2.2. Preparation
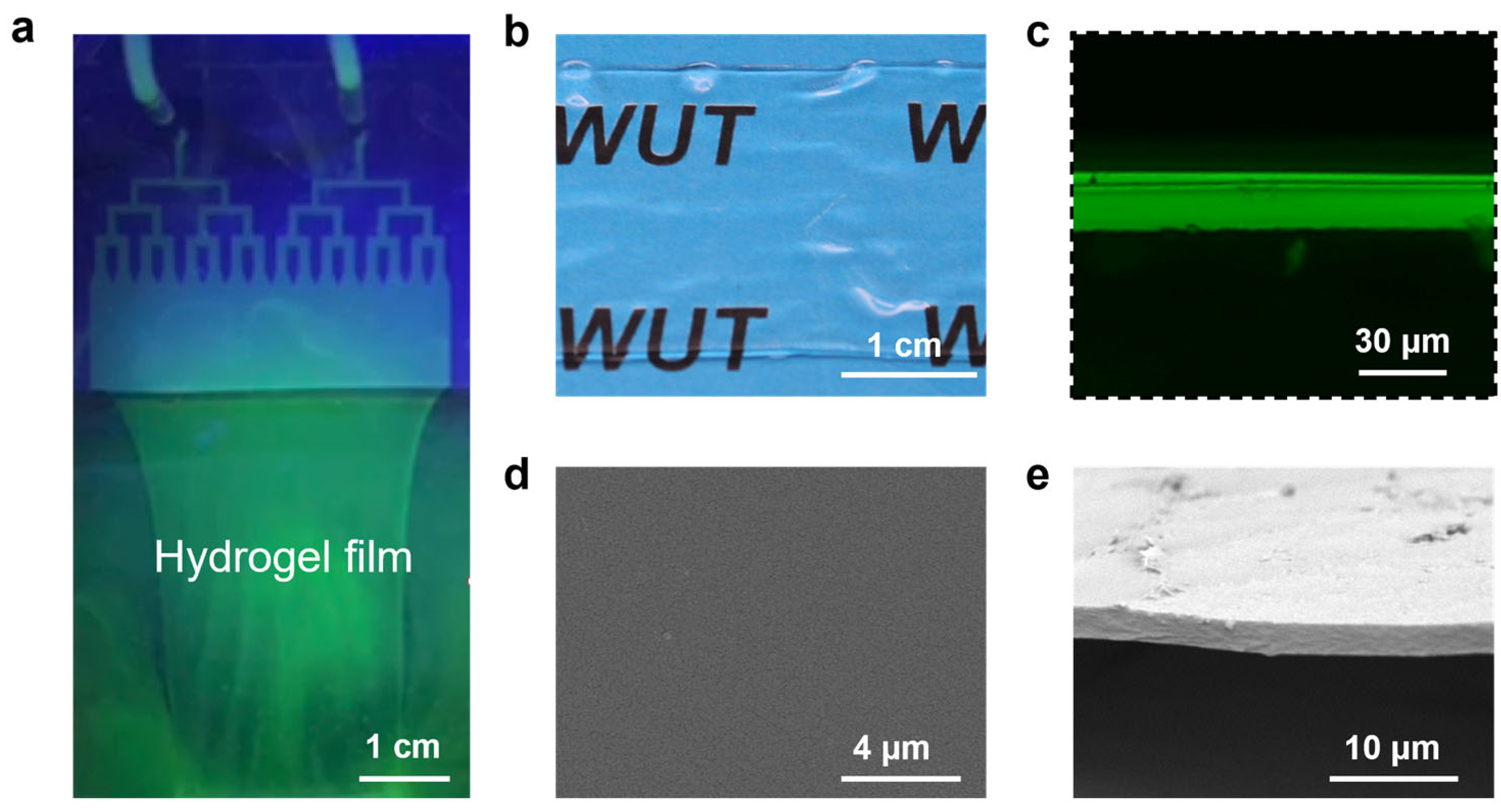
2.3. Instruments and Characterization
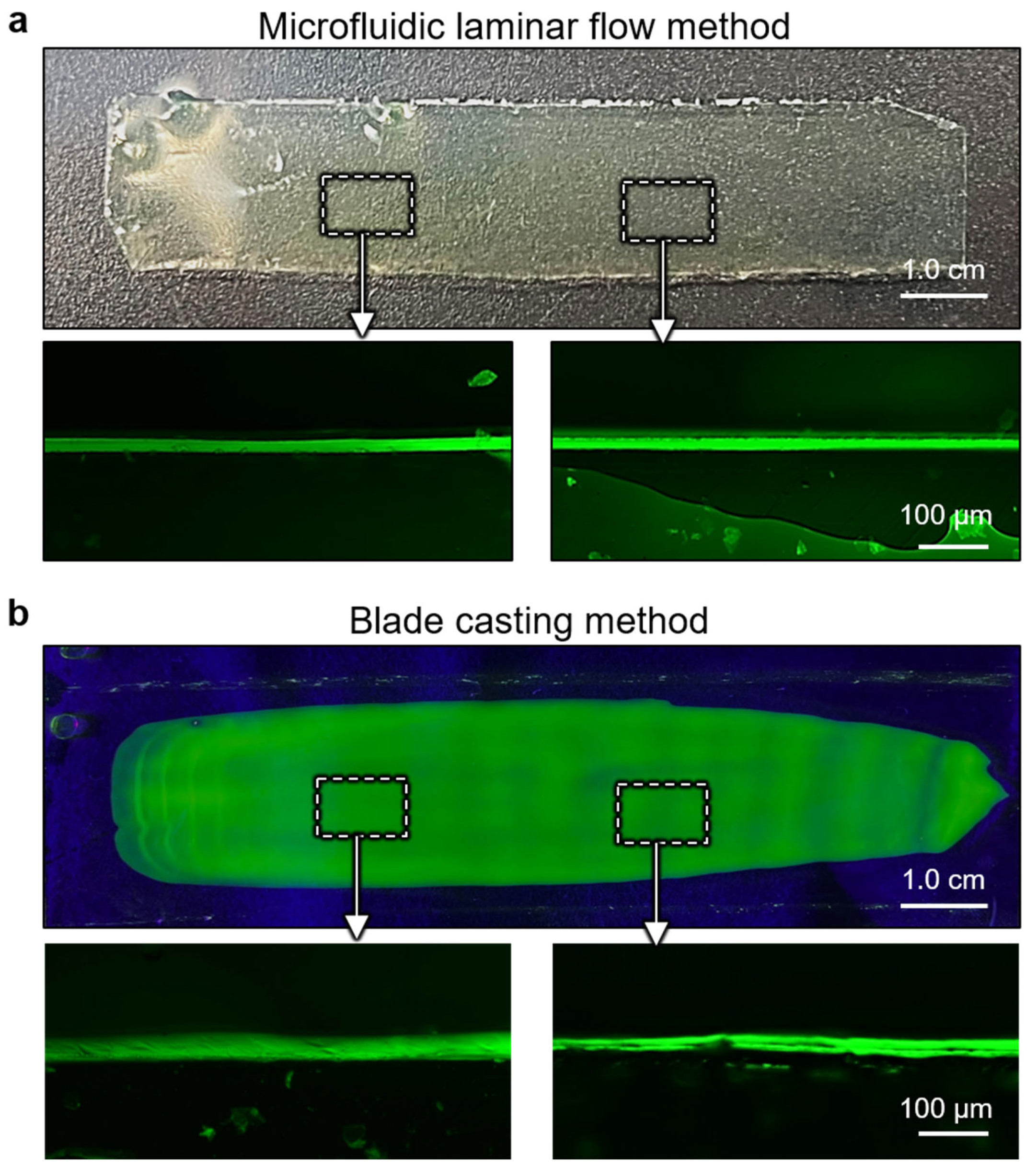
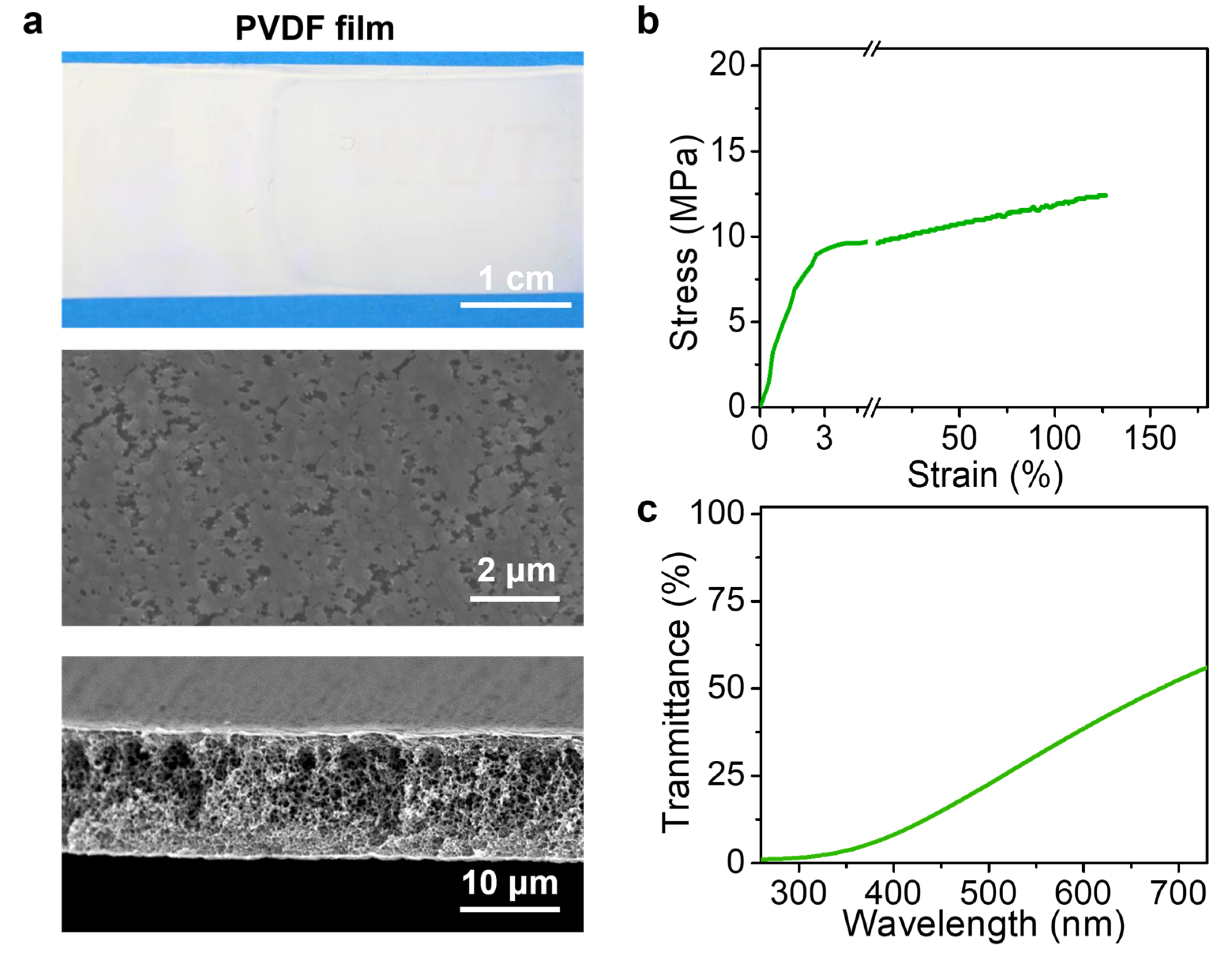
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, S.T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An overview of the development of flexible sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Jung, D.; Lim, C.; Shim, H.J.; Kim, Y.; Park, C.; Jung, J.; Han, S.I.; Sunwoo, S.-H.; Cho, K.W.; Cha, G.D.; et al. Highly conductive and elastic nanomembrane for skin electronics. Science 2021, 373, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Jin, J.; Bae, B.S. Optically transparent multiscale composite films for flexible and wearable electronics. Adv. Mater. 2020, 32, 1907143. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 2018, 30, 1706846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ming, T.; Goulamaly, M.M.; Yao, H.; Nezich, D.; Hempel, M.; Hofmann, M.; Kong, J. Enhancing the sensitivity of percolative graphene films for flexible and transparent pressure sensor arrays. Adv. Funct. Mater. 2016, 26, 5061–5067. [Google Scholar] [CrossRef]
- Jang, J.; Kim, D.W.; Lee, J.H.; Choi, C.; Go, M.; Kim, J.K.; Jeong, U. Triboelectric UV patterning for wearable one-terminal tactile sensor array to perceive dynamic contact motions. Nano Energy 2022, 98, 107320. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Huang, J.; Sha, L.; Lu, Z. Electrically conductive yet insulating aramid nanofiber Janus films via gel–gel assembling for flexible motion sensing and Joule heating. Chem. Eng. J. 2023, 457, 141021. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Yu, X.; Zhang, H.; Han, M. Multilayer flexible electronics: Manufacturing approaches and applications. Mater. Today Phys. 2022, 23, 100647. [Google Scholar] [CrossRef]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020, 32, 2003915. [Google Scholar] [CrossRef]
- Divya, M.; Pradhan, J.R.; Priyadarsini, S.S.; Dasgupta, S. High operation frequency and strain tolerance of fully printed oxide thin film transistors and circuits on PET substrates. Small 2022, 18, 2202891. [Google Scholar] [CrossRef]
