The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Lignin Samples
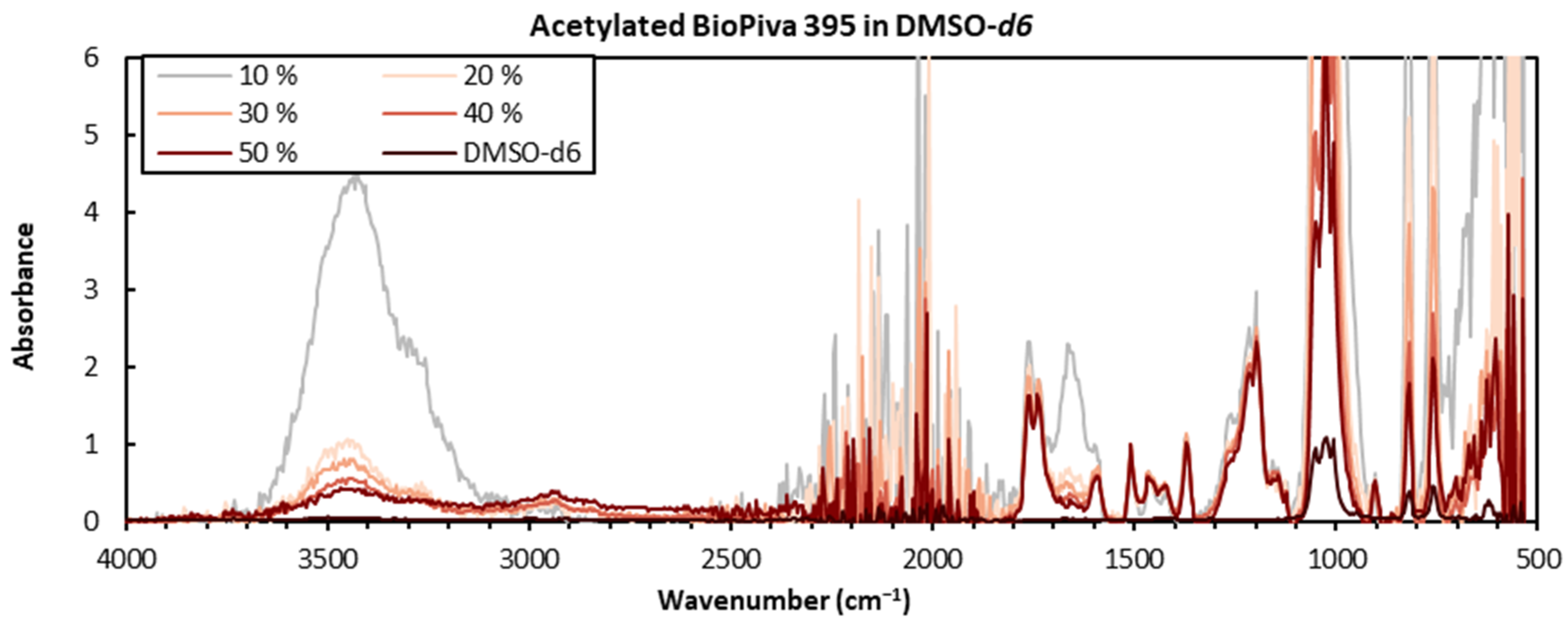
2.2. Acetylation of Lignin Samples
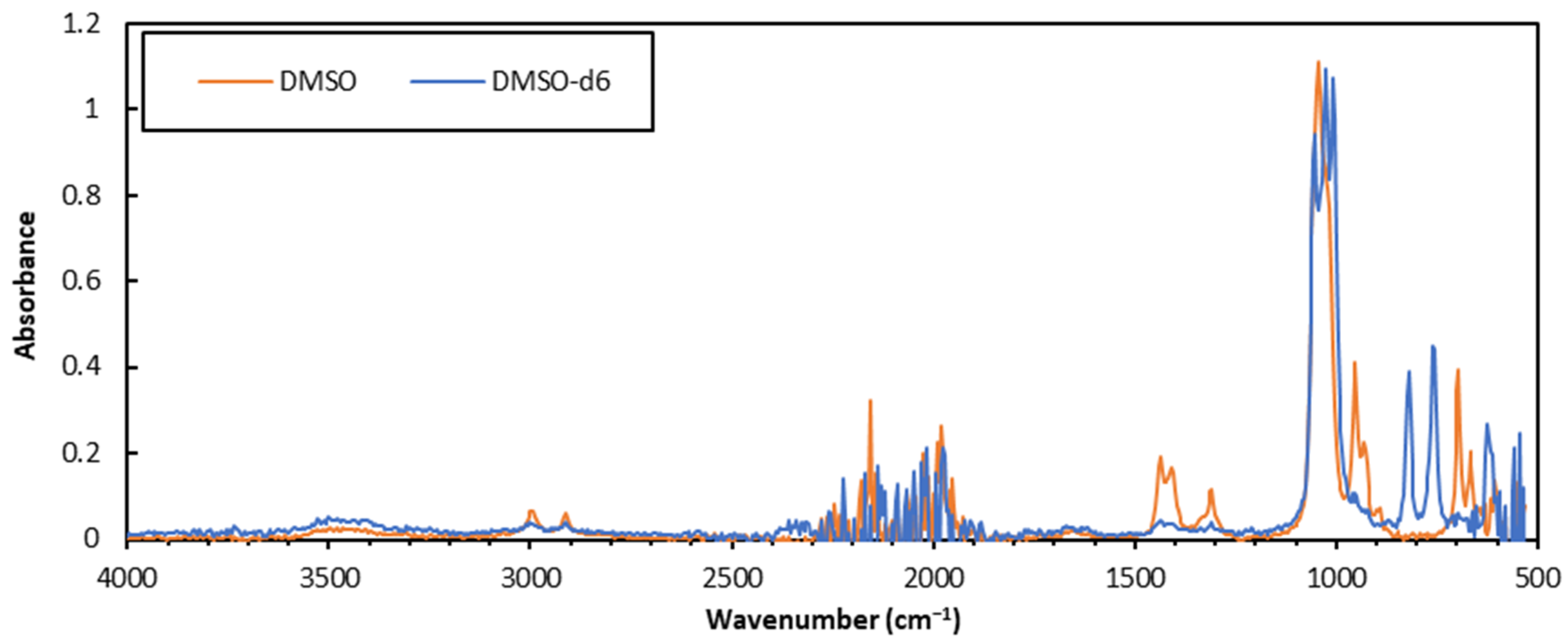
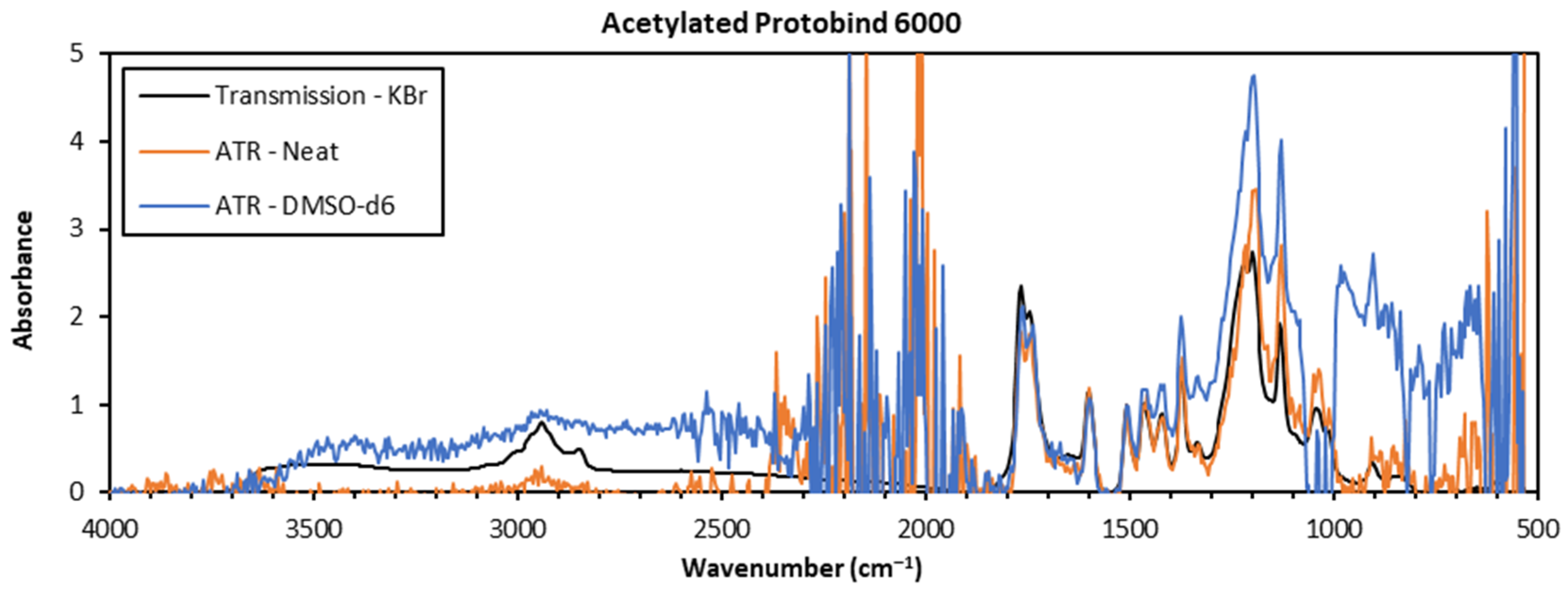
2.3. FTIR Sample Preparation
2.4. FTIR Spectroscopy
2.5. Total OH Content Determination Using the Consumed Acetic Acid Calculation Method
2.6. Non-Aqueous Potentiometric Titration
2.7. SEC Analyses
3. Results
3.1. Comparison of Sample Preparation Techniques
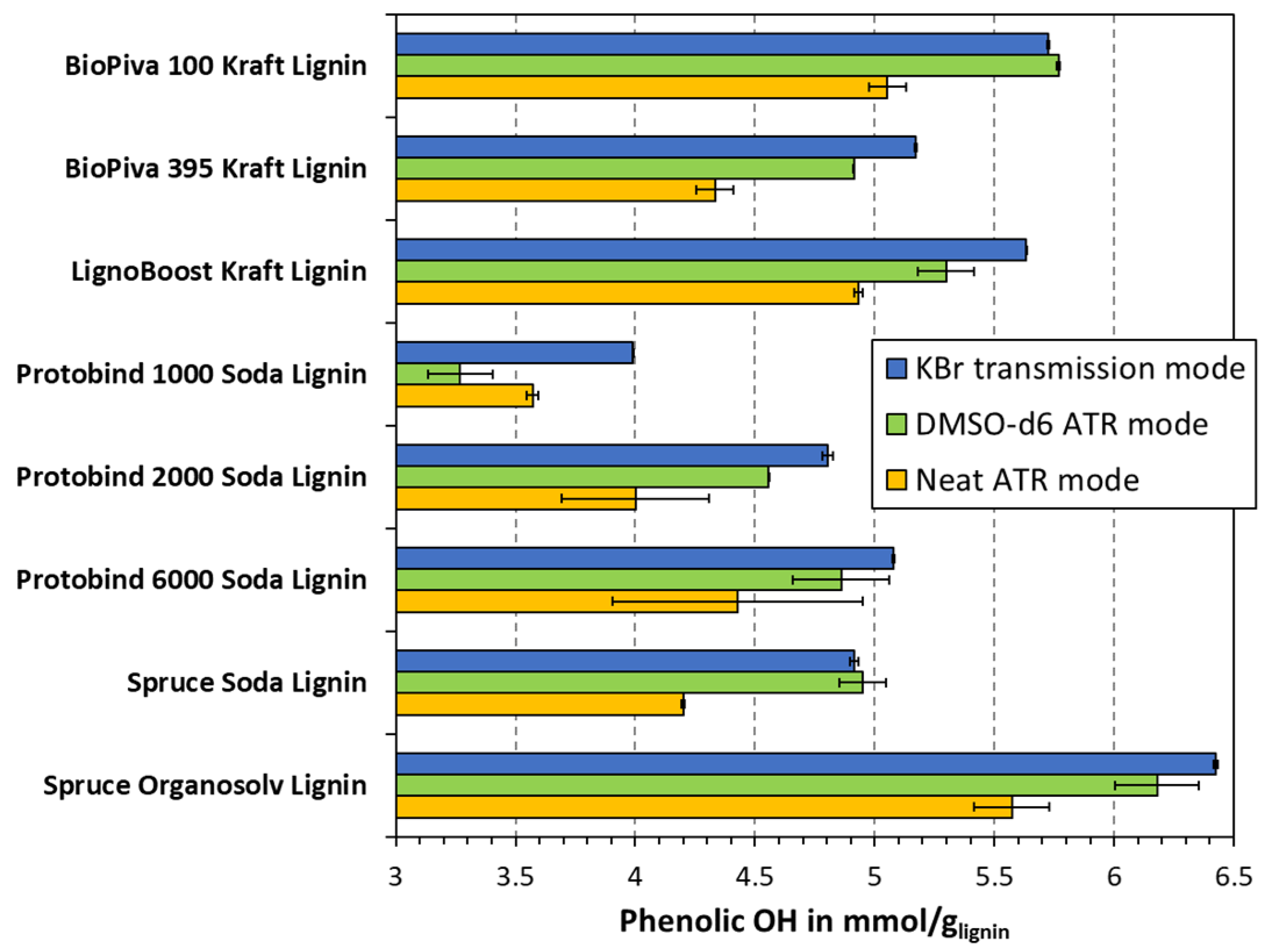
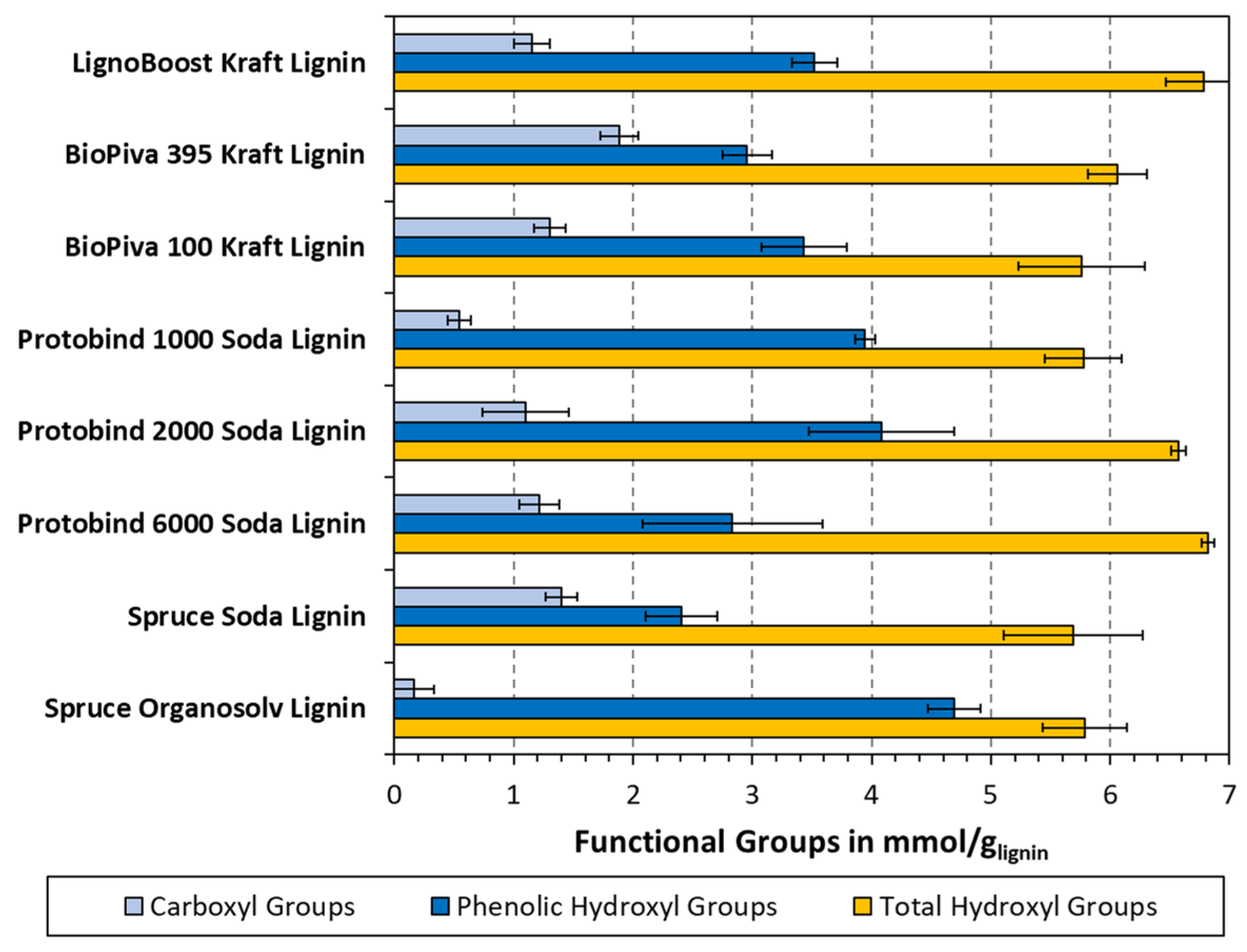
3.2. Phenolic OH Group Content of Lignin Samples in Dependence of FTIR Measuring Mode
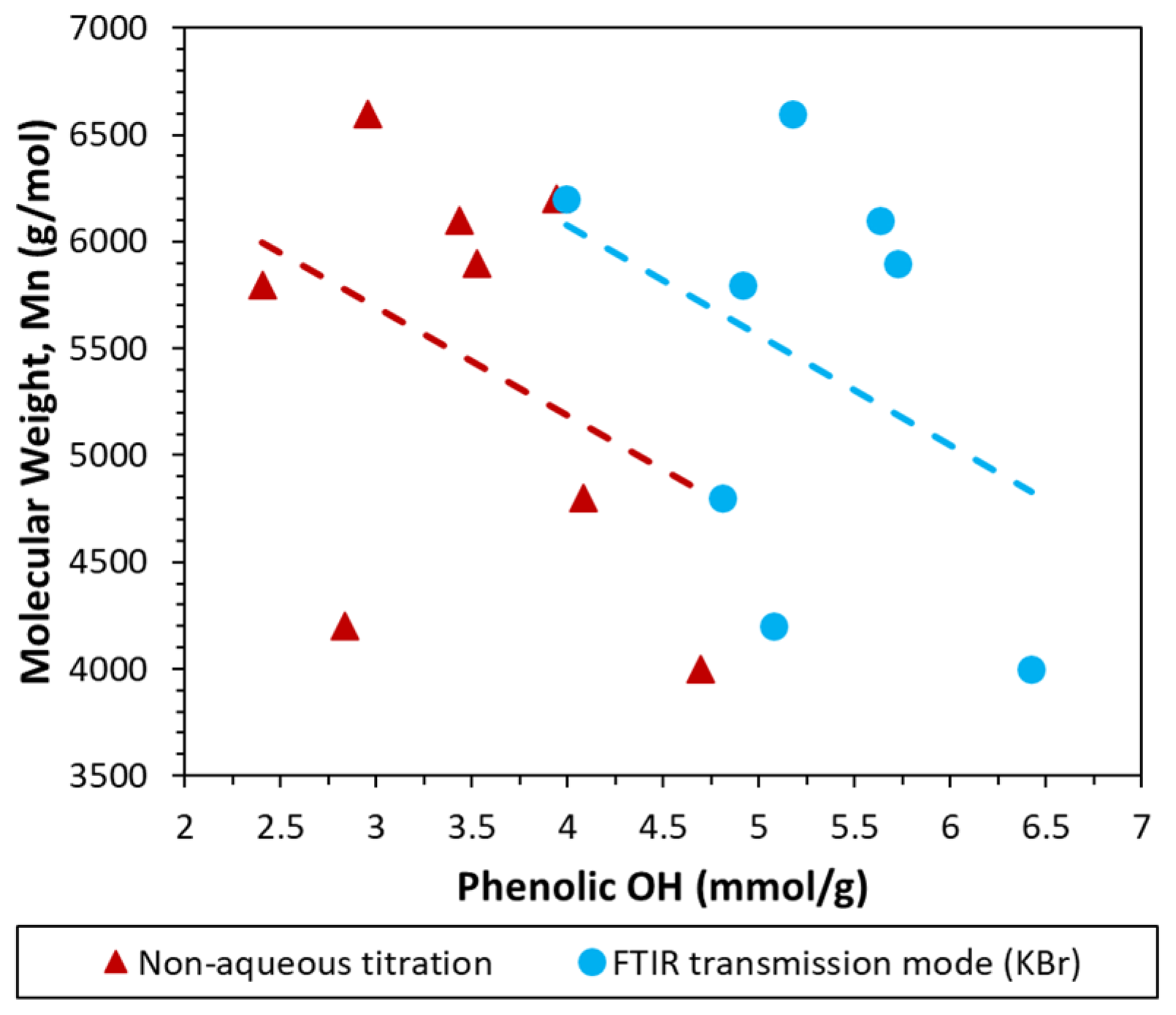
3.3. Comparison with Non-Aqueous Potentiometric Titration and Total OH