- Heo, J.H.; Shin, D.H.; Jang, M.H.; Lee, M.L.; Kang, M.G.; Im, S.H. Highly flexible, high-performance perovskite solar cells with adhesion promoted AuCl3-doped graphene electrodes. J. Mater. Chem. A 2017, 5, 21146–21152. [Google Scholar] [CrossRef]
- Li, W.; Song, X.; Zhao, X.; Zhang, X.; Chen, R.; Zhang, X.; Jiang, C.; He, J.; Xiao, X. Design of wafer-scale uniform Au nanotip array by ion irradiation for enhanced single conductive filament resistive switching. Nano Energy 2020, 67, 104213. [Google Scholar] [CrossRef]
- Shah, V.; Wang, B.; Li, K. High-performance PVDF membranes prepared by the combined crystallisation and diffusion (CCD) method using a dual-casting technique: A breakthrough for water treatment applications. Energ. Environ. Sci. 2021, 14, 5491–5500. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Shi, J.; Li, Y.; You, J.; Bian, F. Crystallization-templated high-performance PVDF separator used in lithium-ion batteries. J. Membr. Sci. 2023, 670, 121359. [Google Scholar] [CrossRef]
- Zou, D.; Lee, Y.M. Design strategy of poly(vinylidene fluoride) membranes for water treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, S.; Lee, T.W.; Jo, J.; Chae, H.Y.; Choi, K.; Kim, J.J.; Lee, J.H.; Yang, C.; Baik, J.M. High-output triboelectric nanogenerator based on dual inductive and resonance effects-controlled highly sransparent polyimide for self-powered sensor network systems. Adv. Energy Mater. 2019, 9, 1901987. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Y.; Wu, Z.; Zhang, Y.; Huang, C.; Cheng, S.; You, Y.; Zhang, P.; Chen, W.; Mao, L.; et al. Mixed matrix membrane with penetrating subnanochannels: A versatile nanofluidic platform for selective metal ion conduction. Angew. Chem. Int. Ed. 2023, 62, e202215906. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry electrodes for human bioelectrical signal monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Zhong, W.; Ke, Y.; Lu, J.; Jia, K.; Ding, X.; Jiang, H.; Li, M.; Wang, D. Isopropanol-regulated adhesion-controllable conductive gels for robust bioelectric signal monitoring and flexible underwater robots. Chem. Eng. J. 2023, 460, 141746. [Google Scholar] [CrossRef]
- Wang, L.; Hao, X.; Gao, Z.; Yang, Z.; Long, Y.; Luo, M.; Guan, J. Artificial nanomotors: Fabrication, iocomotion characterization, motion manipulation, and biomedical applications. Interdiscip. Mater. 2022, 1, 256–280. [Google Scholar] [CrossRef]