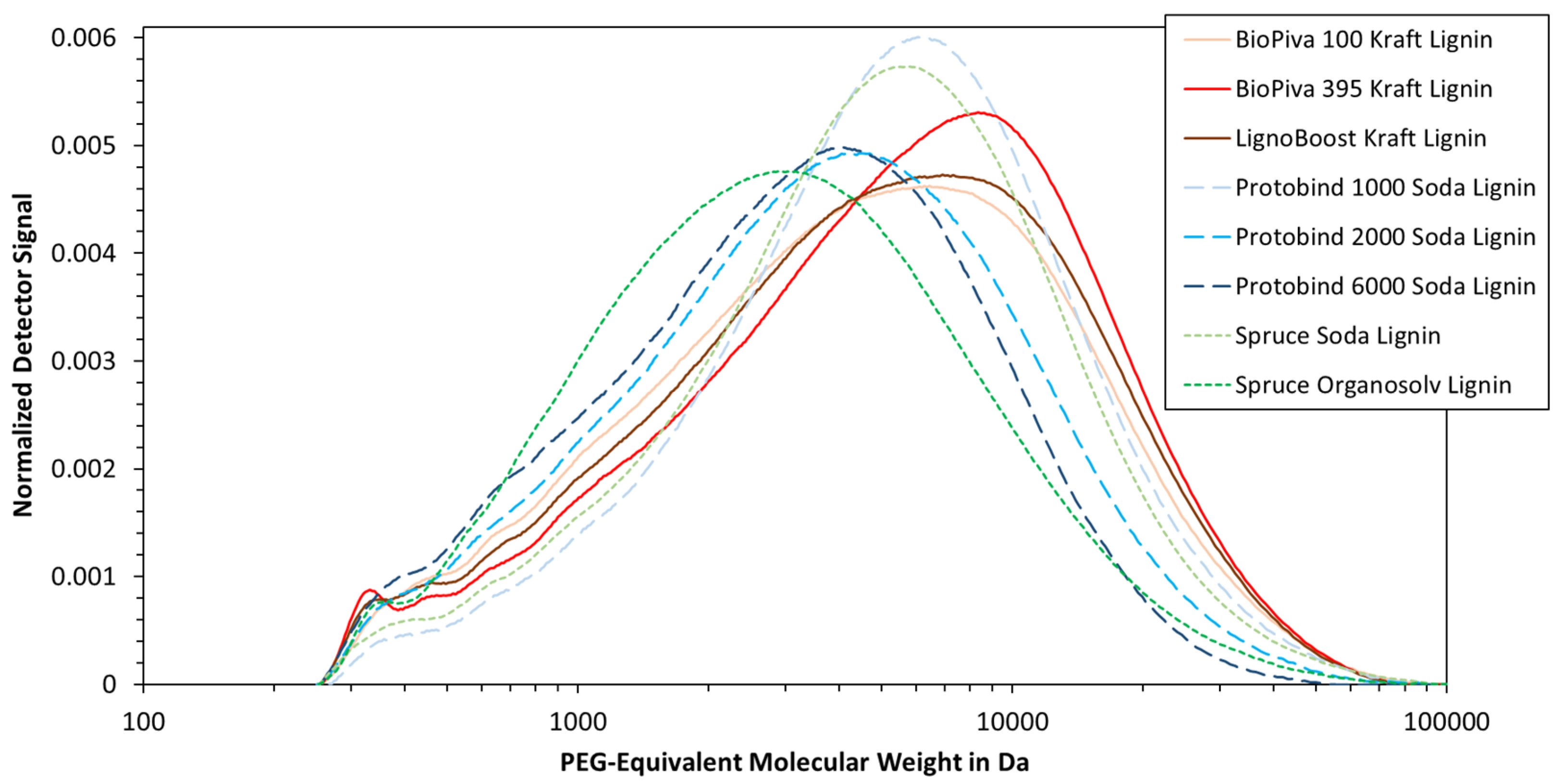
3.4. Lignin Molecular Weight Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Sun, R.C. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Van Wesemael, M.; Feghali, E.; Lufungula, L.L.; Blockhuys, F.; Vanbroekhoven, K.; Eevers, W.; Vendamme, R. Exploring the reactivity of aliphatic and phenolic hydroxyl groups in lignin hydrogenolysis oil towards urethane bond formation. Ind. Crop. Prod. 2022, 180, 114703. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Salvadó, J. Analytical methods for determining functional groups in various technical lignins. Ind. Crop. Prod. 2007, 26, 116–124. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; van Dam, J.E. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Lonchay, W.; Bagnato, G.; Sanna, A. Highly selective hydropyrolysis of lignin waste to benzene, toluene and xylene in presence of zirconia supported iron catalyst. Bioresour. Technol. 2022, 361, 127727. [Google Scholar] [CrossRef]
- Kühnel, I.; Saake, B.; Lehnen, R. Comparison of different cyclic organic carbonates in the oxyalkylation of various types of lignin. React. Funct. Polym. 2017, 120, 83–91. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Hua, Q.; Renneckar, S. A simple route to synthesize esterified lignin derivatives. Green Chem. 2019, 21, 3682–3692. [Google Scholar] [CrossRef]
- de Souza, R.E.; Gomes, F.J.B.; Brito, E.O.; Lelis, R.C.C.; Batalha, L.A.R.; Santos, F.A.; Lounge, D., Jr. A Review on Lignin Sources and Uses. J. Appl. Biotechnol. Bioeng. 2020, 7, 100–105. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- van Aelst, K.; van Sinay, E.; Vangeel, T.; Cooreman, E.; van den Bossche, G.; Renders, T.; van Aelst, J.; van den Bosch, S.; Sels, B.F. Reductive catalytic fractionation of pine wood: Elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.; Wang, H.; Yao, M.; Zhang, L.; Gellerstedt, G.; Lindström, M.E.; Ek, M.; Wang, S.; Min, D. A modified ionization difference UV–vis method for fast quantitation of guaiacyl-type phenolic hydroxyl groups in lignin. Int. J. Biol. Macromol. 2022, 201, 330–337. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E. On the Quantification of Lignin Hydroxyl Groups with 31P and 13C NMR Spectroscopy. J. Wood Chem. Technol. 2015, 35, 220–237. [Google Scholar] [CrossRef]
- McKee, G.A.; Soong, J.L.; Caldéron, F.; Borch, T.; Cotrufo, M.F. An Integrated Spectroscopic and Wet Chemical Approach to Investigate Grass Litter Decomposition Chemistry. Biogeochemistry 2016, 128, 107–123. [Google Scholar] [CrossRef]
- Lee, H.; Feng, X.; Mastalerz, M.; Feakins, S.J. Characterizing lignin: Combining lignin phenol, methoxy quantification, and dual stable carbon and hydrogen isotopic techniques. Org. Geochem. 2019, 136, 103894. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Via, B.K.; Fasina, O.; Han, G. Prediction of mixed hardwood lignin and carbohydrate content using ATR-FTIR and FT-NIR. Carbohydr. Polym. 2015, 121, 336–341. [Google Scholar] [CrossRef]
- Schultz, T.P.; Templeton, M.C.; McGinnis, G.D. Rapid determination of lignocellulose by diffuse reflectance Fourier transform infrared spectrometry. Anal. Chem. 1985, 57, 2867–2869. [Google Scholar] [CrossRef]
- Varol, E.A.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Sills, D.L.; Gossett, J.M. Using FTIR to predict saccharification from enzymatic hydrolysis of alkali-pretreated biomasses. Biotechnol. Bioeng. 2012, 109, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Deus, V.L.; Resende, L.M.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. FTIR and PLS-regression in the evaluation of bioactive amines, total phenolic compounds and antioxidant potential of dark chocolates. Food Chem. 2021, 357, 129754. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.D.; Feliciano, R.P.; Boas, L.V.; Bronze, M.R. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Fourier Transform Infrared Spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 83–109. ISBN 978-3-642-74065-7. [Google Scholar]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Opedal, M.T. Green materials from added-lignin thermoformed pulps. Ind. Crop. Prod. 2022, 185, 115102. [Google Scholar] [CrossRef]
- Hult, E.-L.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crop. Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- ISO 14900:2017(E); Plastics–Polyols for Use in the Production of Polyurethane-Determination of Hydroxyl Number. BSI Publications: London, UK, 2017.