- Zhang, L.; Kan, X.; Huang, T.; Lao, J.; Luo, K.; Gao, J.; Liu, X.; Sui, K.; Jiang, L. Electric field modulated water permeation through laminar Ti(3)C(2)T(x) MXene membrane. Water Res. 2022, 219, 118598. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and eater sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Dong, W.-X.; Deng, Y.; Chen, L.-F.; Zhao, C.-F.; Zhang, C.-L.; Zhou, J.; Qu, Y.-F.; Li, Y.-S.; Li, D.-J.; et al. Biomass-based biomimetic-oriented Janus nanoarchitecture for efficient heavy-metal enrichment and interfacial solar water sanitation. Interdiscip. Mater. 2022, 1, 537–547. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, C.; Zeng, Z.; Pan, F.; Wan, F.; Lei, L.; Nyström, G.; Fu, Z. Bioinspired cellulose-integrated MXene-based hydrogels for multifunctional sensing and electromagnetic interference shielding. Interdiscip. Mater. 2022, 1, 495–506. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Yu, T.; Zhang, J.; Zhang, X.; Zhang, H.; Zhao, K.; Wei, J. Plant-mediated ciosynthesis of iron nanoparticles-calcium alginate hydrogel membrane and its eminent performance in removal of Cr(VI). Chem. Eng. J. 2019, 378, 122120. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.J.; Zhao, J.C.; Guo, Y.; Zhu, P.; Wang, D.Y. Bio-based barium alginate film: Preparation, flame retardancy and thermal degradation behavior. Carbohydr. Polym. 2016, 139, 106–114. [Google Scholar] [CrossRef]
- Yi, J.; Nguyen, K.T.; Wang, W.; Yang, W.; Pan, M.; Lou, E.; Major, P.W.; Le, L.H.; Zeng, H. Polyacrylamide/alginate double-network tough hydrogels for intraoral ultrasound imaging. J. Colloid Interface Sci. 2020, 578, 598–607. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chen, F.; Gu, N. Drug delivery system based on nanobubbles. Interdiscip. Mater. 2022, 1, 471–494. [Google Scholar] [CrossRef]