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 9783642740671. [Google Scholar]
- Gosselink, R.J.A.; Abächerli, A.; Semke, H.; Malherbe, R.; Käuper, P.; Nadif, A.; van Dam, J. Analytical protocols for characterisation of sulphur-free lignin. Ind. Crop. Prod. 2004, 19, 271–281. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of lignin and acetylated lignin in organic solvents. BioResources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Wallace, W.E. Infrared Spectra. Available online: https://doi.org/10.18434/T4D303 (accessed on 17 October 2022).
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated Total Refection Infrared Spectroscopy of Proteins and Lipids in Biological Membranes. Biochim. Biophys. Acta 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Wegener, G.; Strobel, C. Bestimmung Der Phenolischen Hydroxylgruppen in Ligninen Und Ligninfraktionen Durch Aminolyse Und FTIR-Spektroskopie. Holz als Roh-und Werkstoff. 1992, 50, 417–420. [Google Scholar] [CrossRef]
- Liptak, M.D.; Gross, K.C.; Seybold, P.G.; Feldgus, S.; Shields, G.C. Absolute pKa Determinations for Substituted Phenols. J. Am. Chem. Soc. 2002, 124, 6421–6427. [Google Scholar] [CrossRef]
- Gross, K.C.; Seybold, P.G. Substituent Effects on the Physical Properties and PKa of Phenol. Proc. Int. J. Quantum Chem. 2001, 85, 569–579. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef]
- Kazzaz, A.E.; Fatehi, P. Technical lignin and its potential modification routes: A mini-review. Ind. Crop. Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]



| Analysis Type | Lignin Raw Material |
|---|---|
| Neat FTIR-ATR | Acetylated lignin |
| FTIR-ATR on lignin in DMSO-d6 | Acetylated lignin |
| Transmission FTIR in KBr | Acetylated lignin |
| Non-aqueous potentiometric titration | Unmodified lignin |
| Amount of acetic acid consumed (total OH) | Reaction solvent after acetic anhydride hydrolysis, buffering, and removal of lignin via filtration |
| Size exclusion chromatography (SEC) | Unmodified lignin |
| Lignin | Mn (g/mol) | Mw (g/mol) | PI |
|---|---|---|---|
| BioPiva 100 Kraft Lignin | 5900 | 13,400 | 2.3 |
| BioPiva 395 Kraft Lignin | 6600 | 13,800 | 2.1 |
| LignoBoost Kraft Lignin | 6100 | 13,500 | 2.2 |
| Protobind 1000 Soda Lignin | 6200 | 12,300 | 2.0 |
| Protobind 2000 Soda Lignin | 4800 | 10,500 | 2.2 |
| Protobind 6000 Soda Lignin | 4200 | 8100 | 2.0 |
| Spruce Soda Lignin | 5800 | 12,000 | 2.1 |
| Spruce Organosolv Lignin | 4000 | 9200 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blindheim, F.H.; Ruwoldt, J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers 2023, 15, 2901. https://doi.org/10.3390/polym15132901
Blindheim FH, Ruwoldt J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers. 2023; 15(13):2901. https://doi.org/10.3390/polym15132901
Chicago/Turabian StyleBlindheim, Fredrik Heen, and Jost Ruwoldt. 2023. "The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy" Polymers 15, no. 13: 2901. https://doi.org/10.3390/polym15132901
APA StyleBlindheim, F. H., & Ruwoldt, J. (2023). The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers, 15(13), 2901. https://doi.org/10.3390/polym15132901