- Lim, C.; Hong, Y.J.; Kim, J.H.; Lee, S.; Kim, D.-H. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Zhou, J.; Du, F.; Teng, C. Bioinspired, high-strength, and flexible MXene/Aramid fiber for electromagnetic interference shielding papers with joule heating performance. ACS Nano 2022, 16, 6700–6711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Anasori, B.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically crosslinked hydrogel film leads to integrated flexible supercapacitors with superior performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.L.; Deb, N.; Wolfe, R.M.W.; Lo, C.K.; Engmann, S.; Richter, L.J.; Reynolds, J.R. Simple transfer from spin coating to blade coating through processing aggregated solutions. J. Mater. Chem. A 2017, 5, 20687–20695. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Eddaoudi, M. Advanced fabrication method for the preparation of MOF thin films: Liquid-phase epitaxy approach meets spin coating method. ACS Appl. Mater. Interfaces 2016, 8, 20459–20464. [Google Scholar] [CrossRef]
- Fu, X.; Li, J.; Tang, C.; Xie, S.; Sun, X.; Wang, B.; Peng, H. Hydrogel cryo-microtomy continuously making soft electronic devices. Adv. Funct. Mater. 2021, 31, 2008355. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional tissue-adhesive cryogel wound dressing for rapid nonpressing surface hemorrhage and wound repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef]
- Cheng, S.; Lou, Z.; Zhang, L.; Guo, H.; Wang, Z.; Guo, C.; Fukuda, K.; Ma, S.; Wang, G.; Someya, T.; et al. Ultrathin hydrogel flms toward breathable skin-integrated electronics. Adv. Mater. 2023, 35, 2206793. [Google Scholar] [CrossRef]
- Smith Callahan, L.A.; Childers, E.P.; Bernard, S.L.; Weiner, S.D.; Becker, M.L. Maximizing phenotype constraint and extracellular matrix production in primary human chondrocytes using arginine-glycine-aspartate concentration gradient hydrogels. Acta Biomater. 2013, 9, 7420–7428. [Google Scholar] [CrossRef]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, X.; Wang, Z.J.; Pan, P.; Li, F.; Ling, D.; Wu, Z.L.; Zheng, Q. A facile approach to prepare tough and responsive ultrathin physical hydrogel films as artificial muscles. ACS Appl. Mater. Interfaces 2017, 9, 34349–34355. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Xu, Z.; Xia, Z.; Hu, X.; Kou, L.; Peng, L.; Wei, Y.; Gao, C. Wet-spun continuous graphene films. Chem. Mater. 2014, 26, 6786–6795. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, J.; Wang, B.; Qin, L. Microfluidics-based single-cell protrusion analysis for screening drugs targeting subcellular mitochondrial trafficking in cancer progression. Anal. Chem. 2020, 92, 3095–3102. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Yao, J.; Shao, N.; Zhang, K.; Zhou, Y.; Zu, Y.; Wang, B.; Qin, L. High-throughput isolation of cell protrusions with single-cell precision for profiling subcellular gene expression. Angew. Chem. Int. Ed. 2019, 58, 13700–13705. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, D.; Zhao, Z.; Wei, Y.; Xue, J.; Wang, H. Ultrathin and ultrastrong kevlar aramid nanofiber membranes for highly stable osmotic energy conversion. Adv. Sci. 2022, 9, 2202869. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Y.; Cai, L.; Wang, Y.; Shi, K.; Shang, L.; Pan, J.; Zhao, Y. Microfluidics for flexible electronics. Mater. Today 2021, 44, 105–135. [Google Scholar] [CrossRef]
- Wang, B.; Prinsen, P.; Wang, H.; Bai, Z.; Wang, H.; Luque, R.; Xuan, J. Macroporous materials: Microfluidic fabrication, functionalization and applications. Chem. Soc. Rev. 2017, 46, 855–914. [Google Scholar] [CrossRef]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Lyu, Z.; Koh, J.J.; Lim, G.J.H.; Zhang, D.; Xiong, T.; Zhang, L.; Liu, S.; Duan, J.; Ding, J.; Wang, J.; et al. Direct Ink writing of programmable functional silicone-based composites for 4D printing applications. Interdiscip. Mater. 2022, 1, 507–516. [Google Scholar] [CrossRef]
- Dousti, S.; Allaire, P.; Dimond, T.; Cao, J. An extended reynold equation applicable to high reduced reynolds number operation of journal bearings. Tribol. Int. 2016, 102, 182–197. [Google Scholar] [CrossRef]
- Vasukiran, M.; Kishore, N.; Yadav, S. Critical reynolds numbers of shear-thinning fluids flow past unbounded spheres. Powder Technol. 2018, 339, 747–759. [Google Scholar] [CrossRef]
- Schulte, T.H.; Bardell, R.L.; Weigl, B.H. Microfluidic technologies in clinical diagnostics. Clin. Chim. Acta 2002, 321, 1–10. [Google Scholar] [CrossRef]
- Serra, T.; Barcelona, A.; Soler, M.; Colomer, J. Daphnia magna filtration efficiency and mobility in laminar to turbulent flows. Sci. Total Environ. 2018, 621, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Fontes, S.R. Mass transfer in microfiltration with laminar and turbulent flow of macromolecular solutions. J. Membr. Sci. 2005, 249, 207–211. [Google Scholar] [CrossRef]
- Zhang, M.; Jing, Y.; Zhang, J.; Sheng, Z.; Hou, Y.; Xu, J.; Chen, B.; Liu, J.; Wang, M.; Hou, X. Performance prediction of magnetorheological fluid-based liquid gating membrane by kriging machine learning method. Interdiscip. Mater. 2022, 1, 157–169. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Lao, J.; Luo, K.; Liu, X.; Sui, K.; Gao, J.; Jiang, L. Reliable and low temperature actuation of water and oil slugs in Janus photothermal slippery yube. ACS Appl. Mater. Interfaces 2022, 14, 17968–17974. [Google Scholar] [CrossRef]
- Li, J.; He, J. Alginate-based films and membranes: Preparation, characterization and applications. Nanostruct. Polym. Membr. 2016, 1, 457–490. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Rob, V.; Appelqvist, I. Reexamining the egg-box model in calcium-alginate gels with x-ray diffraction. Biomacromolecules 2007, 9, 464–468. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT-Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Zhou, J.; Qi, S.; Yamauchi, Y.; Shi, R.; Fang, R.; Ishida, Y.; Wang, S.; Tomsia, A.P.; et al. Layered nanocomposites by shear-flow-induced alignment of nanosheets. Nature 2020, 580, 210–215. [Google Scholar] [CrossRef]




| Re | μ (mPa.s) | ρ (kg/m3) | v (m/s) | d (μm) |
|---|---|---|---|---|
| 0.00082 | 600 | 1.2 | 0.0046 | 90 |
| 0.00076 | 597 | 1.1 | 0.0046 | 90 |
| 0.00075 | 601 | 1.1 | 0.0046 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, X.; Huang, C.; Cheng, S.; Zhang, P.; Chen, W. Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness. Polymers 2023, 15, 2905. https://doi.org/10.3390/polym15132905
Ouyang X, Huang C, Cheng S, Zhang P, Chen W. Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness. Polymers. 2023; 15(13):2905. https://doi.org/10.3390/polym15132905
Chicago/Turabian StyleOuyang, Xiaozhi, Cheng Huang, Sha Cheng, Pengchao Zhang, and Wen Chen. 2023. "Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness" Polymers 15, no. 13: 2905. https://doi.org/10.3390/polym15132905
APA StyleOuyang, X., Huang, C., Cheng, S., Zhang, P., & Chen, W. (2023). Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness. Polymers, 15(13), 2905. https://doi.org/10.3390/polym15132905








